Abstract
This research describes the use of novel antimalarial combinations of the new artemisinin derivative artemiside, a 10-alkylamino artemisinin. It is a stable, highly crystalline compound that is economically prepared from dihydroartemisinin in a one-step process. Artemiside activity was more pronounced than that of any antimalarial drug in use, both in Plasmodium falciparum culture and in vivo in a murine malaria model depicting cerebral malaria (CM). In vitro high-throughput testing of artemiside combinations revealed a large number of conventional antimalarial drugs with which it was additive. Following monotherapy in mice, individual drugs reduced parasitemias to nondetectable levels. However, after a period of latency, parasites again were seen and eventually all mice became terminally ill. Treatment with individual drugs did not prevent CM in mice with recrudescent malaria, except for piperaquine at high concentrations. Even when CM was prevented, the mice developed later of severe anemia. In contrast, most of the mice treated with drug combinations survived. A combination of artemiside and mefloquine or piperaquine may confer an optimal result because of the longer half life of both conventional drugs. The use of artemiside combinations revealed a significant safety margin of the effective artemiside doses. Likewise, a combination of 1.3 mg/kg of body weight artemiside and 10 mg/kg piperaquine administered for 3 days from the seventh day postinfection was completely curative. It appears possible to increase drug concentrations in the combination therapy without reaching toxic levels. Using the drug combinations as little as 1 day before the expected death of control animals, we could prevent further parasite development and death due to CM or anemic malaria. Earlier treatment may prevent cognitive dysfunctions which might occur after recovery from CM.
INTRODUCTION
Three hundred to 500 million people are infected annually with malaria, and about 1 million, mostly children under 5 years, die (47, 48). Plasmodium falciparum causes most cases of severe malaria, which sometimes is associated with coma and death; young children and pregnant women are especially vulnerable. Splenomegaly, severe headache, cerebral ischemia, hepatomegaly, hypoglycemia, and renal failure may occur (4, 23). Malaria induced by P. falciparum can cause irreversible neurocognitive damage, especially in children. This neurologic damage is associated with cerebral malaria (CM), which is the most severe central nervous system complication of P. falciparum infection (6, 24, 45). A significant number of P. falciparum malaria cases are expressed as CM (24, 32).
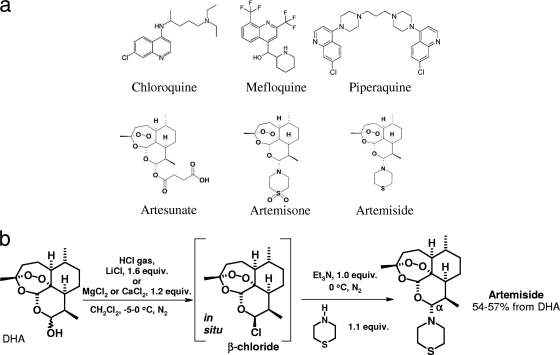
As no successful vaccine for malaria is available, the elimination of the disease is dependent on antimalarial drugs. Unfortunately, in countries where malaria is endemic, many P. falciparum strains now have become resistant to formerly effective antimalarial drugs, making alternative drugs a necessity. Derivatives of the natural compound artemisinin constitute a recent group of antimalarial agents in clinical use or under development (Fig. 1a). These derivatives include dihydroartemisinin (DHA), artesunate, artemether, artemisone, and artemiside, the thiomorpholine precursor of artemisone (21). A key strategy to prevent the emergence and spread of resistance is to use artemisinin derivatives in combination with partner drugs in artemisinin-based combination therapy, termed ACT (2, 33, 49). Because artemisinin monotherapy leads to a high rate of recrudescence, other drugs are required to clear all parasites and prevent recurrence. When used as a drug combination, the artemisinin derivatives are effective in a 3-day course because the partner drug, with a longer plasma half-life, fully eliminates remaining parasites. This combined therapy is more efficient and also protects both components of the ACT from causing the selection of resistant parasites (1, 18).
Fig 1.
(a) Drug structures used for treatment of infected mice. (b) Preparation of artemiside in one step from dihydroartemisinin (DHA). DHA is treated with HCl gas in the presence of a group I or II metal salt, and then, after the degassing of the reaction mixture, with thiomorpholine. The formation of the β-chloride in this reaction is easily detected by conducting 1H nuclear magnetic resonance (NMR) spectroscopic examinations of reactions conducted in deuterated dichloromethane (CD2Cl2). Other approaches also have been used (21).
Among the artemisinin derivatives, artemisone and artemiside are newer semisynthetic 10 alkylaminoartemisinins that can be synthesized from DHA (Fig. 1a). Artemisone is highly effective against P. falciparum in monkeys and has been used in phase IIa clinical trials for nonsevere malaria in humans (34). Recent work also indicates the marked superiority of artemiside over artemisone in both in vitro and in vivo studies involving the apicomplexan parasite Toxoplasma gondii (12). Artemiside has an advantage over artemisone in that its preparation from DHA requires only a one-step procedure (Fig. 1b). Artemiside has been shown to be potent in treating cerebral malaria (46), which we hypothesize is due to its antiplasmodial capacity and its interactions with the immune system.
The hypothesis of this research is that artemiside is a very potent member of its class for the treatment of severe malaria, with an emphasis on CM prevention, especially when used in combination therapy with conventional antimalarial drugs.
MATERIALS AND METHODS
Parasites.
Plasmodium berghei ANKA was maintained in vivo by the serial transfer of parasitized erythrocytes (PE) from infected to naïve mice. Experimental mice were infected by the intraperitoneal (i.p.) injection of 5 × 104 (Jerusalem, Israel) to 5 × 105 (Sydney, Australia) PE from peripheral blood of infected donor mice, an inoculum that caused fatal experimental cerebral malaria (ECM) in at least 80% of infected C57BL/6 mice. The link between early death and ECM in mouse models has been discussed previously (46): mice that died at a parasitemia of 20% or below with accompanying neurological symptoms and drastic reductions in body weight and temperature were considered to have died of ECM, which usually was confirmed by the presence in the central nervous system of hemorrhages, edema, and intravascular leukocyte accumulation upon histopathological analysis. Untreated mice which did not die from ECM went on to succumb to severe anemia and hyperparasitemia, as has been reported in all other cases where mice are resistant to P. berghei ANKA-induced ECM (14, 35).
