Abstract
Daptomycin (DAP) is a new class of cyclic lipopeptide antibiotic highly active against methicillin-resistant Staphylococcus aureus (MRSA) infections. Proposed mechanisms involve disruption of the functional integrity of the bacterial membrane in a Ca-dependent manner. In the present work, we investigated the molecular basis of DAP resistance in a group of isogenic MRSA clinical strains obtained from patients with S. aureus infections after treatment with DAP. Different point mutations were found in the mprF gene in DAP-resistant (DR) strains. Investigation of the mprF L826F mutation in DR strains was accomplished by inactivation and transcomplementation of either full-length wild-type or mutated mprF in DAP-susceptible (DS) strains, revealing that they were mechanistically linked to the DR phenotype. However, our data suggested that mprF was not the only factor determining the resistance to DAP. Differential gene expression analysis showed upregulation of the two-component regulatory system vraSR. Inactivation of vraSR resulted in increased DAP susceptibility, while complementation of vraSR mutant strains restored DAP resistance to levels comparable to those observed in the corresponding DR wild-type strain. Electron microscopy analysis showed a thicker cell wall in DR CB5012 than DS CB5011, an effect that was related to the impact of vraSR and mprF mutations in the cell wall. Moreover, overexpression of vraSR in DS strains resulted in both increased resistance to DAP and decreased resistance to oxacillin, similar to the phenotype observed in DR strains. These results support the suggestion that, in addition to mutations in mprF, vraSR contributes to DAP resistance in the present group of clinical strains.
INTRODUCTION
Staphylococcus aureus is the most common Gram-positive pathogen among skin and soft tissue infections (2). Methicillin resistance in S. aureus is mediated by the acquisition of a penicillin-binding protein (PBP), PBP 2a, which has decreased affinity for β-lactam antibiotics but can continue to cross-link the cell wall once the native PBPs (i.e., PBPs 1 to 4) have been inactivated (23). A distinctive feature for most methicillin-resistant S. aureus (MRSA) strains is the heterogeneous expression of resistance, characterized by a small proportion (≤0.1%) of the population expressing a high level of homogeneous resistance while most of the other isolates in the population express resistance to 10 μg/ml (12, 15, 43). Daptomycin (DAP) is a cyclic anionic lipopeptide antibiotic that is produced by Streptomyces roseosporus (3) and is approved for treatment of skin and skin structure infections as well as treatment of bacteremia and right-side endocarditis caused by MRSA (1). The mechanism of action involves disruption of cytoplasmic membrane function, resulting in depolarization and cell death due to disruption of critical metabolic functions, such as protein, DNA, and RNA synthesis (2).
The incidence of DAP resistance in clinical isolates is very low, and resistant strains display small increases in MIC (2). The exact mechanism of DAP resistance has not yet been fully elucidated, although some characteristics of the resistance phenotype have been described. Genes regulating cell membrane surface charge (e.g., mprF, lysylphosphatidylglycerol [LPG] synthetase) and fatty acid biosynthesis (e.g., yycFG, two-component sensor kinase system) were associated with resistance (34, 40) in both laboratory-derived and clinical S. aureus strains (17). Other studies showed that at least four phenotypic membrane alterations correlated with DAP resistance in S. aureus, notably, in regard to the three major membrane components, phosphatidylglycerol, cardiolipin, and LPG (25). These studies demonstrated that DAP-nonsusceptible isolates exhibited enhanced outer leaflet LPG translocation compared to the daptomycin-susceptible isolates (25). The synthesis and the outer leaflet translocation of LPG are mediated by MprF (40), and a correlation between increased expression of a mutant mprF gene and reduced in vitro DAP susceptibility was recently reported (48). Moreover, it has been demonstrated that the dltABCD operon also contributes to the net membrane positive charge by d-alanylating wall teichoic acids (WTAs) through distinct effector mechanisms (5). Thus, the associated effect of both the mprF and dltABCD mechanisms would result in a reduced access of calcium-DAP to its membrane target. Besides the membrane, changes in the cell wall are suggested to be involved in DAP resistance. Recently, studies by Muthaiyan et al. demonstrated with transcriptional profiling that DAP is an inducer of the cell wall stress stimulon, affecting the expression of both genes that are involved in the peptidoglycan synthesis of S. aureus and those that are known to be under regulation of the VraSR two-component regulatory system (35). In this context, it has been observed in an in vitro-generated, DAP-nonsusceptible derivative strain that vraSR was upregulated, while expression of mprF was repressed; no mutations in mprF were observed in this strain (7).
