Abstract
Complete sequencing of plasmid pOXA-48a carrying the blaOXA-48 gene from a Klebsiella pneumoniae isolate was performed. Its backbone corresponded to that of an IncL/M-type plasmid, in which the blaOXA-48 gene had been integrated through the acquisition of the Tn1999 composite transposon without any other antibiotic resistance gene. Molecular epidemiology using a collection of international OXA-48 producers revealed the wide diffusion of pOXA-48a or closely related plasmids.
TEXT
Carbapenem-hydrolyzing β-lactamases belonging to Ambler classes A, B, and D have been found worldwide among the Enterobacteriaceae (24). Although carbapenem-hydrolyzing class D β-lactamases (CHDLs) are identified mainly in Acinetobacter species (19, 23), OXA-48 has been identified only in the Enterobacteriaceae. The blaOXA-48 gene was first identified in a Klebsiella pneumoniae isolate from Turkey (22). Since then, several other OXA-48-producing isolates of various enterobacterial species (including Citrobacter freundii and Escherichia coli) have been reported, first mainly in Turkey and then in Belgium, France, Lebanon (16), Egypt, Israel (12), Senegal (18), Morocco (25), and Tunisia (9). In addition, there have been recent reports of OXA-48-related enzymes, such as OXA-181, being identified in India (7) and in other countries but always with a link to India (26), as well as OXA-163 from Argentina (21). It is noteworthy that OXA-48 and OXA-181 hydrolyze penicillins and carbapenems but spare extended-spectrum cephalosporins (27, 28). Many OXA-48 producers coexpress an extended-spectrum β-lactamase (ESBL) (most often CTX-M-15 or SHV-12), making those isolates resistant to all β-lactams available.
Previous studies indicated that plasmids carrying the blaOXA-48 gene from different enterobacterial isolates, different clones, and different countries may share very similar features (6, 10). They were self-conjugative, very similar in size (reported to be ca. 60 to 70 kb), did not encode additional resistance markers, and were not typeable by using PCR-based replicon typing (PBRT) (4). This suggested the possibility of a wide spread of a single plasmid at the origin of dissemination of this carbapenemase gene. This prompted us to determine the sequence of one of these plasmids, to compare the identified structure with those of other OXA-48-positive plasmids, and to determine if some particular features could explain its successful spread. It is noteworthy that a 7.6-kb plasmid carrying the blaOXA-181 gene and recovered from a K. pneumoniae isolate from Oman was fully sequenced. This revealed that it belonged to the broad-host-range ColE family of plasmids and that the blaOXA-181 gene had been acquired by an ISEcp1-related one-ended transposition mechanism (27), two features clearly differentiating it from the blaOXA-48 plasmids.
Plasmid pOXA-48a was extracted from the E. coli transconjugant recovered from the K. pneumoniae 11978 clinical isolate (22) by using the Qiagen Maxi kit (Qiagen, Courtaboeuf, France). The complete sequencing work flow of the Illumina genome analyzer IIx system was performed by the DNA Vision company (Gosselles, Belgium).
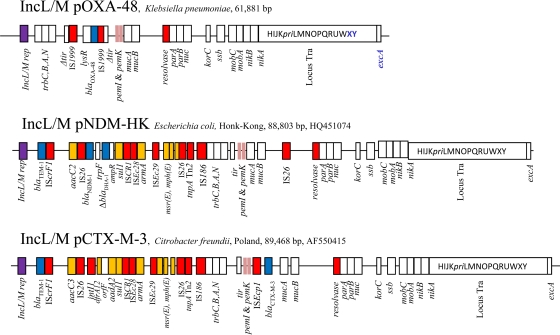
Sequence analysis of pOXA-48a revealed that it was 61,881 bp in size, with an average G+C content of 51.1% (Fig. 1). Each predicted protein was compared against the GenBank protein database using BlastP (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with a minimum cutoff of 30% identity over 80% length coverage. Gene sequences were further compared and aligned with GenBank data using BlastN and ClustalW software. The IncL/M plasmids pCTX-M3 (GenBank accession number AF550415), which harbors the blaCTX-M-3 ESBL gene, and pHK-NDM (GenBank accession number HQ451074), which harbors the metallo-β-lactamase blaNDM-1 gene, were used as references for annotating pOXA-48a.
Fig 1.
Major structural features of plasmid pOXA-48a from K. pneumoniae 11978 (22) in comparison with the IncL/M plasmids pCTX-M-3 (11) and pNDM-HK (14). White boxes indicate plasmid scaffold regions that are in common among plasmids. The locus Tra within the box is indicated. Resistance genes are indicated by orange boxes, except the β-lactamase genes, which are indicated by blue boxes. Transposon-related genes (tnpA, tnpR, and tnpM) and insertion sequences are indicated by red boxes. Replicase genes are indicated by purple boxes.