The 3D7 strain of P. falciparum (purchased from the American Type Culture Collection [ATCC]) was grown in culture as specified later.
Animals.
C57BL/6 mice (C57BL/6 from Harlan, Israel, or from the Animal Resource Centre, Perth, Australia) aged 7 to 8 weeks were used in all experiments, with 6 to 10 mice per group. The mice were housed under standard light and temperature conditions and provided with unlimited access to water and food. All experiments were carried out in accordance with institutional guidelines for animal care by following protocols approved by the Animal Ethical Care Committee of The Hebrew University of Jerusalem or the University of Sydney. Parasitemia was monitored every other day, from the time of inoculation until death or sacrifice, by thin-blood Giemsa-stained smears prepared from tail blood. The slides were examined under a light microscope, and parasitemia was determined as the number of infected red blood cells per 10,000 erythrocytes. Clinical score was evaluated and used for scoring disease severity (using a scoring chart [46]). Mice were euthanized when they reached a degree of disease severity that inevitably would have led to their death.
Histology.
Mice were deeply anesthetized with isoflurane and sacrificed by terminal intracardial perfusion with 10 ml ice-cold phosphate-buffered saline (PBS). Organs were removed and fixed overnight in 10% (vol/vol) neutral buffered formalin (Fronine, Australia). Paraffin-embedded tissues were cut into 5- to 7-μm slices, deparaffinated, and stained with hematoxylin and eosin before coverslipping.
Drugs.
DHA and artesunate were purchased from the Kunming Pharmaceutical Corporation.
Artemisone was synthesized from DHA and purified by flash column chromatography, followed by recrystallization according to the procedure previously reported (21).
Artemiside was prepared according to a new procedure (Fig. 1b) as follows. HCl gas was passed over a stirred mixture of DHA (5.0 g; 17.6 mmol) and LiCl (1.2 g; 28.3 mmol) in dichloromethane (80 ml) at 0°C. The reaction mixture changed from colorless to pale purple. The system was gently flushed with nitrogen gas for 5 min. After degassing further under reduced pressure with alternate nitrogen flushing, CH2Cl2 (20 ml) was added to rinse the reaction flask and to dilute the reaction mixture. The reaction mixture was treated with the slow dropwise addition of a mixture of thiomorpholine (2.0 ml, 2.1 g, and 19.9 mmol) and triethylamine (2.5 ml, 1.8 g, and 18.0 mmol). The color of the reaction mixture changed to pale yellow. After 45 min, the reaction mixture was quenched with brine (50 ml). The organic layer was separated, and the aqueous layer was extracted with dichloromethane (twice with 20 ml). The combined organic extracts were dried (MgSO4). The filtration and evaporation of the filtrate gave a pale yellow solid, which was recrystallized by the addition of methanol (80 ml) to give 10α-(thiomorpholino)dihydroartemisinin as a fine white microcrystalline solid (3.58 g; 55%). Further material was able to be recovered from the mother liquor by concentration and crystallization. An analytical sample of artemiside was obtained by recrystallization from methanol to give the product as white needles. m.p. 147.0 to 147.6°C; [α]D20 = + 17° (c 0.021, CHCl3); IR (film) νmax 2,924, 2,872, 1,454, 1,418, 1,376, 1,326, 1,278, 1,226, 1,198, 1,184, 1,154, 1,130, 1,100, 1,056, 1,038, 1,018, 988, 940, 926, 880, 850, 828, 756; δH 5.26 (1H, s, H-12), 3.93 (1H, d, J = 10.21 Hz, H-10), 3.23 to 3.31 (2H, m), 2.92 to 2.96 (2H, m), 2.60 to 2.67 (5H, m), 2.25 to 2.45 (1H, m), 1.95 to 2.05 (1H, m), 1.80 to 1.92 (1H, m), 1.67 to 1.70 (2H, m), 1.14 to 1.52 (5H, m), 1.36 (3H, s, H-14), 0.90 to 1.04 (1H, m), 0.91 (3H, d, J = 6.14 Hz, H-16), 0.76 (3H, d, J = 7.18 Hz, H-15); δC 104.20, 92.28, 91.42, 80.11, 52.20, 51.30, 46.60, 37.90, 36.80, 34.90, 28.45, 26.35, 25.1, 22.10, 20.85, 13.90; MS (CI, NH3) m/z 370 (M++1, 100), 324 (70), 310 (10); Anal. Calc. for C19H31NO4S: C, 61.76; H, 8.46; N, 3.79; found C, 62.04; H, 8.39; N, 3.65.
Piperaquine was donated by Cipla Ltd., Mumbai, India. It was dissolved in distilled deionized water at pH 3.5 (reached with HCl) and injected in 100 μl of solution.
Dimethyl sulfoxide (DMSO) and chloroquine diphosphate were purchased from Sigma-Aldrich, Ltd. All artemisinin derivatives were prepared in DMSO according to the required dosage and administered in a volume of 20 μl by intraperitoneal injection.
Chloroquine diphosphate (Sigma) was dissolved in PBS and administered in a 50-μl volume by intraperitoneal injection.
Mefloquine (Sigma) was dissolved in DMSO and used as the artemisinin derivative.
Drug structures that were used in the current research are presented in Fig. 1a. Drugs were injected six times, either (i) starting in the evening of day 5 postinfection (after inoculation with parasites), twice on days 6 and 7, and once on day 8 (morning), or (ii) twice a day on days 7 to 9 postinfection. All drugs were injected twice a day except for piperaquine, which was injected once a day due to its longer retention time in mice.
Cytotoxicity assay in THP-1 cell line.