The present work aimed to investigate the molecular basis of DAP resistance in pairs of DAP-susceptible (DS) and DAP-resistant (DR) isogenic MRSA clinical strains obtained as a result of DAP treatment failure. We found that both mprF point mutations and differential expression levels of vraSR observed between DS and DR strains were mechanistically linked to the DAP-resistant phenotype. Moreover, we observed that decreased susceptibility to DAP was associated in the majority of strains with increased susceptibility to oxacillin, a phenotype associated with their heterotypic mode of β-lactam resistance expression, i.e., in heterogeneous but not homogeneous MRSA strains. These results support the concept that increased expression of vraSR represents an additional factor contributing to the development of daptomycin resistance in these clinical MRSA strains.
MATERIALS AND METHODS
Materials and media.
Trypticase soy agar (TSA II) with 5% sheep blood (BBL, Sparks, MD) and Mueller-Hinton agar (MHA; BBL Microbiology Systems, Cockeysville, MD) with and without additives (Sigma, St. Louis, MO; United States Biochemicals, Cleveland, OH) were used for subculture and maintenance of S. aureus strains.
Bacterial strains.
All of the strains and primers used in this study are listed in Tables 1 and 2. Pulsed-field gel electrophoresis (PFGE) was used to determine the clonality of the isogenic strains as previously described (4, 45).
Table 1.
Description of strains and plasmids used in this study
| Straina | Relevant genotype and phenotype | Reference or source |
|---|---|---|
| S. aureus | ||
| COL | Methicillin-resistant SCCmec type I | 10 |
| N315 | Methicillin-resistant SCCmec type II | 29 |
| USA300 | Methicillin-resistant SCCmec type IV | 9, 27 |
| CK1001 | RN4220 ΔmprF::cat | 22 |
| KVR | N315ΔvraSR::cat | 28 |
| RN4220 | Restriction-deficient mutagenized RN450 | 15 |
| CB5011, pair A | DS, SCCmec type II (USA100) | Cubist Pharmaceuticals |
| CB5012, pair A | DR isogenic to CB5011; MprF L826F | Cubist Pharmaceuticals |
| CB5013, pair B | DS, SCCmec type II (USA100) | Cubist Pharmaceuticals |
| CB5014, pair B | DR isogenic to CB5013, MprF S377L | Cubist Pharmaceuticals |
| CB1631, pair C | DS, SCCmec type II (USA100) | Cubist Pharmaceuticals |
| CB1634, pair C | DR isogenic to CB1631, MprF L826F | Cubist Pharmaceuticals |
| CB5048, pair D | DS, SCCmec type IV (USA300) | Cubist Pharmaceuticals |
| CB5051, pair D | DR isogenic to CB5048 | Cubist Pharmaceuticals |
| CB5035, pair E | DS, SCCmec type II (USA100) | Cubist Pharmaceuticals |
| CB5036, pair E | DR isogenic to CB5035, MprF P314L | Cubist Pharmaceuticals |
| CB182, pair F | DS, SCCmec type IV (USA300) | Cubist Pharmaceuticals |
| CB183, pair F | DR isogenic to CB182, MprF L341S | Cubist Pharmaceuticals |
| Derivative S. aureus strains | ||
| MAR-1 | CB5011 ΔmprF::cat | This study |
| MAR-2 | MAR-1 + pMPRF-1 (wild-type mprF cloned into pPV72-2) | This study |
| MAR-3 | MAR-1 + pMPRF-2 (mutated mprF L826F cloned into pPV72-2) | This study |
| MAR-4 | CB5012+ ΔmprF::cat | This study |
| MAR-5 | MAR-4 + pMPRF-1 | This study |
| MAR-6 | MAR-4 + pMPRF-2 | This study |
| MAR-7 | CB5012 ΔvraSR::cat | This study |
| MAR-8 | MAR-7 + pVRASR-2 | This study |
| MAR-9 | CB5011 expressing pVRASR-2 | This study |
| MAR-10 | CB5013 expressing pVRASR-2 | This study |
| MAR-11 | CB1631 expressing pVRASR-2 | This study |
| MAR-12 | CB5048 expressing pVRASR-2 | This study |
| MAR-13 | CB5035 expressing pVRASR-2 | This study |