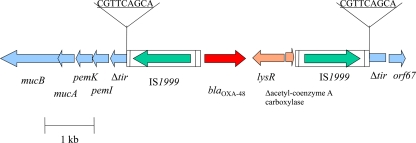
In silico analysis showed that pOXA-48a possessed the highest degree of sequence identity and gene synteny within its entire scaffold with the reference plasmid pCTX-M3 (97% identity at the nucleotide level) (11), with the exception of two loci. One region that differs from pCTX-M3 corresponded to Tn1999, which consists of two copies of the same insertion sequence, IS1999, flanking a DNA fragment that includes the blaOXA-48 gene (1, 21). As previously shown, transposon Tn1999 was inserted within a tir gene similar to that responsible for inhibition of transfer of plasmid R100 (17). A target site duplication of 9 bp (CGTTCAGCA) was identified on each extremity of Tn1999 (1) (Fig. 2). Downstream of blaOXA-48, a lysR gene encoding a regulatory protein of 304 amino acids was identified (98% amino acid identity with that of Shewanella oneidensis MR1) (13), followed by a truncated fragment of a gene encoding an acetyl coenzyme A carboxylase sharing 100% amino acid identity with that of S. oneidensis MR1 (13). In pCTX-M3, the ISEcp1-blaCTX-M-3 transposable locus was inserted close to this target site for Tn1999, suggesting that this locus could possibly be a hot spot for integrating foreign DNA within IncL/M-type plasmids. The other region differing from other IncL/M plasmids was located between the replication module and the trb transfer locus. In pCTX-M3, this 27-kb resistance island was composed of a copy of Tn2 in which transposon Tn1548 carried the armA methylase encoding gene (2). That region contained at least 19 open reading frames (ORFs), including in particular genes encoding resistance to aminoglycosides (aadA2 and armA), trimethoprim (dfrA12), sulfonamides (sul1), and macrolides (mphE and msrE) (Fig. 1). In pHK-NDM, the blaNDM-1 gene locus was inserted within the transposon carrying the armA gene, replacing the class 1 integron structure identified in pCTX-M3. In pOXA-48a, no foreign DNA was inserted in the backbone at this locus.
Fig 2.
Genetic environment and content of Tn1999. The different gene names are indicated. The sequence of the 9-bp target site duplication is underlined.
The RepA replication protein of pOXA-48a exhibited only two amino acid differences, out of 351 amino acids, from RepA of both plasmids pCTX-M3 and pHK-NDM. Only one replication module was identified in pOXA-48a, as for other IncL/M-type plasmids known to possess broad-host-range properties that have since been identified in the Enterobacteriaceae, Erwinia amylovora, Ralstonia eutropha, Pseudomonas aeruginosa, and Agrobacterium tumefaciens (11, 17). Altogether, these results indicate that plasmids pOXA-48a, pCTX-M3, and pHK-NDM may have a common origin in the environment, a hypothesis which is reinforced by the identification of blaOXA-48-like genes in the waterborne environmental species S. oneidensis and Shewanella xiamenensis (23, 28).
In order to better address whether plasmid pOXA-48a may possess a broad host range of replication, electrotransformation assays were carried out using DNA of pOXA-48a and different recipient species, namely, E. coli JM109 (control), Acinetobacter baumannii CIP70.10, Pseudomonas aeruginosa PU21, and S. oneidensis MR-1, with selection based on ticarcillin (50 μg/ml) (13, 29). Different protocols were used depending on the species of the recipient strain (8, 30). Whereas no transformant was obtained with A. baumannii and P. aeruginosa, pOXA-48a replicated efficiently in E. coli and S. oneidensis. This indicates that pOXA-48a possesses a broad host range of replication and that such an IncL/M-type scaffold could have been at the origin of the capture of the blaOXA-48 gene from its natural progenitor (Shewanella spp.) and its dissemination among the Enterobacteriaceae. Our results also suggest that dissemination of the blaOXA-48 gene in A. baumannii or P. aeruginosa is unlikely to occur if it remains associated only with this type of plasmid.
