Abstract
Despite significant improvements, antiretroviral therapies against HIV-1 are plagued by a high frequency of therapeutic failures that have been associated with acquisition of drug resistance. We recently reported that HIV-1 exploits a host glycan binding protein, galectin-1, to increase its attachment to host cells, thereby increasing its overall infectivity in susceptible cells. This finding suggests that host molecules such as galectin-1 could reduce the expected efficiency of HIV-1 drugs targeting early steps of the replicative cycle, such as attachment and entry processes. Thus, new classes of drugs that would interfere with galectin-1/HIV-1 interactions could benefit the current antiretroviral therapy. To further explore this possibility, experiments were conducted to discover leading compounds showing specific inhibition of galectin-1 activity in a cellular model of HIV-1 infection. Three lactoside compounds were found to modestly inhibit the interaction of galectin-1 with primary human CD4+ T cells. Interestingly, these same inhibitors reduced the galectin-1-mediated increase in HIV-1 attachment to target cells in a much more efficient manner. More important, the tested lactoside derivatives also significantly decreased the galectin-1-dependent enhancement of HIV-1 infection. These observations deserve further attention when considering that the development of new drugs to prevent and treat HIV-1 infection remains a priority.
INTRODUCTION
HIV-1 is the etiologic agent responsible for AIDS (6, 23), which has already killed more than 25 million people (76). Even though the transmission rate following unprotected sexual intercourse is relatively low (20, 57), a successful transmission event results in devastating effects on the immune system, since it depletes more than 90% of gut-associated CD4+ T cells in a relatively short time period (10, 31, 45). So far, the life expectancy of HIV-1-infected individuals has been improved by the development of highly active antiretroviral therapy (HAART) (58) targeting primarily the virus-encoded reverse transcriptase and protease enzymes. However, many therapeutic failures have resulted from the emergence of resistant viruses and adverse side effects (17, 34, 58). Thus, the novel antiviral drugs now target other viral processes, such as adhesion and entry steps (9, 14, 37, 47), which require specific interactions between the external viral envelope glycoprotein gp120 (Env) and cell surface host molecules, such as CD4, and a chemokine receptor, such as CCR5 or CXCR4.
Accumulating studies indicate that in a physiological setting, other host factors may participate in the establishment of HIV-1 infection (12, 26, 38, 70, 75). Unlike other enveloped viruses, HIV-1 carries a limited number of Env spikes, which are required for its adsorption to target cells (14, 24). This represents a significant bottleneck for efficiently establishing an initial replicative focus. HIV-1 is thought to circumvent this limiting factor by exploiting the host's membrane adhesion molecules or soluble proteins that can promote attachment of viral particles to target cells (22, 26, 36, 38, 41, 44, 46, 54, 68, 75). One of the host molecules exploited by HIV-1 is galectin-1, which has been reported to enhance both HIV-1 binding and infectivity in CD4+ T cells and macrophages by increasing viral adsorption to target susceptible cells (46, 54, 67). Since galectin-1 is abundantly found in organs rich in CD4+ T cells, such as lymphoid tissues and tissues surrounding the lamina propria of the genital and gut mucosa (50, 59, 69), it may play a significant role in HIV-1 transmission. Since galectin-1 can significantly reduce HIV-1 sensitivity to entry inhibitors (e.g., CXCR4 ligand SDF-1 and fusion inhibitors T-20 and TAK779) in vitro, it may compromise the efficacy of emerging drugs targeting viral attachment (46, 54). Thus, specific inhibition of galectin-1 could represent an interesting avenue to chemically interfere with HIV-1 propagation and to maximize the efficacy of HIV-1 attachment/entry inhibitors.
Galectins are soluble glycan-binding proteins harboring one or two carbohydrate recognition domains (CRDs), defined by conserved peptide sequences of approximately 130 amino acids that are responsible for their β-galactoside-binding specificity (4, 5). Despite the similarity of their CRDs, each galectin displays a unique ligand preference that depends on the β-galactoside structure as well as its substitutions (32). For example, galectin-3, as opposed to galectin-1, does not bind very efficiently to HIV-1 or its primary cellular receptor, CD4 (67, 68). This suggests a specific interaction between galectin-1 and HIV-1, which could be relevant for AIDS pathogenesis. So far, 15 galectins have been identified in mammals. Classification of galectins relies on the structural presentation of their CRD (i.e., prototype, chimera, and tandem repeated) (33, 40). For example, galectin-1 is a prototype galectin, while galectin-4 is a member of the tandem-repeat type and galectin-3 is the sole representative of the chimera type. Galectins are involved in a wide variety of biological processes influencing different steps in the immunological response. Some activities overlap across several members of the galectin family, while others are unique to one galectin only. Galectin-3 has been proposed to promote cell surface retention of receptors like epidermal growth factor receptor and T-cell receptor, leading to an increase in cell signaling (19, 39). Other reports suggest that galectin-1 and -3 could modulate macrophage activation and differentiation toward a wound-healing phenotype (7, 15, 43). In addition, both galectin-1 and -3 were postulated to participate in cell migration and angiogenesis (48, 74), while galectin-1 has been shown to contribute to tumor cell evasion from immune responses (42, 62). Thus, while galectins share relatively similar CRDs, their functions often differ significantly, implying the necessity of developing specific antagonists/inhibitors for each galectin.