A monocyte THP-1 cell line (ATCC) was cultured in suspension in flat-bottom flasks (Nunc) at 37°C in a humidified 5% CO2 incubator. The growth medium (Biological Industries, Beit-Haemek, Israel) was RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 μg/ml), and streptomycin (100 μg/ml). Before each experiment the cells were washed, and an appropriate volume was centrifuged, resuspended, and diluted in growth medium to the desired cell concentration. Drug toxicity was determined in THP-1 cells using an alamarBlue viability assay. THP-1 cells were plated into 96-well flat-bottom plates (Nunc) at a density of 4 × 105 to 8 cells/ml, which corresponds to 0.5 × 105 to 1 × 105 cells/well. Using an automated dispenser, 125 μl cell suspension was added to the wells. Compounds to be tested were diluted in complete medium, and 125 μl was aliquoted in triplicate to the wells. After 48 h of incubation at 37°C, the cell viability indicator alamarBlue (AbD Serotec, United Kingdom) was added (25 μl/well), the plates were incubated for an additional 3 h at 37°C, and the fluorescence (excitation wavelength, 544 nm; emission wavelength, 590 nm) was read using a microplate reader (Fluoroskan Ascent FL, Finland). The percent growth inhibition of the cells was calculated according to the equation % inhibition = [(fluorescence control − fluorescence test)/(fluorescence control)] × 100.
High-throughput analysis of in vitro antiplasmodial activity. (i) P. falciparum drug susceptibility assay.
Parasites were grown as previously described (44). For drug susceptibility assays, 20 μl of RPMI 1640 (l-glutamine and HEPES; Invitrogen) with 5 μg/ml gentamicin was dispensed in each well with a liquid dispenser (Matrix Wellmate; Thermo Scientific) in an assay plate (384-well black polystyrene microplate, clear bottom, tissue culture treated; Corning). DMSO inhibitor stock solutions were pin transferred (V&P Scientific) into the assay plate. Twenty μl of a synchronized culture suspension (1% rings, 4% hematocrit) was added to each well, thus making a final hematocrit and parasitemia of 2 and 1%, respectively. Assay plates were incubated for 72 h, and parasite development was determined based on a modified protocol (42). Briefly, 10 μl of mixed DNA dye solution in RPMI (10× SYBR green I, 1% [vol/vol] Triton X-100, 1 mg/ml saponin) was added to each well. Assay plates were shaken for 30 s at 2,000 rpm and incubated in the dark for 4 h, and then fluorescence was measured (excitation at 485 nm, emission at 535 nm) in an Envision plate reader (Perkin Elmer).
(ii) IC50 determination and combination experiments in synergy experiments.
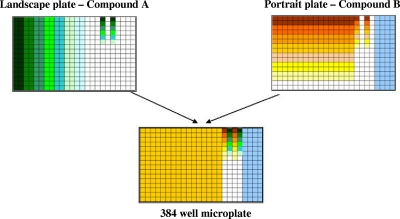
To determine the 50% inhibitory concentrations (IC50s) of drugs on cultures of P. falciparum, 3-fold serial dilutions resulting in 10 different concentrations in DMSO were prepared from stock solutions. Tests were run in triplicate in two independent runs to determine IC50s against the 3D7 strain for each drug. Based on IC50s, the top concentration for each compound was calculated using the following formula to center the compound's IC50 around the median dilution point of the plate: top concentration = IC50 × 1.5 × 2.53. If IC50s were greater than 0.5 μM, then a top concentration of 10 μM was used. Landscape and portrait compound plates for drug combination experiments (Fig. 2) were made with a liquid handler (Tecan robot Freedom EVO; Tecan Group Ltd.).
Fig 2.
Combination experiment strategy. Compound A was dispensed in all wells of column 1 and then 2.5-fold serial diluted down the row (landscape plate) from left to right until column 16, each in duplicate. Compound B was dispensed in wells A1 to A16 and then 2.5-fold serial diluted from top to bottom from row A through P, each in duplicate. Compounds A and B also were 2.5-fold serial diluted in columns 17 and 19 (landscape plate) and in columns 18 and 20 (portrait plate) to determine their individual IC50s. Controls included the following columns: 22, 100% DMSO as a negative control (0% growth inhibition); 23, 10 mM mefloquine solution as a positive control (100% growth inhibition); 21 and 24, 3-fold serial dilutions of 10 mM mefloquine and 10 mM chloroquine, respectively. Binary combinations were performed by combining a portrait plate (compound A) and a landscape plate (compound B) in a 384-well microplate. Additional controls included combining a landscape plate with its portrait counterpart.
(iii) Data analysis.
Data were parsed with an in-house algorithm, RISE (for robust investigation of screening experiments). IC50s were calculated using Pipeline Pilot v7.5 and GraphPad software. Synergy or antagonism was determined using the following models.
(a) Bliss independence (3).
The Bliss noninteraction for two drugs in combination is expressed by the following equation: Eab = Ea + Eb − (Ea × Eb), where E is the fractional effect and a and b are the doses of two compounds. The expected effect (Eab) is compared to the experimental data and plotted as a heat map for visual analysis. A modified version of the Bliss analysis was done using a Z score. The average Z score was derived from controls in which a compound was tested against itself.
(b) Fifty percent fractional inhibition concentration (5) (FIC50).
A derivative of the Loewe additivity model, FIC was determined using the following equation: FIC50 = IC50a/IC50A + IC50b/IC50B, where IC50a and IC50b are the respective IC50s of the individual drug in the combination and IC50A and IC50B are the respective IC50 of each drug alone.
Drug combinations were defined as nonadditive when more than one binary combination effect lay outside the predicted effect (Bliss) and when more than one FIC value was outside the 95% confidence interval of the control FIC50 (using a compound against itself as a control).
Manual analysis of in vitro antiplasmodial activity.