The transfer operon identified in pOXA-48a was very similar to the transfer operon in pCTX-M-3, being formed by two distinct regions. This common feature suggests that the propensity for spreading of the blaOXA-48 gene in the Enterobacteriaceae might be as high as that of the blaCTX-M-3 gene. This transfer operon included the trb and tra operons, known to encode type IV pili, which are involved in plasmid conjugation. The tra operon was very similar to that of pCTX-M-3, except for three genes, namely, traX-traY-excA, encoding proteins more distantly related (54%, 79%, and 34% identities, respectively) to those encoded by pCTX-M-3. Due to the significant divergence of this gene array, a recombination event could explain such gene array replacement. The lack of identification of pOXA-48a as an IncL/M plasmid by the PBRT method can be explained by the location of one of the corresponding primers in the excA gene.
In order to evaluate whether the modifications observed in pOXA-48a could influence its conjugation rate compared to that of another IncL/M-type plasmid, conjugation frequencies were determined by using as a donor the same E. coli J53 background harboring either pOXA-48a or pNDM-HK, along with E. coli JM109 as the recipient, with a selection based on ticarcillin (50 μg/ml) and nalidixic acid (20 μg/ml). No difference was observed, conjugation rates being 3.3 × 10−5 and 2.5 × 10−5 for pOXA-48a and pNDM-HK, respectively).
Since the PBRT technique was inefficient for classifying pOXA-48a, three primer pairs were newly designed to amplify conserved regions of its backbone (Table 1). They were designed in the repA, traU, and parA genes, encoding proteins implicated in replication, transfer, and partitioning, respectively. The presence of the blaOXA-48 gene was confirmed using previously designed primers (Table 1). An international collection of strains (n = 19) harboring the same blaOXA-48 gene was used for typing blaOXA-48-positive plasmids. Plasmid analysis performed by using the Kieser technique (15) confirmed that they were all ca. 62 kb in size. PCR experiments confirmed that this same plasmid backbone was identified in all the blaOXA-48-positive isolates (Table 2). In addition, PCR performed as described previously (17) confirmed that transposon Tn1999 targeted the same location in all plasmids. Altogether, our results show that the current dissemination of OXA-48 producers, mostly in countries located near the Mediterranean Sea and in Western Europe, may be due largely to the spread of a single plasmid.
Table 1.
Primers used in this study
| Primer | Sequence (5′–3′) | Reference |
|---|---|---|
| OXA-48A | TTGGTGGCATCGATTATCGG | 22 |
| OXA-48B | GAGCACTTCTTTTGTGATGGC | 22 |
| PBRT incL/M for | GGATGAAAACTATCAGCATCTGAAG | 4 |
| PBRT incL/M rev | CTGCAGGGGCGATTCTTTAGG | 4 |
| RepA-A | GACATTGAGTCAGTAGAAGG | This study |
| RepA-B | CGTGCAGTTCGTCTTTCGGC | This study |
| TraU-A | ATCTCACGCAATCTTACGTC | This study |
| TraU-B | TCGCGTCATGCGTGATCTTC | This study |
| ParA-A | GCAGTGAAAACGTTGATCAG | This study |
| ParA-B | GATCGCAATGCGTCTTGGTG | This study |
Table 2.
Distribution of IncL/M-type plasmids carrying the blaOXA-48 gene
| Strain | Country | Size (kb)a | Plasmid feature |
Plasmid-associated resistance marker | Reference | |||
|---|---|---|---|---|---|---|---|---|
| RepA | TraU | ParA | Inc group | |||||
| K. pneumoniae 11978 | Turkey | ca. 62 | + | + | + | L/M | None | 22 |
| K. pneumoniae Bel | Belgium | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae Lib | Lebanon | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae 8 | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae Egy | Egypt | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae 5A | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae 7A | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae Bey | Lebanon | ca. 62 | + | + | + | L/M | None | 16 |
| K. pneumoniae 3A | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae 4A | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae 17A | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| K. pneumoniae Bou | France | ca. 62 | + | + | + | L/M | None | 10 |
| K. pneumoniae Dia | France | ca. 62 | + | + | + | L/M | None | 9 |
| K. pneumoniae C2 | Morocco | ca. 62 | + | + | + | L/M | None | Unpublished |
| K. oxytoca A7 | Morocco | ca. 62 | + | + | + | L/M | None | Unpublished |
| E. coli 1 | Turkey | ca. 62 | + | + | + | L/M | None | 5 |
| E. cloacae 501 | France | ca. 62 | + | + | + | L/M | None | 25 |
| E. cloacae Bou | Morocco | ca. 62 | + | + | + | L/M | None | 25 |
| E. cloacae D4 | Morocco | ca. 62 | + | + | + | L/M | None | Unpublished |
Sizes were often reported to be ca. 70 kb according to the size markers used, but present comparison with plasmid pOXA-48 from K. pneumoniae 11978 revealed the same size.