A unique feature of galectins is their capacity to promote cell-cell or cell-pathogen interactions by directly cross-linking different entities. Previous studies have documented the role of galectin-1, -3, and -9 as critical mediators of heterotypic interactions between immune cells and pathogens. For example, galectin-1 enhances the interaction of human T-cell leukemia virus type 1 (HTLV-1) (25) and Trichomonas vaginalis (52) with their target cells. Galectin-3 increases binding of Trypanosoma cruzi to smooth muscle cells (35), while galectin-9 increases internalization of Leishmania major by macrophages (55). Such recognition can initiate immune responses that can either lead to the clearance of microorganisms or, alternatively, help their persistence in the infected host. In the context of HIV-1, it has been previously reported that galectin-1 is able to cross-link molecules found on the exterior of both virions and target cells, thus resulting in a significant enhancement of HIV-1 infection (46, 54, 67, 68).
Due to the peculiar ability of galectin-1 to specifically bind to clustered complex type glycans on HIV-1 and increase virus infectivity (67), new inhibitors that interfere with galectin-1-mediated interactions could be clinically relevant. Several recent studies have been carried out to find specific glycan derivatives that inhibit various galectins by using biochemical parameters, such as fluorescence polarization or enzyme-linked lectin assays (64, 65). Some of the compounds that were found had a low dissociation constant (Kd) for some galectins (16, 18, 66, 71–73), but while many inhibitors for galectin-3 have been reported, the search for a specific inhibitor for galectin-1 continues. In most cases, the selection of these inhibitors was achieved through techniques involving the use of soluble glycans. Since glycans are mostly present in a clustered fashion in physiological settings, it may affect their preference for selected galectins, as we have previously shown in the case of the interaction between galectin-1 and HIV-1 (67).
Interaction of a galectin with a pathogen can contribute to its infectivity, virulence, or persistence in the host. Therefore, we endeavored to find synthetic compounds derived from the lactoside or galactoside molecule that could specifically inhibit galectin-1 activity in a cellular model of HIV-1 infection by altering their attached aglycone structures of lactoside or galactoside. While lactose has been used to inhibit galectins' activities in many investigations, it is known to target every β-galactoside binding lectin and requires high concentrations (at least 10 mM) to be effective. Variations in aglycone structures, which modify the charge density or multivalency of lactoside derivatives, allow lactosides to have more stable and specific interactions with the CRDs of selected galectins. These synthetic compounds were first evaluated for their ability to inhibit hemagglutination induced by different galectins, followed by their capacity to modulate both HIV-1 binding and virus infection.
MATERIALS AND METHODS
Reagents.
Chemicals and other reagents were obtained from Sigma-Aldrich (St-Louis, MO) unless otherwise specified. Lactoside derivatives that were used as specific galectin-1 inhibitors were synthesized and purified as described previously (28–30).
Recombinant proteins.
Recombinant human galectin-1 and -3 were purified by affinity chromatography using an established procedure (54, 68) and were run through Acticlean ETOX endotoxin-removing gels (Sterogene, Carlsbad, CA). Alexa 488-labeled galectin-1 was prepared following the manufacturer's instructions (Molecular Probes, Eugene, OR) with a slight modification as described previously (55, 56).
Cell line and primary cells.
The LuSIV cell line is derived from the CEMx174 cell line and stably expresses a luciferase reporter gene driven by the SIVmac 239 long terminal repeat (LTR) region (obtained from the NIH AIDS Research and Reference Reagent Program, Germantown, MD). This indicator cell line was grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 300 μg/ml of hygromycin B as previously published (61, 68). LuSIV cells allow the quantitative evaluation of single-cycle HIV-1 infection events through transcriptional activation of the integrated LTR region, which drives luciferase reporter gene transcription following the production of the viral protein Tat by de novo viral infection (77). The LuSIV reporter cell line expresses only CXCR4 but not CCR5 and is thus not susceptible to infection by R5-utilizing virus. Peripheral blood mononuclear cells (PBMCs) were purified from healthy donors by Ficoll-Hypaque centrifugation, and CD4+ T cells were purified from PBMCs by using the human CD4+ T cell enrichment kit from Stemcell Technologies Inc. (Vancouver, Canada) according to the manufacturer's instructions. PBMCs and CD4+ T cells were maintained in RPMI 1640 medium supplemented with 10% FBS.
Virus stocks.
Virus particles were prepared from the culture medium of human embryonic kidney 293T cells that were transiently transfected with the infectious molecular clone pNL4-3 (X4 tropic) as previously published (1, 68). Titers of virus particles were normalized by assessing the p24 content as determined by an in-house sandwich-type enzyme-linked immunosorbent assay (ELISA) (8, 68) Briefly, flat-bottom 96-well plates were initially coated with 183 H12-5C, a monoclonal anti-p24 antibody (NIH AIDS Research and Reference Reagent Program, Germantown, MD). After washing and blocking with 1% bovine serum albumin (Sigma, St. Louis, MO), viral lysates were added to the wells. Plates were incubated for 1 h at 37°C and washed, and a biotinylated anti-p24 monoclonal antibody (clone 31-90-25; NIH AIDS Research and Reference Reagent Program, Germantown, MD) was then added. After 1 h of incubation at 37°C, the plates were washed and incubated with a streptavidin-peroxidase conjugate (streptavidin-HRP-40; Research Diagnostics, Inc., Flanders, NJ) for 30 min. Following extensive washes, the TMB-S substrate (Research Diagnostics, Inc.) was added to measure the activity of peroxidase bound to the plates. The reaction was terminated by adding H3PO4, and the absorbance was measured at 450 nm. The level of p24 in the samples were calculated based on the standard curve using recombinant p24gag/SF2, which was kindly supplied by Chiron Corporation.
Virus attachment assay.
LuSIV cells or CD4+ T cells were incubated with galectin-1 or -3 and HIV-1 (5 ng p24 per 105 cells) in the absence or presence of a potential antagonist/inhibitor for 1 h at 4°C. After two washes with cold phosphate-buffered saline (PBS), cells were lysed immediately and viral attachment was estimated by measuring p24 levels.
Infection assay.