| MAR-14 | CB1631 ΔmprF::cat | This study |
| MAR-15 | MAR-14 pMPRF-1 (wild-type mprF cloned into pPV72-2) | This study |
| MAR-16 | MAR-14 pMRPF-2 (mutated mprF L826F cloned into pPV72-2) | This study |
| MAR-17 | CB1634 ΔmprF::cat | This study |
| MAR-18 | MAR-17(pMPRF-1) (wild-type mprF cloned into pPV72-2) | This study |
| MAR-19 | MAR-17(pMRPF-3) (mutated mprF L826F cloned into pPV72-2) | This study |
| E. coli (PCR2.1-TOPO) | Ampr Kanr | Invitrogen |
| S. aureus (pPV72-2) | Shuttle vector; E. coli-S. aureus Tetr | 31 |
| S. aureus RN4220 (pVRASR-2) | Entire vraS/vraR cloned into pAW8 | 6 |
| S. aureus RN4220 (pMPRF-1) | Entire mprF cloned into pPV72-2 | This study |
| S. aureus RN4220 (pMPRF-2) | Full-length mprF containing L826F site-directed mutation from CB5012 cloned into pPV72-2 | This study |
| S. aureus RN4220 (pMPRF-3) | Full-length mprF containing L826F site-directed mutation from CB1634 cloned into pPV72-2 | This study |
Pair A, parent strain CB5011 and its derivative, CB5012; pair B, CB5013 and derivative CB5014; pair C, CB1631 and derivative CB1634; pair D, CB5048 and derivative CB5051; pair E, CB5035 and derivative CB5036; pair F, CB182 and derivative CB183.
Table 2.
Primers used in this studya
| Primers | 5′–3′ |
|---|---|
| 16S-F | TCCGGAATTATTGGGCGTAA |
| 16S-R | CCACTTTCCTCTTCTGCACTCA |
| vraSR-R | GGTGCAACGTTCCATATTGTATTGT |
| vraSR-F | GGCTTCAACTCATGGGCTTTGGCAA |
| mecA-F | TGCCTAATCTCATATGTGTTCCTGTAT |
| mecA-R | CGGTGCTGAAACTTATTCACAATATAAT |
| mprF-F | GTGGCGACATTCTTCACTTACG |
| mprF-R | GCCAGAAGTAATAGCGCAATACAG |
| mprF-F2 | ATGTTGGGCAGTTACATTTATGAT |
| mprF-R2 | GACTTAACTTAAGCTCATTTC |
All primers were prepared for this study.
Antibiotics.
Standard reference powders were obtained from Sigma-Aldrich, St. Louis, MO (oxacillin and vancomycin); DAP was provided by Cubist Pharmaceuticals, Lexington, MA. Susceptibilities to oxacillin and vancomycin were determined according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) (36). Daptomycin MICs were determined by Etest (AB Biodisk, Solna, Sweden).
Population analysis.
Population analysis profiles were determined as described by Chambers et al. (10). In the case of DAP population analysis, profiles were performed by using MHA supplemented with calcium (50 μg/ml) and containing increasing concentrations of DAP, and 109 CFU from an overnight culture was serially diluted as the inoculum.
Comparison of relative net cell surface charge.
Previous investigations indicated that DR S. aureus strains are associated with frequent alteration of the net cell surface charge compared to that of the respective DS parental strains (25, 32). For these assays, we compared the relative net cell surface charge of pairs CB5011/CB5012, CB5035/CB5036, and CB182/CB183 by quantifying the association of the highly cationic molecule cytochrome c (pI 10; Sigma) to the staphylococcal surface. The amount of cytochrome c remaining in the postcentrifugation supernatant after a 10-min binding interaction with S. aureus cells was quantified spectrophotometrically at an optical density at 530 nm (OD530). The more unbound cytochrome c that was detected in the supernatant, the more that a positive charge existed on the bacterial cell surface. Strains CB685 and CB687 were used as controls (dltA positive and negative), respectively (41). The data shown are the means (± standard deviations [SDs]) of the amount of unbound cytochrome c from three independent experiments.