The effect of piperaquine and its combinations with artemiside against P. falciparum were manually evaluated in vitro by a luciferin-luciferase bioluminescence assay. For this experiment, we use erythrocytic stages of P. falciparum stably expressing the luciferase gene by the hrp2 promoter from a chromosomal locus (Pf:LUC). Cultures containing predominantly ring forms were used. Upon reaching 3% parasitemia, cultures were transferred to a 96-well flat-bottom sterile plate (200 μl/well). Diluted drugs were added to the appropriate wells and tested in triplicate. The plate was incubated in a sealed culture chamber for 24 h in an atmosphere of 5% O2, 5% CO2, and 90% N2. After 48 h the medium was removed and the erythrocytes lysed by lysis buffer of the Bright-Glo luciferase assay system (100 μl/well). Fifty μl of the Bright-Glo substrate was added to each well, and the luminescence was measured by a luminometer (Fluoroskan Ascent FL; Thermo).
Statistics.
When comparing parasitemia, P values were calculated using Student's t test; for the analysis of survival curves, the Kaplan-Meier test was employed. In both cases, values below 0.05 were considered significant.
RESULTS
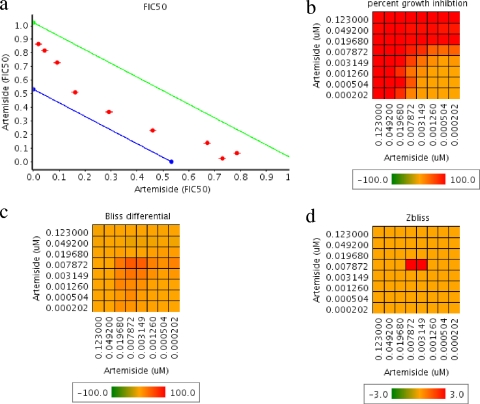
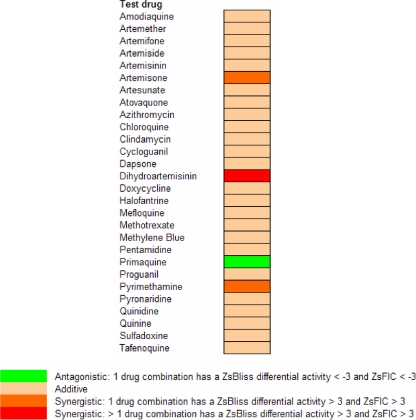
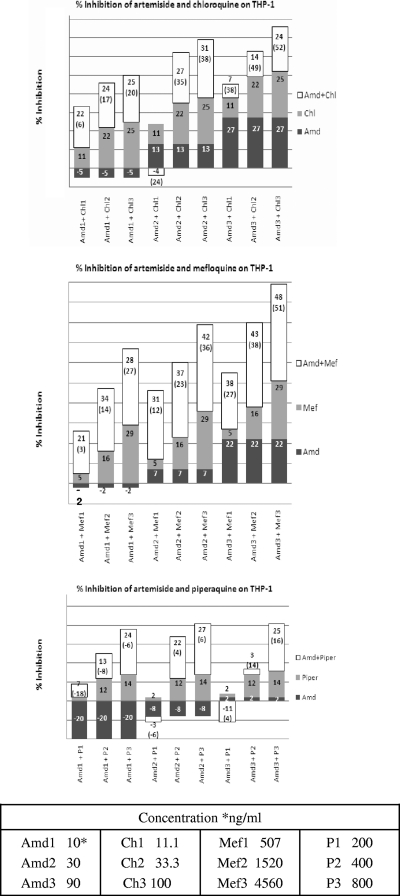
Drug susceptibility assays were performed in P. falciparum cultures using high-throughput techniques. Piperaquine and its combinations with artemiside were manually examined in microplates. The IC50s for chloroquine, mefloquine, piperaquine, artesunate, artemisone, and artemiside are shown in Table 1. All IC50s were within an average of 2.3 to 52.4 nM. The counts for individual drugs were within 10% of the average result. To evaluate synergistic combinations, we used two orthogonal methods (FIC50 and Bliss independence), and the combination of artemiside with itself was used to define the additive background (Fig. 3). A summary of the overall results of examining combinations of artemiside with conventional antimalarial drugs is shown in Fig. 4. Primaquine was the only antagonistic drug, while most drugs were additive. The artemiside-artemiside combination was additive, as expected, but some other artemiside-artemisinin derivative combinations were synergistic in one run (and additive in two runs). Synergism among various artemisinins could be explained by the fact that they may affect the parasites by multiple mechanisms (see Discussion) that are effective at different concentrations. In view of these results and the availability of reference results of other ACTs (18, 36, 37, 43), we conducted in vitro cytotoxicity experiments with THP1 cells and in vivo combination experiments to monitor artemiside combinations with mefloquine and piperaquine. Chloroquine also was examined due to its proven in vivo synergistic effect with artemisone, another 10-alkyl amino artemisinin (46). The cytotoxicity results show that all compounds tested, including their combinations with artemiside, are nontoxic at doses much higher than needed to kill P. falciparum in culture (Fig. 5).
Table 1.
IC50s of individual drugs in P. falciparum cultures
| Compound | IC50 of P. falciparum strain 3D7 in: |
|
|---|---|---|
| nM | ng/ml | |
| Artemiside | 5.70 | 2.10 |
| Artemisone | 2.30 | 0.92 |
| Artesunate | 9.70 | 3.70 |
| Chloroquine | 42.9 | 13.7 |
| Mefloquine | 31.2 | 11.8 |
| Piperaquinea | 52.4 | 28.1 |
Piperaquine was examined manually (i.e., not by the high-throughput system).
Fig 3.
Artemiside pairwise combinations examined in vitro. Artemiside combined with itself was used as a control for additivity. (a) Fractional IC50 of artemiside in combination with itself. The surface between the green and the blue lines defines the additivity area. The distance between these two lines equals 95% of the FIC50 confidence interval. (b) Heat map representing the percentage of the growth inhibition of the tested drug combinations. (c) Heat map representing the Bliss differential of the drug combinations, the difference in growth inhibition between observed and predicted values. (d) Zbliss is derived from data shown in panel c using Z scores, considering all Bliss differential values as additive values. Value in panels b, c, and d are averages from four dependent replicates.
Fig 4.