LuSIV cells or CD4+ T cells were incubated with galectin-1 or -3 and HIV-1 (5 ng of p24 per 105 cells) in the absence or presence of a potential antagonist/inhibitor for 1 h at 4°C. After two washes with PBS, cells were transferred at 37°C for 24 to 72 h before lysis. In the case of LuSIV cells, the infection level was evaluated by measuring the luciferase activity as previously described (53). Virus replication in CD4+ T cells was evaluated at 48 to 72 h following virus infection by estimating p24 levels.
Hemagglutination assay.
Hemagglutination assays were used to evaluate the inhibitory potential of synthetic compounds on the cross-linking-mediated aggregation of red blood cells (RBC) by galectins through their affinity for glycans at the surface of RBC. Briefly, type O RBC were purified, fixed with 3% glutaraldehyde, and resuspended at 3 to 4% in PBS with sodium azide. Serial dilutions of compounds were placed in a U-shape 96-well plate, and appropriate amounts of RBC and galectins (1 to 2 μM) were added. After 30 min of incubation at 37°C, the MIC of each molecule was evaluated by comparing with controls (11). Since the output of the hemagglutination assay is digital (i.e., positive or negative), an inhibition curve could not be determined with precision. Thus, the concentration of a compound that inhibits 100% of galectin-induced hemagglutination was used as its MIC to compare the inhibitory property of each compound.
Cell viability.
Cell viability was evaluated by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] test, using the CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega, Madison, WI). Following the manufacturer's instructions, MTS reagent was added to LuSIV or CD4+ T cells (2 × 105 cells/well) that had been pretreated or not with different synthetic inhibitors (200 μM) and galectin-1 (2 μM) for 1 h at 4°C, as for virus attachment and infection assays. Cells were then incubated at 37°C for 4 h in the presence of MTS, which was reduced to formazan by metabolically active cells. The absorbance was detected at 490 nm with a Wallac Victor microplate reader (Perkin Elmer Life Sciences, Waltham, MA). Metabolic activity was finally compared to that of untreated cells to evaluate the toxicities of inhibitors.
Flow cytometry (galectin-1 binding).
Freshly isolated CD4+ T cells (2 × 105 cells/ml) were resuspended in PBS containing Alexa 488-labeled galectin-1 (2 μM) with or without different inhibitors (200 μM). After incubation at 4°C for 1 h, cells were washed twice with PBS-bovine serum albumin (BSA)-sodium azide (NaN3) and fixed in PBS containing 2% formaldehyde. The percentages of cells labeled with galectin-1 were estimated by detecting fluorescence with a Beckman-Coulter flow cytometer.
Statistical analysis.
Statistical significance was analyzed with the GraphPad Prism software program (GraphPad Software, La Jolla, CA) using the Student t test. P values of less than 0.05 were deemed statistically significant.
RESULTS
Galectin-3 does not affect the galectin-1-mediated increase in HIV-1 infection.
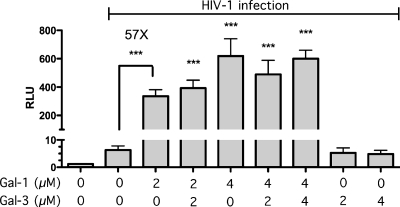
It is well known that galectins can modulate the inflammatory response through interactions with ligands expressed on a single cell or through cross-linking ligands present on different entities. It has been previously shown that the prototype galectin-1, which is actively secreted in secondary lymphoid tissues, binds to HIV-1 Env and CD4 and greatly enhances the kinetics of HIV-1 infection in vitro in various cell types, including primary human CD4+ T cells and macrophages (46, 54, 67, 68). Experiments conducted in the LuSIV reporter cell line confirm that galectin-1 promotes HIV-1 infection, since treatment with this lectin leads to a stastistically significant increase in luciferase activity (Fig. 1). A similar enhancement in HIV-1 infection is not seen with galectin-3. However, since galectin-3 shares some glycan binding preferences with galectin-1, it remained undefined whether or not galectin-3 could influence the galectin-1-mediated effect on virus infection. Data displayed in Fig. 1 indicate that galectin-3 does not compete with galectin-1 for their respective ligands. These results suggest a high specificity of galectin-1 with respect to its possible modulatory effect on the life cycle of HIV-1 and thus indicate that it can be possible to develop inhibitors specific for this soluble glycan-binding protein (i.e., galectin-1).
Fig 1.
Modulation of HIV-1 infectivity by galectins. LuSIV cells were infected for 48 h with NL4-3 in the absence or presence of the listed concentrations of galectin-1 and/or -3. Virus infection was evaluated by measuring luciferase activity. Data shown represent the means ± standard errors of the means (SEM) of data for triplicate samples and are representative of three different experiments. The statistical significance of differences between results for untreated/infected and treated/infected cells is denoted by asterisks (***, P < 0.001).
Galectin-1-dependent formation of cell-cell conjugates is inhibited by some lactoside derivatives.