Determination of SCCmec types.
Chromosomal DNA was prepared by using a Qiagen (Valencia, CA) genomic DNA preparation kit according to the manufacturer's instructions. Staphylococcal cassette chromosome mec (SCCmec) types were determined as previously described (38). S. aureus strains COL (SCCmec type I), N315 (SCCmec type II), ANS46 (SCCmec type III), and USA300 (SCCmec type IV) were used as positive controls.
Analysis of gene expression by real-time RT-PCR, Northern blotting, and microarray transcriptional profiling.
RNA extractions for real-time reverse transcription-PCR (RT-PCR) and Northern blot analyses were performed as previously described (12, 19, 44). Total RNA was extracted using an RNeasy isolation kit (Qiagen); all RNA samples were analyzed by A260/A280 spectrophotometry and gel electrophoresis to assess concentration and integrity and cleaned of potential DNA contamination by treating them with DNase as per manufacturer recommendations (Ambion, Inc., Austin, TX). Real-time reverse transcription-PCR analysis was done using a SensiMix SYBR One-Step kit (Quantace/Bioline, Taunton, MA) according to the manufacturer's protocol. Gene expression was compared according to the threshold cycle (CT) values converted to fold change with respect of a sample considered the reference (value = 1) using log2 −(ΔΔCT). The change (n-fold) in the transcript level was calculated using the following equations: ΔCT = CT(test DNA) − CT(reference cDNA), ΔΔCT = ΔCT(target gene) − ΔCT(16S rRNA), and ratio = 2−ΔΔCT (30). The quantity of cDNA for each experimental gene was normalized to the quantity of 16S cDNA in each sample determined in a separate reaction. Each RNA sample was run in triplicate; values represent the means of at least three separate RNA samples. Oligonucleotide primers are shown in Table 2.
Northern blot analysis was performed by using 7 μg of RNA separated through formaldehyde-containing 1% agarose. The intensities of the 23S and 16S rRNAs were visualized using a 254-nm UV shortwave lamp, and quantities were adjusted so that the same amount of RNA was loaded for each sample. RNA was transferred from agarose to positively charged nylon membranes (Stratagene, La Jolla, CA) by capillary action (44). Labeling and hybridization were done using digoxigenin labeling and detection kits (Roche, Indianapolis, IN) according to the manufacturer's instructions. Oligonucleotide primers are shown in Table 2.
Microarray transcriptional profiling was carried out as previously described (12, 19) by using a spotted DNA microarray (J. Craig Venter Institute, version 6 S. aureus slides) containing 4,546 oligonucleotides (70-mer) covering the genomes of S. aureus COL (2,654 open reading frames [ORFs]), S. aureus N315 (2,623 ORFs), S. aureus Mu50 (2,748 ORFs), MRSA 252 (2,744 ORFs), MSSA 476 (2,619 ORFs), and pLW043 (62 ORFs). Pairwise comparisons, performed in triplicate, between isogenic pairs A (CB5011 versus CB5012), E (CB5035 versus CB5036), and F (CB182 versus CB183) were made, as indicated in Table 1.
The comparisons were performed between DS and DR MRSA isogenic pairs grown in drug-free LB medium collected at exponential growth (log) phase (OD600, 0.6). The results are based on a series of statistical analyses (filtering) where ratios of Cy3 and Cy5 were converted to log2 values and the cutoff was set at above 1 (present) or below −1 (absent) (8, 12). Median signal intensity values, calculated from each set of in-slide replicates and flipped-dye experiments, were used to calculate log2 and n-fold changes in gene expression. The data set was normalized by applying the LOWESS algorithm (block mode; smooth parameter, 0.33) and using MIDAS software (http://www.jcvi.org/cms/research/software/), and significant changes were identified with SAM (significance analysis of microarrays) software (http://www-stat.stanford.edu/tibs/SAM/index.html) (8, 12).
Cloning, transformation, and DNA manipulation.