We report here the whole sequence of the major vehicle of the blaOXA-48 carbapenemase gene. Although some other widespread carbapenemase determinants, such as the blaKPC and blaNDM genes, have been shown to disseminate through different plasmid scaffolds (3), we showed here that the current spread of the blaOXA-48 gene is linked to the wide diffusion of an identical IncL/M plasmid scaffold.
Nucleotide sequence accession number.
The nucleotide sequence reported in this work has been deposited in the GenBank nucleotide sequence database under accession no. JN626286.
ACKNOWLEDGMENT
This work was funded by a grant from the INSERM (U914).
Footnotes
Published ahead of print 14 November 2011
REFERENCES
- 1. Aubert D, Naas T, Héritier C, Poirel L, Nordmann P. 2006. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 188:6506–6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey JK, Pinyon JL, Anantham S, Hall RM. 2011. Distribution of the blaTEM gene and blaTEM-containing transposons in commensal Escherichia coli. J. Antimicrob. Chemother. 66:745–751 [DOI] [PubMed] [Google Scholar]
- 3. Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carattoli A, et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 5. Carrër A, et al. 2008. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 52:2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrër A, et al. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob. Agents Chemother. 54:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castanheira M, et al. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY antimicrobial surveillance program, 2006–2007. Antimicrob. Agents Chemother. 55:1274–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397 [DOI] [PubMed] [Google Scholar]
- 9. Cuzon G, Naas T, Lesenne A, Benhamou M, Nordmann P. 2010. Plasmid-mediated carbapenem-hydrolysing OXA-48 β-lactamase in Klebsiella pneumoniae from Tunisia. Int. J. Antimicrob. Agents 36:91–93 [DOI] [PubMed] [Google Scholar]
- 10. Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. 2011. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae in France. Antimicrob. Agents Chemother. 55:2420–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golebiewski M, et al. 2007. Complete nucleotide sequence of the pCTX-M3 plasmid and its involvement in spread of the extended-spectrum β-lactamase gene blaCTX-M-3. Antimicrob. Agents Chemother. 51:3789–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goren MG, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2011. Plasmid-encoded OXA-48 carbapenemase in Escherichia coli from Israel. J. Antimicrob. Chemother. 66:672–673 [DOI] [PubMed] [Google Scholar]
- 13. Heidelberg JF, et al. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118–1123 [DOI] [PubMed] [Google Scholar]
- 14. Ho PL, et al. 2011. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One 6:e17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36 [DOI] [PubMed] [Google Scholar]
- 16. Matar GM, et al. 2008. Oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Lebanon. Clin. Microbiol. Infect. 14:887–888 [DOI] [PubMed] [Google Scholar]
- 17. Mierzejewska J, Kulińska A, Jagura-Burdzy G. 2007. Functional analysis of replication and stability regions of broad-host-range conjugative plasmid CTX-M3 from the IncL/M incompatibility group. Plasmid 57:95–107 [DOI] [PubMed] [Google Scholar]
- 18. Moquet O, et al. 2011. Class D OXA-48 carbapenemase in multidrug-resistant enterobacteria, Senegal. Emerg. Infect. Dis. 17:143–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poirel L, Bonnin RA, Nordmann P. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species. IUBMB Life, in press [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Poirel L, et al. 2011. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 55:2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poirel L, Naas T, Nordmann PP. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 54:24–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poirel L, Pitout JD, Nordmann P. 2007. Carbapenemases: molecular diversity and clinical consequences. Future Microbiol. 2:501–512 [DOI] [PubMed] [Google Scholar]
- 25. Poirel L, et al. 2011. Cross-border transmission of OXA-48-producing Enterobacter cloacae from Morocco to France. J. Antimicrob. Chemother. 66:1181–1182 [DOI] [PubMed] [Google Scholar]
- 26. Poirel L, et al. 2011. Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other carbapenemases identified in a patient returning from India. Antimicrob. Agents Chemother. 55:447–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potron A, et al. 2011. Characterization of OXA-181, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae. Antimicrob. Agents. Chemother. 55:4896–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Potron A, Poirel L, Nordmann P. 2011. Origin of OXA-181, an emerging carbapenem-hydrolyzing oxacillinase, as a chromosomal gene in Shewanella xiamenensis. Antimicrob. Agents Chemother. 55:4405–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Potron A, Poirel L, Nordmann P. 2011. Plasmid-mediated transfer of the blaNDM-1 gene in Gram-negative rods. FEMS Microbiol. Lett. 324:111–116 [DOI] [PubMed] [Google Scholar]
- 30. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]