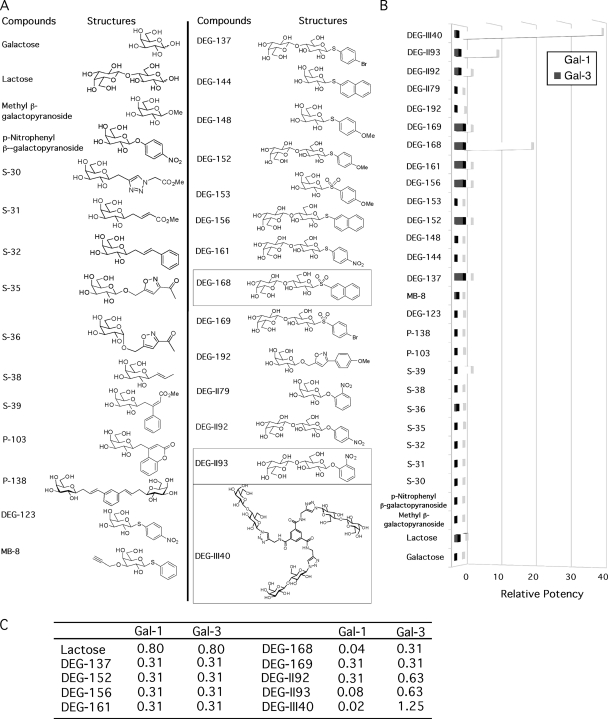
We next performed a well-established hemagglutination test using galectin-1 and -3 along with a series of different synthetic galactose and lactose derivatives (Fig. 2A) (11). This test can evaluate the inhibitory strength of molecules for galectin-induced erythrocyte aggregation, which occurs through cross-linking of cell surface glycoconjugates by the multivalent lectin. Compounds showing an inhibition of galectin-mediated hemagglutination at doses higher than 1 mM were not studied further since the central objective of this study was to discover compounds with antagonistic potential for galectins higher than that of lactose, which was used as a control in this study and displays a MIC of 0.8 mM (Fig. 2C). Some lactoside derivatives showed a noteworthy inhibitory effect on galectin-1-dependent hemagglutination compared to lactose (Fig. 2B). For example, the potencies (relative to that of lactose) of DEG-II93, DEG-168, and DEG-III40 were 10, 20, and 40, respectively. In the case of DEG-II93, the MIC for galectin-1 was 80 μM, while it required a concentration nearly 8 times higher to inhibit galectin-3 (Fig. 2C). This represents a significant improvement in selectivity for galectin-1. DEG-168 and DEG-III40 exhibited the lowest MICs for galectin-1 (40 μM and 20 μM, respectively) (Fig. 2C). Importantly, these compounds also exhibited preferential inhibition of galectin-1 over galectin-3, since their MICs for galectin-3 were 7.83- and 62.5-fold higher, respectively. These results suggest that such inhibitors are specific for galectin-1 and could be used to interfere with the galectin-1-mediated enhancement of HIV-1 infection.
Fig 2.
Inhibition of galectin-induced erythrocyte hemagglutination by galactoside and lactoside derivatives. (A) Structures of the studied compounds. Compounds showing high preference for galectin-1, as shown in panels B and C, are marked with squares and were selected for further studies. (B) Compounds were evaluated for their ability to block the cell-cell aggregation of RBC mediated by galectins. Data shown represent the means of data for triplicate samples and are representative of three different experiments. Potencies of the compounds relative to that of lactose is shown. (C) The minimum inhibitory concentrations (mM) of some compounds are shown. Experiments were done twice, and representative data are shown in the table. For panels B and C, no SEM is shown due to minimal differences between triplicate samples.
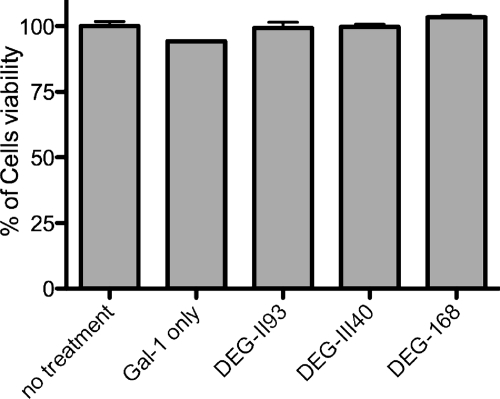
Lactoside derivatives do not affect cell viability.
HIV-1 infectivity can be evaluated using luciferase activity in a reporter cell line or by the de novo production of viral particles, two different methods requiring metabolically active cells. Thus, we assessed the cytotoxicities of the selected synthetic compounds in our experimental model system. By using a cell viability assay based on the reduction of the tetrazolium ring into formazan in active mitochondria, the cytotoxicity of each potential galectin-1 inhibitor was assessed. As shown in Fig. 3, no significant cytotoxic effects were observed following treatment of cells with the most potent galectin-1 inhibitors at the highest concentration tested (i.e., 200 μM).
Fig 3.
Impact of synthetic lactoside derivatives on cellular toxicity. LuSIV cells were incubated for 1 h in absence or presence of the listed lactoside derivatives (200 μM), followed by incubation with the MTS substrate. Metabolic activity was evaluated by measuring the optical density at 490 nm, and results are presented as a percentage of viability compared with untreated controls. Data shown represent the means ± SEM of triplicate samples and are representative of three distinct experiments.
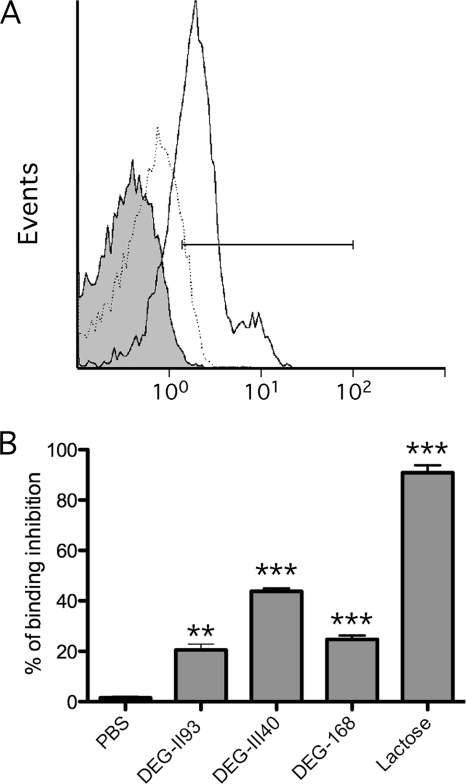
Effect of lactoside derivatives on the capacity of galectin-1 to bind to CD4+ T cells.