All restriction endonuclease digestions and ligations were performed in accordance with the manufacturer's (New England BioLabs, Beverly, MA) specifications. Chromosomal DNA was prepared by using a Qiagen genomic DNA preparation kit according to the manufacturer's directions. Sequencing of all PCR amplification products was performed by the Nucleic Acid Research Facility at Virginia Commonwealth University (Richmond, VA). Sequence analysis of mprF from clinical isolates was performed using chromosomal DNA isolated from DS/DR strains at SeqWright (Houston, TX); primers used were previously described (17, 26, 42). Consensus sequences were assembled from both orientations with Vector NTI Advance 10 software for Windows (InforMax, Bethesda, MD). S. aureus N315 (GenBank accession number BA000018) was used as a positive control.
Mutational insertion inactivation of mprF and complementation.
mprF-null mutants were constructed by moving ΔmprF::cat from strain CK1001 (22) into the clinical DR MRSA strains CB5012 and CB1634 and their DS counterparts, CB5011 and CB1631, respectively, by general transduction using 80α phage (37). Transcomplementation of mprF was performed by using a construct encompassing the complete mprF gene as well as the upstream region (425 bp) including the putative ribosomal binding site and promoter using mprF primers mprF-F2 and mprF-R2, shown in Table 2. The 3.0-kb PCR fragment products were purified using a QIAquick gel extraction kit, ligated into the ligase-independent cloning site of the PCR2.1-TOPO vector (Invitrogen, Carlsbad, CA), and transformed into chemically competent Escherichia coli TOP10 cells (Invitrogen). A staphylococcal origin of replication was introduced by cloning plasmid pPV72-2, an S. aureus replicon (31), into the unique BamHI site on PCR 2.1-TOPO (pMPRF-1; Table 1); the construct was moved into S. aureus RN4220 by electroporation (15). Transcomplementation of mprF mutants was obtained by transduction of plasmid pMPRF-1 (pPV72-2 vector containing wild-type mprF) from RN4220 by phage 80α into DR mprF mutants CB5012 ΔmprF::cat and CB1634 ΔmprF::cat and into their counterpart DS mprF mutants, CB5011 ΔmprF::cat and CB1631ΔmprF::cat, respectively. In addition, CB5012 ΔmprF::cat and CB5011 ΔmprF::cat mutants were transduced with pMPRF-2 (pPV72-2 harboring full-length mprF containing an L826F site-directed mutation), obtaining MAR-3 (CB5011ΔmprF::cat) and MAR-6 (CB5012 ΔmprF::cat), respectively. This was similarly performed for mutants CB1631ΔmprF::cat and CB1634 ΔmprF::cat by introducing pMPRF-3 (pPV72-2 harboring full-length mprF containing L826F), obtaining MAR-16 and MAR-19, respectively. All constructs were verified by both sequence and restriction enzyme analyses.
Construction of vraSR-null mutants and complementation.
A mutant MAR-7 strain (Table 1) was obtained by transducing the deletion vraSR mutant (ΔvraSR::cat) by ϕ11 phage from strain KVR (28) into DR CB5012 (37). The mutant MAR-7 was transcomplemented with a shuttle plasmid, pAW8, containing a 3.3-kb fragment corresponding to the entire vraSR operon (6) by transduction, resulting in the MAR-8 strain (Table 1). Overexpression of vraSR in DS strains CB5011, CB5013, CB1631, CB5048, and CB5035 was performed by phage 80α-mediated transduction of shuttle plasmid pAW8 containing the entire vraSR operon (6), resulting in strains MAR-9 to MAR-13, respectively (Table 1).
Electron microscopy (EM) evaluation of cell wall thickness.
The preparation of S. aureus cells for transmission electron microscopy from DS parent CB5011, DR derivative CB5012, and genetically modified mutant derivative strains was performed using standard procedures described by Cui et al. (11). Briefly, morphometric evaluation of cell wall thickness was performed by using photographic images at a final magnification of ×30,000. Thirty cells of each strain with nearly equatorial cut surfaces were measured for the evaluation of cell wall thickness, and results were expressed as means ± SDs. All of the cell wall thickness measurements were performed by two of the listed authors (S.M. and K.B.P.), who were blinded to the identities of the organisms.
RESULTS
Phenotypic studies between DS and DR MRSA pairs.