Artemiside pairwise combinations examined in vitro. A color-coded summary of the pairwise combinations examined by the high-throughput system is shown. For each pair, FIC50s and Bliss values were calculated (as shown in Fig. 3), and the degree of synergy was estimated according to defined criteria based on Z scores (Zs).
Fig 5.
Cytotoxicity assays of artemiside (Amd) combinations with the standard antimalarial drugs chloroquine (Ch), mefloquine (Mef), and piperaquine (P). The numbers represent the experimental results. The numbers in parentheses are the theoretical additive values, e.g., Amd2 (30.0 ng/ml), Ch2 (33.3 ng/ml), and their combination inhibit 13, 22, and 27% of untreated THP-1 growth, respectively. The theoretical additive value is 35% (13% ± 22%).
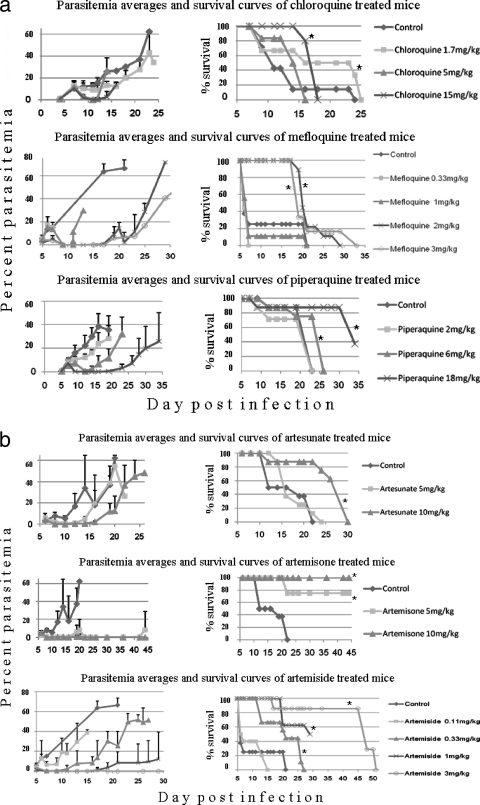
The individual effects of artemiside, chloroquine, mefloquine, and piperaquine on P. berghei ANKA infections in C57BL/6 mice were determined. Artesunate also was used for comparison with artemiside. Figures 6a and b depict the resulting parasitemias and survival curves. Following treatment, parasitemias were reduced and survival prolonged, in parallel with an (temporary) increase in body weight and temperature (data not shown). All parameters were affected in a dose-dependent manner. However, the most pronounced effects were recorded for artemiside: 0.66 mg/kg treatment generally was as beneficial as 10-fold greater concentrations of the other compounds. In general, individual drugs at the concentrations that were examined failed to prevent malarial death or CM (Table 2). However, most of the mice were rescued by using high concentrations of artemisone and artemiside (>5 and >3 mg/kg for artemisone and artemiside, respectively). The results obtained with the individual drugs enabled us to select concentrations that induced a temporary reduction in parasitemia (Fig. 6a and b) and clinical score (data not shown) for further in vivo combination experiments.
Fig 6.
(a) Treatment of P. berghei ANKA-infected mice with individual conventional antimalarials. An asterisk indicates significance (P < 0.01). (b) Treatment of P. berghei ANKA-infected mice with individual artemisinin derivatives. An asterisk indicates significance (P < 0.01) in each panel.
Table 2.
Summary of in vivo treatmentsa
| Compound(s) (in mg/kg) | No. of mice |
||
|---|---|---|---|
| dCM | dSM | Survived | |
| Control saline | 17 | 8 | 0 |
| Control DMSO | 13 | 4 | 0 |
| Chloroquine (1.7) | 3 | 3 | 0 |
| Chloroquine (5) | 4 | 2 | 0 |
| Chloroquine (15) | 7 | 5 | 0 |
| Mefloquine (0.33) | 8 | 1 | 0 |
| Mefloquine (1) | 8 | 1 | 0 |
| Mefloquine (2) | 7 | 2 | 0 |
| Mefloquine (3) | 5 | 1 | 0 |
| Piperaquine (2) | 4 | 3 | 0 |
| Piperaquine (6) | 3 | 5 | 0 |
| Piperaquine (10) | 0 | 7 | 1 |
| Piperaquine (18) | 2 | 4 | 2 |
| Artesunate (5) | 6 | 2 | 0 |
| Artesunate (10) | 3 | 5 | 0 |
| Artemisone (5) | 8 | 3 | 5 |
| Artemisone (10) | 4 | 3 | 8 |
| Artemiside (0.11) | 9 | 1 | 0 |
| Artemiside (0.33) | 3 | 6 | 0 |
| Artemiside (0.66) | 8 | 0 | 0 |
| Artemiside (1) | 3 | 1 | 4 |
| Artemiside (3) | 5 | 0 | 2 |
| Chloroquine (5) and artemisone (10) | 1 | 0 | 6 |
| Chloroquine (15) and artemisone (10) | 0 | 0 | 7 |
| Piperaquine (10) and artemisone (5) | 0 | 0 | 8 |
| Chloroquine (10) and artemiside (0.66) | 1 | 3 | 2 |
| Mefloquine (2) and artemiside (0.33) | 2 | 3 | 4 |
| Mefloquine (1) and artemiside (1) | 1 | 0 | 5 |
| Mefloquine (2) and artemiside (1) | 1 | 0 | 7 |
| Piperaquine (10) and artemiside (0.66) | 0 | 0 | 9 |
The table summarizes in vivo experiments in which mice were injected with P. berghei ANKA and treated with drugs alone or in combination with artemiside. Mice that died at a parasitemia below 20% with accompanying neurological symptoms, matching clinical score, and brain histology were considered to have died of CM (dCM). Mice which did not die of CM went on to die at higher parasitemias, with severe anemia (dSM).