We next evaluated the capacity of lactoside derivatives to inhibit binding of galectin-1 to primary human CD4+ T cells. As shown in Fig. 4A, in the absence of inhibitors, Alexa 488-labeled galectin-1 binds to more than 75% of cells and this binding is inhibited by lactose (50 mM), which is used as a positive control. Interestingly, at the dose that efficiently blocked cell-cell conjugate formation (as defined with the hemagglutination assay), the studied lactoside derivatives were not as efficient as lactose in diminishing attachment of galectin-1 to CD4+ T cells, since they exhibited a mere 20 to ∼40% reduction of galectin-1 binding (Fig. 4B). These results suggest that inhibiting the cross-linking activity of galectin-1 requires a lower concentration of lactoside derivatives than the inhibition of galectin-1 binding to the cell surface.
Fig 4.
Inhibition of galectin-1 binding to HIV-1-susceptible cells by lactoside derivatives. (A) Binding of galectin-1 (2 μM) to primary human CD4+ T cells was monitored by flow cytometry analysis. Typical histogram of Alexa 488-labeled galectin-1 binding (continuous line) is shown, as well as its inhibition by lactose (50 mM) (dotted line). The gray area is the background fluorescence from cells without galectin-1. Cells in the gated region were considered galectin-1 positive. (B) Cells were either left untreated or treated with either the listed lactose derivatives (200 μM) or lactose (50 mM) before addition of fluorophore-labeled galectin-1. Levels of inhibition are shown by percent reduction calculated with comparison to fluorescent levels of cells in the absence of inhibitors. Data shown represent the means ± SEM of data for triplicate samples and are representative of three different experiments. The statistical significance of differences between untreated and treated cells is denoted by asterisks (**, P < 0.01; ***, P < 0.001).
Effect of lactoside derivatives on galectin-1-mediated HIV-1 binding and infection.
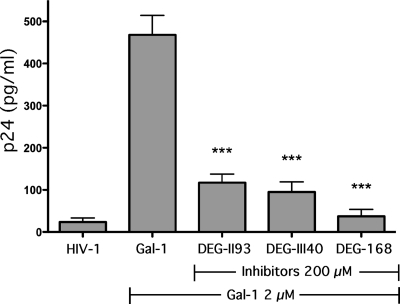
In order to examine whether or not the studied lactoside derivatives can interfere with the enhancement of HIV-1 infectivity mediated by galectin-1, a virus binding assay on LuSIV cells was first performed in the absence or presence of each inhibitor. A significant diminution of the galectin-1-mediated increase in HIV-1 binding to target cells was seen when using DEG-II93 and DEG-III40 (Fig. 5). A more impressive decrease in the lectin-mediated enhancing effect on virus attachment was obtained with DEG-168.
Fig 5.
Inhibition of galectin-1-mediated increase in HIV-1 binding by lactoside derivatives. LuSIV cells were either left untreated or treated with galectin-1 (2 μM) and treated or not with the listed galectin-1 inhibitors before exposure to HIV-1. Cells were next washed extensively, and the quantity of cell-associated virus was estimated by measuring p24 levels. Data shown represent the means ± SEM of data for triplicate samples and are representative of three distinct experiments. The statistical significance of differences between cells left untreated or treated with the studied lactoside derivatives is denoted by asterisks (***, P < 0.001).
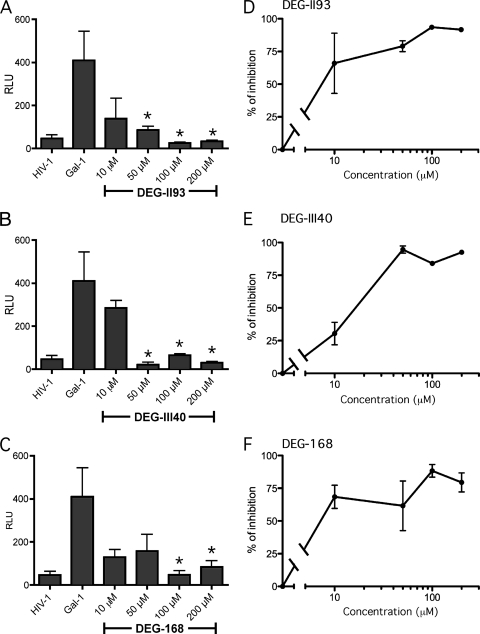
The final step was to determine whether these lactose derivatives can similarly modulate the galectin-1-dependent enhancement of HIV-1 infection. As shown in Fig. 6, each of these inhibitors was able to potently reduce the galectin-1-directed increase in virus infectivity. Importantly, even at the lowest concentration tested (i.e., 10 μM), all inhibitors still induced a reduction in the galectin-1-mediated enhancement of HIV-1 replication. Together, these data indicate that galectin-1-specific lactoside derivative inhibitors can repress efficiently both galectin-1-mediated HIV-1 binding and infection of host cells.
Fig 6.
Inhibition of galectin-1-mediated enhancement in HIV-1 infection by lactoside derivatives. LuSIV cells were first either left untreated or treated with galectin-1 (2 μM) and next subjected to a treatment with DEG-II93 (A), DEG-III40 (B), or DEG-168 (C) at the indicated concentrations before exposure to HIV-1. Cells were washed extensively and cultured at 37°C for 48 h. Finally, virus infection was assessed by monitoring luciferase activity (expressed in relative light units/RLU). Percentages of inhibition of HIV-1 infection are shown for DEG-II93 (D), DEG-III40 (E), and DEG-168 (F). Data shown represent the means ± SEM of data for triplicate samples and are representative of three different experiments. The statistical significance of differences between cells untreated or treated with the studied lactoside derivatives is denoted by asterisks (*, P < 0.05).