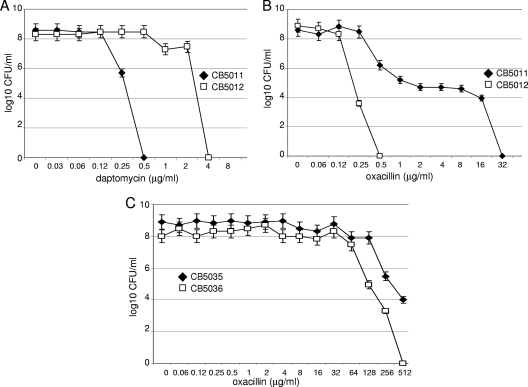
DAP, oxacillin, and vancomycin MICs were determined in six isogenic DS/DR strains labeled pairs A, B, C, D, E, and F by using standard procedures, including the Etest (AB Biodisk, Dalvagen, Sweden) in medium supplemented with calcium (50 μg/ml CaCl2). We found an increase in DAP MICs in all DR strains (2 to 4 μg/ml) that was associated with a slight decrease in vancomycin susceptibility (1 to 3 μg/ml); baseline daptomycin and vancomycin MICs were 0.25 to 0.5 μg/ml and 1 to 2 μg/ml for the DS and DR strains, respectively (Table 3). No changes in susceptibility to cationic molecules such as gentamicin were observed between DS-DR strains (data not shown). We next investigated the expression of resistance to DAP by population analysis profile. As shown in Fig. 1A, for pair A, 5 × 105 DS CB5011 cells were inhibited at 0.5 μg/ml, while 3 × 108 DR CB5012 cells were still growing at the same DAP concentration. Similar results were obtained for pairs D and E (data not shown). Interestingly, susceptibility to oxacillin was reduced from 16 to 32 μg/ml to 1 to 0.25 μg/ml in DR strains for pairs A, C, D, and F; pairs B and E did not show changes in oxacillin MICs (512 μg/ml) (Table 3). Analysis of oxacillin resistance expression showed a typical heterogeneous MRSA profile in pairs A, C, D, and F, in which, for example, 9 × 103 DS CB5011 cells grew at an oxacillin concentration of 32 μg/ml, while its DR CB5012 counterpart (4 × 103 cells) was inhibited at an oxacillin concentration of 0.25 μg/ml (Fig. 1B). In contrast, pairs B and E displayed a homogeneous oxacillin expression profile with no reduction in oxacillin susceptibility (Fig. 1C). These results suggest that in this group of strains, DAP resistance is associated with a concomitant decrease in oxacillin resistance in MRSA strains expressing heterogeneous β-lactam resistance.
Table 3.
MICs of S. aureus DS and DR strains against DAP, oxacillin, and vancomycin
| Pair | Strain (DAP susceptibility) | MIC (μg/ml)a |
||
|---|---|---|---|---|
| DAP | OXA | VAN | ||
| A | CB5011 (DS) | 0.5 | 32 | 1 |
| CB5012 (DR) | 4 | 0.25 | 2 | |
| B | CB5013 (DS) | 0.5 | 512 | 1 |
| CB5014 (DR) | 4 | 512 | 2 | |
| C | CB1631 (DS) | 0.5 | 32 | 1 |
| CB1634 (DR) | 4 | 0.5 | 2 | |
| D | CB5048 (DS) | 0.25 | 32 | 1.5 |
| CB5051 (DR) | 2 | 1 | 2 | |
| E | CB5035 (DS) | 0.25 | 512 | 1.5 |
| CB5036 (DR) | 2 | 512 | 3 | |
| F | CB182 (DS) | 0.5 | 32 | 1 |
| CB183 (DR) | 4 | 1 | 2 | |
OXA, oxacillin; VAN, vancomycin.
Fig 1.
Population analysis profiles. Aliquots (10 μl) from overnight LB cultures of the DS CB5011 and DR CB5012 (A and B) and DS CB5035 and DR CB5036 (C) S. aureus strains were inoculated to MHA plates containing increasing concentrations of daptomycin (with Ca2+ at 50 mg/liter) (A and B) and oxacillin (C) to determine the mode of phenotypic expression of resistance by population analysis, as described in Materials and Methods.