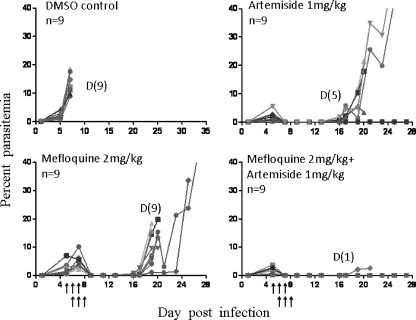
Figure 7 depicts a representative experiment in which artemiside, mefloquine, and their combination were used. Vehicle control (DMSO) and mefloquine alone failed to prevent the death of the infected mice. Mefloquine and artemiside prevented the early death of the mice by the initial reduction of the parasitemias to a nondetectable level. Both drugs delayed recrudescence by about a week. However, artemiside reduced the death to 4 out of 9 mice, while all mefloquine-treated mice eventually developed fatal illness and had to be euthanized. All control mice died of CM at parasitemias lower than 20%. Most animals that died in the mefloquine and artemiside groups also suffered from CM following recrudescence. In contrast, most mice (8/9) that were treated by the drug combination were free of parasites even after three injections and survived. One animal in this group died of CM at day 23 postinfection.
Fig 7.
Artemiside-mefloquine effects in P. berghei ANKA-infected mice. Each line represents parasitemias of a single mouse. Arrows denote treatment times. n, number of mice in the group; D, number of dead mice in the group.
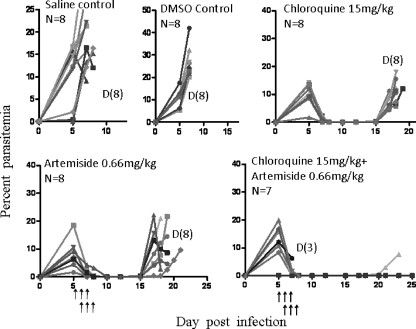
Figure 8 depicts a representative experiment that is similar in its design to the previous one, and it demonstrates the effect of artemiside combined with chloroquine. The results generally are similar to those obtained with the artemiside-mefloquine combination: the drugs delayed recrudescence after the initial reduction of parasitemia, and the combination gave a significant increase in survival.
Fig 8.
Artemiside-chloroquine effects in P. berghei ANKA-infected mice. Each line represents parasitemias of a single mouse. Arrows denote treatment times. n, number of mice in the group; D, number of dead mice in the group.
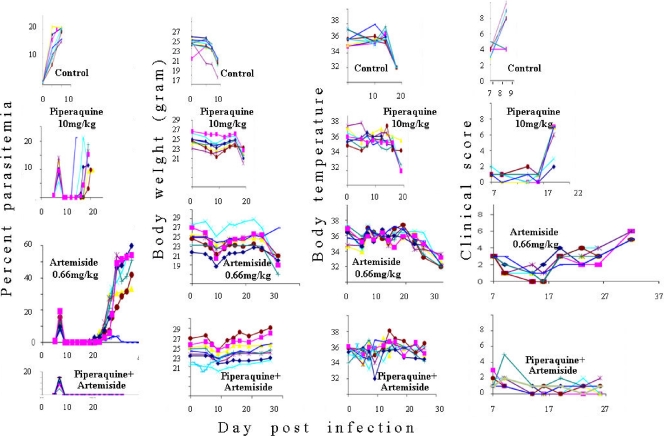
Figure 9 shows the results of treatment using an artemiside-piperaquine combination. The figure depicts the results of individual mice to demonstrate the reproducibility of the results. Drugs alone (artemiside at 0.66 mg/kg and piperaquine at 10 mg/kg) reduced the initial peak parasitemia and delayed the recrudescence but did not prevent the mortality (except for that of one mouse in the piperaquine group). In contrast, the combination reduced the initial peak, there was no recrudescence, and all mice survived. Parasitemia monitoring in this experiment was accompanied by measuring weight and temperature and the estimation of the clinical score. Weight, temperature, and clinical score were in accordance with the parasitemias.
Fig 9.
Artemiside-piperaquine effects in P. berghei ANKA-infected mice. Each line represents a single mouse. Artemiside treatment was twice a day and piperaquine once a day on days 7 to 9 postinfection. Each group included eight mice.
DISCUSSION
The decreasing effectiveness of the antimalarial arsenal due to drug resistance necessitates constant efforts to develop new drugs. Artemisinin derivatives are considered the front line of treatment, but there already are indications of resistance to the currently used artemisinins by P. falciparum, especially to artemether and artesunate (11, 18). Consequently, it is critical to evaluate the antimalarial effect of newer artemisinins that could be developed as ACT formulations (31). While one important consideration is that the drug combination should assist in countering the development of resistance, it must be recognized that artemisinins have short half-lives, and to avoid recrudescence the combination partner should have a long half-life (13). For this reason, mefloquine was first used in combination with artemisinin by Li and coworkers, where it was most effective in curing chloroquine-resistant malaria on Hainan Island (27).
The research here describes the use of novel combinations of the new artemisinin compound artemiside, a 10-alkylamino artemisinin derivative. Artemiside was prepared in a one-step process from DHA that requires no chromatographic purification, and because of the relative accessibility of one of the other starting materials, thiomorpholine (Fig. 1b), artemiside is more economically prepared than is artemisone, the 10-alkylamino artemisinin derivative that is currently under development for clinical use. Artemiside is a stable, highly crystalline compound that appears to have a stability profile similar to that of artemisone (results not shown). We found in P. falciparum cultures that its activity is more pronounced than that of artesunate and artemisone. The differences in the activity of the individual drugs also were expressed in our in vivo model of C57BL/6 mice that were infected with P. berghei ANKA.
The testing of the antiplasmodial activity of various artemiside combinations in vitro revealed a relatively large number of conventional antimalarials that were additive with artemiside. These results and the in vivo testing of candidate drugs enabled the selection of drug concentrations for monotherapies that had a significant effect on the course of experimental malaria but did not prevent death. Subsequent drug combination studies used these concentrations. Following monotherapy, individual drugs reduced parasitemias to nondetectable levels. However, after a period of latency, parasites again appeared in the blood and eventually all mice died. The treatments did not prevent CM in the mice with malaria recrudescence, except for piperaquine, which decreased CM frequency at its higher concentrations. Even when CM was prevented, the mice died later of severe anemia. In contrast, most of the mice treated by drug combinations survived.