DISCUSSION
Although CRDs of galectins display remarkable similarity, accumulating evidences indicate that while some functions overlap, each galectin often exhibits unique functions (60). The present and previous studies suggest that galectin-1 but not galectin-3 can significantly enhance HIV-1 infectivity (46, 54, 67, 68), while involvement of other members of galectins has remained elusive. Interestingly, although galectin-3 often exhibits high avidity for glycans that are present on cells susceptible to productive HIV-1 infection, it failed to interfere with galectin-1's ability to increase HIV-1 binding. Nevertheless, galectin-3 is reported to play important roles in the recruitment of neutrophils, the maintenance of epithelium integrity, and mucosal natural defenses (3, 13, 21, 49). Thus, specific inhibition of a galectin might represent an important avenue not only for understanding the biological significance of each member of this protein family but also as a basis for the development of future therapeutic interventions.
Pioneering studies led by Nilsson and colleagues and Giguere and colleagues reported the development of specific galectin inhibitors by using soluble glycans in fluorescence polarization assays (27, 51, 63, 64, 73). Other findings also reported that a multimeric or a clustered arrangement of lactoside derivatives may enhance their affinity for specific galectins (2, 29). While the majority of those compounds are antagonists for both galectin-1 and -3, a few galectin antagonist candidates could be relatively specific for galectin-1 over galectin-3 (73). In the current work, lactoside-derived compounds were first screened for a specific inhibition of galectin-1 using homotypic aggregation of RBC. Compounds were screened for their potency, which relates to their structure or their charge density. This cell-based test is ideal to screen for inhibition of the cross-linking ability of each galectin in native settings, which more closely approximates the HIV-1 attachment step to the cell surface. Three distinct lactoside derivatives were identified as highly specific for galectin-1 in this assay. All of these galectin-1-specific inhibitors bear aglycones which have electron donors close to the O-1 hydroxyl group of their glucose residues, suggesting that having both the electrostatic and steric states in proximity to the OH-1 group may be critical for their preference for galectin-1. Further studies are necessary to exploit such a possibility and to develop galectin-1 inhibitors displaying a higher specificity and potency.
During an initial virus transmission event, adhesion and fusion of HIV-1 viral particles to susceptible CD4+ T cells represent potential limiting steps that galectin-1 can help to overcome. Therefore, inhibition of the galectin-1-mediated effect on the first step in HIV-1 replication (i.e., attachment) could reduce transmission risks in the early stages of infection and thus avoid chronic infection, life-long monitoring, and costly antiretroviral therapies. At least three promising lactoside derivatives identified in the hemagglutination assay were potent at blocking the galectin-1-mediated enhancement in virus binding. Furthermore, these compounds can also inhibit the ability of galectin-1 to enhance HIV-1 infectivity without affecting cell viability. Since cytotoxicity was not evaluated with a wide range of concentrations of the studied galectin-1-specific inhibitors, we could not establish a therapeutic index per se. The absence of toxicity with the highest dose of each inhibitor is a proof of concept that inhibition of HIV-1 binding and infection is directly related to the modulation of the activity of galectin-1. However, the possible cytotoxic effects of the tested compounds were not measured over an extended time period. Therefore, long-term treatment toxicity studies will be needed if galectin-1-specific inhibitors are ever used in clinical settings.
This effect seen with galectin-1 inhibitors might be associated, at least in part, with the destabilization of galectin-1's interaction with susceptible cells. Interestingly, although the studied lactoside derivatives could only weakly inhibit the binding of galectin-1 to individual cells, the effect of galectin-1 on HIV-1 infection was effectively abolished by the same concentration of each compound. This suggests that inhibition of only one of the binding sites of homodimeric galectin-1 is sufficient to interfere with the cross-linking ability of galectin-1. In summary, our results show that inhibition of the galectin-1-mediated increase in HIV-1 binding and infection can be achieved by using some specific lactoside derivatives. Thus, further modifications of these leading compounds are expected to increase their potency and specificity as galectin-1 antagonists and could possibly enable their use as promising new antiretroviral strategies, either to prevent sexual HIV-1 transmission or to be used in combination with existing entry/fusion inhibitors.
ACKNOWLEDGMENTS
We acknowledge the contribution of Laurence Bérubé-Cooey for hemagglutination assays.
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) to S.S. and M.J.T. (MOP-89743). C.S.-P. holds a Graduated Scholarship Award from CIHR, S.S. held a Scholarship Award (senior level) from the Fonds de la Recherche en Santé du Québec, and M.J.T. is the recipient of a Tier 1 Canada Research Chair in Human Immuno-Retrovirology.
Footnotes
Published ahead of print 7 November 2011
REFERENCES
- 1. Adachi A, et al. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andre S, Kaltner H, Furuike T, Nishimura S, Gabius HJ. 2004. Persubstituted cyclodextrin-based glycoclusters as inhibitors of protein-carbohydrate recognition using purified plant and mammalian lectins and wild-type and lectin-gene-transfected tumor cells as targets. Bioconjug. Chem. 15:87–98 [DOI] [PubMed] [Google Scholar]
- 3. Argueso P, et al. 2009. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 284:23037–23045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barondes SH, et al. 1994. Galectins: a family of animal beta-galactoside-binding lectins. Cell 76:597–598 [DOI] [PubMed] [Google Scholar]
- 5. Barondes SH, Cooper DN, Gitt MA, Leffler H. 1994. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 269:20807–20810 [PubMed] [Google Scholar]
- 6. Barre-Sinoussi F, et al. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871 [DOI] [PubMed] [Google Scholar]
- 7. Barrionuevo P, et al. 2007. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J. Immunol. 178:436–445 [DOI] [PubMed] [Google Scholar]
- 8. Bounou S, Leclerc JE, Tremblay MJ. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boussard C, Klimkait T, Mahmood N, Pritchard M, Gilbert IH. 2004. Design, synthesis and evaluation of potential inhibitors of HIV gp120-CD4 interactions. Bioorg. Med. Chem. Lett. 14:2673–2676 [DOI] [PubMed] [Google Scholar]
- 10. Brenchley JM, et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butler WT. 1963. Hemagglutination studies with formalinized erythrocytes. Effect of bis-diazo-benzidine and tannic acid treatment on sensitization by soluble antigen. J. Immunol. 90:663–671 [PubMed] [Google Scholar]
- 12. Cantin R, Fortin JF, Tremblay M. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372–381 [DOI] [PubMed] [Google Scholar]
- 13. Cao Z, et al. 2002. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J. Biol. Chem. 277:42299–42305 [DOI] [PubMed] [Google Scholar]
- 14. Chan DC, Kim PS. 1998. HIV entry and its inhibition. Cell 93:681–684 [DOI] [PubMed] [Google Scholar]
- 15. Correa SG, Sotomayor CE, Aoki MP, Maldonado CA, Rabinovich GA. 2003. Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology 13:119–128 [DOI] [PubMed] [Google Scholar]
- 16. Cumpstey I, Carlsson S, Leffler H, Nilsson UJ. 2005. Synthesis of a phenyl thio-beta-D-galactopyranoside library from 1,5-difluoro-2,4-dinitrobenzene: discovery of efficient and selective monosaccharide inhibitors of galectin-7. Org. Biomol. Chem. 3:1922–1932 [DOI] [PubMed] [Google Scholar]
- 17. Dahl V, Palmer S. 2009. Establishment of drug-resistant HIV-1 in latent reservoirs. J. Infect. Dis. 199:1258–1260 [DOI] [PubMed] [Google Scholar]
- 18. Delaine T, et al. 2008. Galectin-inhibitory thiodigalactoside ester derivatives have antimigratory effects in cultured lung and prostate cancer cells. J. Med. Chem. 51:8109–8114 [DOI] [PubMed] [Google Scholar]
- 19. Demetriou M, Granovsky M, Quaggin S, Dennis JW. 2001. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409:733–739 [DOI] [PubMed] [Google Scholar]
- 20. Derdeyn CA, et al. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019–2022 [DOI] [PubMed] [Google Scholar]
- 21. Farnworth SL, et al. 2008. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am. J. Pathol. 172:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feinberg H, Mitchell DA, Drickamer K, Weis WI. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163–2166 [DOI] [PubMed] [Google Scholar]
- 23. Gallo RC, et al. 1983. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 220:865–867 [DOI] [PubMed] [Google Scholar]
- 24. Gallo SA, et al. 2003. The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 1614:36–50 [DOI] [PubMed] [Google Scholar]
- 25. Gauthier S, et al. 2008. Induction of galectin-1 expression by HTLV-I Tax and its impact on HTLV-I infectivity. Retrovirology 5:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geijtenbeek TB, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597 [DOI] [PubMed] [Google Scholar]
- 27. Giguere D, et al. 2011. Inhibitory potential of chemical substitutions at bioinspired sites of beta-D-galactopyranose on neoglycoprotein/cell surface binding of two classes of medically relevant lectins. Bioorg. Med. Chem. 19:3280–3287 [DOI] [PubMed] [Google Scholar]
- 28. Giguere D, et al. 2008. Synthesis of stable and selective inhibitors of human galectins-1 and -3. Bioorg. Med. Chem. 16:7811–7823 [DOI] [PubMed] [Google Scholar]
- 29. Giguere D, et al. 2006. Carbohydrate triazoles and isoxazoles as inhibitors of galectins-1 and -3. Chem. Commun. (Camb.) 2006:2379–2381 [DOI] [PubMed] [Google Scholar]
- 30. Giguere D, Sato S, St-Pierre C, Sirois S, Roy R. 2006. Aryl O- and S-galactosides and lactosides as specific inhibitors of human galectins-1 and -3: role of electrostatic potential at O-3. Bioorg. Med. Chem. Lett. 16:1668–1672 [DOI] [PubMed] [Google Scholar]
- 31. Guadalupe M, et al. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirabayashi J, et al. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572:232–254 [DOI] [PubMed] [Google Scholar]
- 33. Hirabayashi J, Kasai K. 1993. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3:297–304 [DOI] [PubMed] [Google Scholar]
- 34. Johnson VA, et al. 2010. Update of the drug resistance mutations in HIV-1: December 2010. Top. HIV Med. 18:156–163 [PubMed] [Google Scholar]
- 35. Kleshchenko YY, et al. 2004. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect. Immun. 72:6717–6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135–144 [DOI] [PubMed] [Google Scholar]
- 37. Labrecque J, et al. 2011. HIV-1 entry inhibition by small-molecule CCR5 antagonists: a combined molecular modeling and mutant study using a high-throughput assay. Virology 413:231–243 [DOI] [PubMed] [Google Scholar]
- 38. Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. 2008. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood 112:1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lau KS, et al. 2007. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129:123–134 [DOI] [PubMed] [Google Scholar]