Compared to artemisinins in current use, artemisone is nonneurotoxic both in vitro and in vivo (9). Artemiside, the thiomorpholine precursor of artemisone, is more lipophilic (log P, 4.97; calculated log P, 3.98) than artemisone (log P, 2.49; calculated log P, 2.08); however, in line with the facile metabolism of drugs containing thioether linkages, it is expected to be irreversibly metabolized in vivo into artemisone (15, 39). In in vitro neurotoxicity screens, artemiside has IC50s that are approximately two orders of magnitude higher than those of artemisinin (21). In vivo pilot tolerability screens involving the treatment of male rats with artemiside (10 mg/kg for 14 days by gavage) in sesame oil (a vehicle that exacerbates neurotoxic effects) elicited no clinical effects. In contrast to artemisone, which has no effect, artemiside administered at 50 mg/kg for 14 days induced body weight loss, reduced motility, uncoordinated gait, the discoloration of feces, and piloerection. However, according to our current results, artemiside is a more efficient antiplasmodial drug than artemisone in vivo. Moreover, the use of artemiside combinations with other drugs revealed a significant safety margin of the effective artemiside doses. For example, a combination of 1.3 mg/kg artemiside and 10 mg/kg piperaquine administered for 3 days from the seventh day postinfection was completely curative. Overall, in view of the outcome of the in vitro antiplasmodial and cytotoxicity tests and our in vivo results, it is most probable that the concentrations of the drugs used in the combination therapy can be increased without reaching toxic levels. Consequently, the parasites will be completely eliminated.
There are a few mechanisms of action proposed for artemisinins (10, 17, 38). It is commonly accepted that artemisinins are accumulated in parasitized erythrocytes, and that they increase oxidant stress within the parasitized cell. Most recently, it has been shown that artemisinins potently interfere with flavin cofactors that are essential for the functioning of flavin disulfide reductases that maintain redox homeostasis in the intraerythrocytic parasite (19, 20). Other mechanisms have been suggested. Therefore, it is possible that there is more than one mechanism of action for a single artemisinin compound, and that different mechanisms are active at different drug concentrations. This also should be considered in selecting a drug combination (where the result could be a potentiation of different mechanisms). It may be possible to elucidate the use of individual drugs that are less effective alone because of parasite resistance but are efficient and synergistic in a combination (e.g., Malarone contains atovaquone and proguanil, and P. falciparum resistance to both of these has been shown). It has been suggested that artemisinin drugs induce P. falciparum dormancy, and this is the reason for recrudescence, but a recent paper by Codd et al. (8) suggests that the dormancy recovery hypothesis is not valid. In any case, a drug combination should prevent (in high enough doses) the reappearance of plasmodia. We achieved such an effect by using the artemiside-piperaquine combination. Using the drug combinations as little as 1 day before the anticipated death of control animals, we could prevent further parasite development and death due to CM or anemic malaria. However, if treatment was started at a later stage (12 h before the death of control mice), even higher doses of the drugs could not prevent terminal illness (data not shown). Obviously, the earlier the antimalarial treatment is given to a patient, the better the chance that the treatment will be successful. This is especially vital if the pathogenic process leading to CM has been initiated already, which in ECM occurs at least several days before terminal illness (7). In this case, a delayed treatment may eliminate the parasites and prevent death but not neurological damage (25, 28). Cognitive dysfunction might be sustained after rescue therapy in ECM, but it is reduced by additive antioxidant therapy (40). This might be a hint to consider drug combinations that also include components that reduce oxidant stress. In addition, immunomodulators that could alleviate CM should be considered for adjunctive therapy due to the inflammatory etiological nature of CM (and severe malaria in general) in both human malaria and experimental models (16, 22, 26, 29, 30, 41). Obviously, an adjunctive drug also should be examined in combination with the antiplasmodial drugs to exclude any deleterious antagonistic effects.
ACKNOWLEDGMENTS
We thank Lee Kin-Wo and Wu Yuet from the Hong Kong University of Science and Technology (HKUST) for their technical help in the synthesis of artemiside.
We thank Cipla Ltd., Mumbai, India, for the kind donation of piperaquine.
The work at The Hebrew University was supported by grants from the Gretel B. Bloch Charitable Trust, the Sir Zelman Cowen Universities Fund, and the Israel Science Foundation.
Work at HKUST was carried out in the Open Laboratory of Chemical Biology of the Institute of Molecular Technology for Drug Discovery and Synthesis with financial support from the Government of the HKSAR University Grants Committee Areas of Excellence Fund, project no. AoE P/10-01/01-02-I and AOE/P-10/01-2-II, and the University Grants Council (grant no. HKUST 6493/06 M and 600507).
The work at the University of Sydney was supported by the National Health and Medical Research Council of Australia and the Sir Zelman Cowen Universities Fund.