- 40. Leffler H. 2002. Special issue on galectins. Glycoconj. J. 19:433–630 [DOI] [PubMed] [Google Scholar]
- 41. Lin G, et al. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu FT, Rabinovich GA. 2005. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 5:29–41 [DOI] [PubMed] [Google Scholar]
- 43. MacKinnon AC, et al. 2008. Regulation of alternative macrophage activation by galectin-3. J. Immunol. 180:2650–2658 [DOI] [PubMed] [Google Scholar]
- 44. McDonald D, et al. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297 [DOI] [PubMed] [Google Scholar]
- 45. Mehandru S, et al. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mercier S, et al. 2008. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371:121–129 [DOI] [PubMed] [Google Scholar]
- 47. Murakami T, et al. 1999. Inhibitory mechanism of the CXCR4 antagonist T22 against human immunodeficiency virus type 1 infection. J. Virol. 73:7489–7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nangia-Makker P, et al. 2000. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 156:899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. 2008. Role of Galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J. Immunol. 180:2466–2473 [DOI] [PubMed] [Google Scholar]
- 50. Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. 2009. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J. Histochem. Cytochem. 57:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oberg CT, Leffler H, Nilsson UJ. 2011. Inhibition of galectins with small molecules. Chimia (Aarau) 65:18–23 [DOI] [PubMed] [Google Scholar]
- 52. Okumura CY, Baum LG, Johnson PJ. 2008. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 10:2078–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ouellet M, Barbeau B, Tremblay MJ. 1999. p56(lck), ZAP-70, SLP-76, and calcium-regulated effectors are involved in NF-kappaB activation by bisperoxovanadium phosphotyrosyl phosphatase inhibitors in human T cells. J. Biol. Chem. 274:35029–35036 [DOI] [PubMed] [Google Scholar]
- 54. Ouellet M, et al. 2005. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 174:4120–4126 [DOI] [PubMed] [Google Scholar]
- 55. Pelletier I, et al. 2003. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 278:22223–22230 [DOI] [PubMed] [Google Scholar]
- 56. Pelletier I, Sato S. 2002. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 277:17663–17670 [DOI] [PubMed] [Google Scholar]
- 57. Piot P, Bartos M, Ghys PD, Walker N, Schwartlander B. 2001. The global impact of HIV/AIDS. Nature 410:968–973 [DOI] [PubMed] [Google Scholar]
- 58. Pomerantz RJ, Horn DL. 2003. Twenty years of therapy for HIV-1 infection. Nat. Med. 9:867–873 [DOI] [PubMed] [Google Scholar]
- 59. Rabinovich G, Castagna L, Landa C, Riera CM, Sotomayor C. 1996. Regulated expression of a 16-kd galectin-like protein in activated rat macrophages. J. Leukoc. Biol. 59:363–370 [DOI] [PubMed] [Google Scholar]
- 60. Rabinovich GA, Toscano MA. 2009. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9:338–352 [DOI] [PubMed] [Google Scholar]
- 61. Roos JW, Maughan MF, Liao Z, Hildreth JE, Clements JE. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307–315 [DOI] [PubMed] [Google Scholar]
- 62. Rubinstein N, et al. 2004. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; a potential mechanism of tumor-immune privilege. Cancer Cell 5:241–251 [DOI] [PubMed] [Google Scholar]
- 63. Salameh BA, Leffler H, Nilsson UJ. 2005. 3-(1,2,3-Triazol-1-yl)-1-thio-galactosides as small, efficient, and hydrolytically stable inhibitors of galectin-3. Bioorg. Med. Chem. Lett. 15:3344–3346 [DOI] [PubMed] [Google Scholar]
- 64. Sorme P, Kahl-Knutson B, Wellmar U, Nilsson UJ, Leffler H. 2003. Fluorescence polarization to study galectin-ligand interactions. Methods Enzymol. 362:504–512 [DOI] [PubMed] [Google Scholar]
- 65. Sorme P, et al. 2003. Design and synthesis of galectin inhibitors. Methods Enzymol. 363:157–169 [DOI] [PubMed] [Google Scholar]
- 66. Sorme P, Qian Y, Nyholm PG, Leffler H, Nilsson UJ. 2002. Low micromolar inhibitors of galectin-3 based on 3′-derivatization of N-acetyllactosamine. Chembiochem 3:183–189 [DOI] [PubMed] [Google Scholar]
- 67. St-Pierre C, et al. 2011. Host soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 85:11742–11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. St-Pierre C, Ouellet M, Tremblay MJ, Sato S. 2010. Galectin-1 and HIV-1 infection. Methods Enzymol. 480:267–294 [DOI] [PubMed] [Google Scholar]
- 69. Stillman BN, et al. 2006. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176:778–789 [DOI] [PubMed] [Google Scholar]
- 70. Tardif MR, Tremblay MJ. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299–12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tejler J, Leffler H, Nilsson UJ. 2005. Synthesis of O-galactosyl aldoximes as potent LacNAc-mimetic galectin-3 inhibitors. Bioorg. Med. Chem. Lett. 15:2343–2345 [DOI] [PubMed] [Google Scholar]
- 72. Tejler J, Skogman F, Leffler H, Nilsson UJ. 2007. Synthesis of galactose-mimicking 1H-(1,2,3-triazol-1-yl)-mannosides as selective galectin-3 and 9N inhibitors. Carbohydr. Res. 342:1869–1875 [DOI] [PubMed] [Google Scholar]
- 73. Tejler J, Tullberg E, Frejd T, Leffler H, Nilsson UJ. 2006. Synthesis of multivalent lactose derivatives by 1,3-dipolar cycloadditions: selective galectin-1 inhibition. Carbohydr. Res. 341:1353–1362 [DOI] [PubMed] [Google Scholar]
- 74. Thijssen VL, et al. 2006. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. U. S. A. 103:15975–15980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Turville S, Wilkinson J, Cameron P, Dable J, Cunningham AL. 2003. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 74:710–718 [DOI] [PubMed] [Google Scholar]
- 76. UNAIDS 2010. Global report: UNAIDS report on the global AIDS epidemic 2010. http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf
- 77. Wu Y. 2004. HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]