Footnotes
Published ahead of print 17 October 2011
REFERENCES
- 1. Adjuik M, et al. 2002. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet 359:1365–1372 [DOI] [PubMed] [Google Scholar]
- 2. Ashley EA, White NJ. 2005. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 18:531–536 [DOI] [PubMed] [Google Scholar]
- 3. Bliss CI. 1939. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26:585–615 [Google Scholar]
- 4. Buffet PA, et al. 2011. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117:381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canfield CJ, Pudney M, Gutteridge WE. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373–381 [DOI] [PubMed] [Google Scholar]
- 6. Carter JA, Neville BGR, Newton C. 2003. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res. Rev. 43:57–69 [DOI] [PubMed] [Google Scholar]
- 7. Chanling T, Neill AL, Hunt NH. 1992. Early microvascular changes in murine cerebral malaria detected in retinal wholemounts. Am. J. Pathol. 140:1121–1130 [PMC free article] [PubMed] [Google Scholar]
- 8. Codd A, Teuscher F, Kyle DE, Cheng Q, Gatton ML. 2011. Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malar. J. 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Alessandro S, et al. 2007. Differential effects on angiogenesis of two antimalarial compounds, dihydroartemisinin and artemisone: Implications for embryotoxicity. Toxicology 241:66–74 [DOI] [PubMed] [Google Scholar]
- 10. Ding XC, Beck HP, Raso G. 2011. Plasmodium sensitivity to artemisinins: magic bullets hit elusive targets. Trends Parasitol. 27:73–81 [DOI] [PubMed] [Google Scholar]
- 11. Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunay IR, Chan WC, Haynes RK, Sibley LD. 2009. Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob. Agents Chemother. 53:4450–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 7:864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engwerda C, Belnoue E, Gruner AC, Renia L. 2005. Experimental models of cerebral malaria, p. 103–143 In Langhorne J. (ed), Immunology and immunopathogenesis of malaria, vol. 297 Springer-Verlag, Berlin, Germany: [PubMed] [Google Scholar]
- 15. Fogli S, et al. 2010. Therapeutic potential of sulindac hydroxamic acid against human pancreatic and colonic cancer cells. Eur. J. Med. Chem. 45:5100–5107 [DOI] [PubMed] [Google Scholar]
- 16. Golenser J, McQuillan J, Hee L, Mitchell AJ, Hunt NH. 2006. Conventional and experimental treatment of cerebral malaria. Int. J. Parasitol. 36:583–593 [DOI] [PubMed] [Google Scholar]
- 17. Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. 2006. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 36:1427–1441 [DOI] [PubMed] [Google Scholar]
- 18. Hastings I. 2011. How artemisinin-containing combination therapies slow the spread of antimalarial drug resistance. Trends Parasitol. 27:67–72 [DOI] [PubMed] [Google Scholar]
- 19. Haynes RK, et al. 2010. Facile oxidation of leucomethylene blue and dihydroflavins by artemisinins: relationship with flavoenzyme function and antimalarial mechanism of action. ChemMedChem 5:1282–1299 [DOI] [PubMed] [Google Scholar]
- 20. Haynes RK, et al. 2011. Reactions of antimalarial peroxides with each of leucomethylene blue and dihydroflavins: flavin reductase and the cofactor model exemplified. ChemMedChem 6:279–291 [DOI] [PubMed] [Google Scholar]
- 21. Haynes RK, et al. 2006. Artemisone–a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Ed. Engl. 45:2082–2088 [DOI] [PubMed] [Google Scholar]
- 22. Ho M, et al. 1998. Endogenous interleukin-10 modulates proinflammatory response in Plasmodium falciparum malaria. J. Infect. Dis. 178:520–525 [DOI] [PubMed] [Google Scholar]
- 23. Hunt NH, et al. 2006. Immunopathogenesis of cerebral malaria. Int. J. Parasitol. 36:569–582 [DOI] [PubMed] [Google Scholar]
- 24. Idro R, Jenkins NE, Newton C. 2005. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 4:827–840 [DOI] [PubMed] [Google Scholar]
- 25. Idro R, et al. 2010. Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res. Notes 3:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Idro R, Marsh K, John CC, Newton CRJ. 2010. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 68:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang JB, Guo XB, Li GQ, Kong YC, Arnold K. 1982. Antimalarial activity of mefloquine and qinghaosu. Lancet ii:285–288 [DOI] [PubMed] [Google Scholar]
- 28. John CC, et al. 2008. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 122:E92–E99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaewpongsri S, et al. 2011. The presence of leukocytes in ex vivo assays significantly increases the 50-percent inhibitory concentrations of artesunate and chloroquine against Plasmodium vivax and Plasmodium falciparum. Antimicrob. Agents Chemother. 55:1300–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kossodo S, et al. 1997. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91:536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin JT, Juliano JJ, Wongsrichanalai C. 2010. Drug-resistant malaria: the era of ACT. Curr. Infect. Dis. Rep. 12:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maitland K, Newton C. 2005. Acidosis of severe falciparum malaria: heading for a shock? Trends Parasitol. 21:11–16 [DOI] [PubMed] [Google Scholar]
- 33. Mutabingwa TK. 2005. Artemisinin-based combination therapies (ACTS): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 95:305–315 [DOI] [PubMed] [Google Scholar]
- 34. Nagelschmitz J, et al. 2008. First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob. Agents Chemother. 52:3085–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neill AL, Hunt NH. 1992. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology 105:165–175 [DOI] [PubMed] [Google Scholar]
- 36. Nosten F, White NJ. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181–192 [PubMed] [Google Scholar]
- 37. Obaldia N, et al. 2009. Evaluation of artemisone combinations in aotus monkeys infected with Plasmodium falciparum. Antimicrob. Agents Chemother. 53:3592–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Neill PM, Barton VE, Ward SA. 2010. The molecular mechanism of action of artemisinin–the debate continues. Molecules 15:1705–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reddy GM, Mukkanti K, Bhaskar BV, Reddy PP. 2009. Synthesis of metabolites and related substances of rabeprazole, an anti-ulcerative drug. Synth. Commun. 39:278–290 [Google Scholar]
- 40. Reis PA, et al. 2010. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog. 6:e1000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serghides L, et al. 2009. Rosiglitazone modulates the innate immune response to plasmodium falciparum infection and improves outcome in experimental cerebral malaria. J. Infect. Dis. 199:1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smithuis F, et al. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect. Dis. 10:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675 [DOI] [PubMed] [Google Scholar]
- 45. van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. 1997. Residual neurologic sequelae after childhood cerebral malaria. J. Pediatr. 131:125–129 [DOI] [PubMed] [Google Scholar]
- 46. Waknine-Grinberg JH, et al. 2010. Artemisone effective against murine cerebral malaria. Malar. J. 9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. WHO 2011. Global plan for artemisinin resistance containment. WHO, Geneva, Switzerland [Google Scholar]
- 48. WHO 2010. Guidelines for the treatment of malaria. WHO, Geneva, Switzerland [Google Scholar]
- 49. Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ. 2004. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am. J. Trop. Med. Hyg. 71:179–186 [PubMed] [Google Scholar]