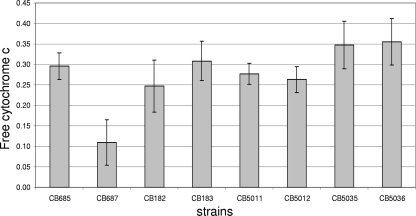
Previous studies have shown that DR S. aureus strains displayed a frequent alteration of the net cell surface charge compared to their DS counterparts (14). By spectrophotometrically quantifying the amount of bound cytochrome c, we found no significant changes between DS/DR strain pairs CB5011/CB5012, CB5035/CB5036, and CB182/CB183 (Table 1; Fig. 2). The pair CB685 and CB687 (dltA positive and negative, respectively) was used as a positive control. As previously reported (41), strain CB687displayed increased levels of bound cytochrome c compared to strain CB685 (Fig. 2). The resistant isolates tested herein (Fig. 2) were not significantly different from their parent, susceptible isolates, suggesting that these data are consistent with, although do not completely exclude, the possibility that changes in cell surface charge are not a significant contributor to the mechanism associated with the resistance phenotype in the present group of clinical strains.
Fig 2.
Binding of positively charged cytochrome c to whole DS-DR MRSA cells. The graph shows the percentage of cytochrome c unbound after 10 min incubation with the DS-DR cells at room temperature. Strains CB685 and CB687 (dltA positive and negative, respectively) were used as positive controls. Data represent the means and standard deviations from three independent experiments.
Genotypic analysis of DS/DR MRSA strains.
To determine whether DAP resistance was linked to a specific SCCmec structure, we performed a multiplex PCR analysis of the ccrAB genes, implicated in the excision and integration of SCCmec to the S. aureus chromosome (38). We determined that pairs A, B, C, and E display an SCCmec type II structure characterized by the presence of mecA, the regulators mecI/mecR1, and the ccrAB2 genes, a profile that was identical to that for the positive-control S. aureus N315 (SCCmec type II element) strain and related to that of USA100-type strains. In contrast, pairs D and F harbored SCCmec type IV and were related to USA300-type strains, with the insertion of the IS1272 element at the 3′-terminal portion of the regulator mecR1 and deletion of the repressor mecI (data not shown). In addition, PFGE analysis performed with DS/DR pair strains indicated clonality with an absence of genomic rearrangements (data not shown). Together, these results led us to infer that the decrease in oxacillin resistance observed in the above-mentioned DR strains was not due to deletion of SCCmec and/or mecA (data not shown).
Mutations in the mprF gene contribute to DAP resistance in DS/DR MRSA pairs.
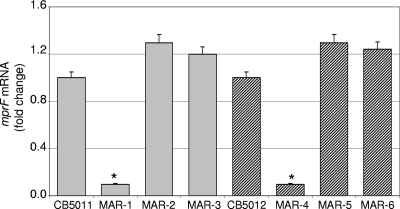
Previous studies have shown that resistance to DAP in either clinical MRSA or laboratory mutant strains may be associated with changes in mprF, including increased transcription and/or point mutations leading to a gain-of-function phenotype (40, 48). To determine whether similar events may account for resistance to DAP in our clinical MRSA strains, we performed expression analysis of mprF by real-time RT-PCR using RNA extracted from DS and DR cells collected at both exponential and stationary phases of growth (OD600s, 0.6 and 1.0, respectively). No significant difference between DS and DR strains was observed (data not shown), suggesting that DAP resistance in our clinical DR MRSA strains is not mediated through increased mprF expression. Sequence analysis of mprF revealed the presence of the following mutations: pair A, DR CB5012, one amino acid substitution, leucine 826 to phenylalanine (L826F); pair B, DR CB5013, S377L; pair C, DR CB1631, L826F; pair E, DR CB5036, P314L; and pair F, DR CB183, L341S (Table 1). Three single amino acid substitutions were mapped to the hydrophobic N-terminal translocase domain of MprF (i.e., S377L, P314L, and L341S), while the substitution L826F localized to the hydrophilic Lys1-phosphatidylglycerol synthase C-terminal portion (14). To determine the potential role of mprF mutations in DAP resistance, an mprF null mutant was generated, using as background the DS CB5011 strain, by 80α-mediated transduction from mprF-null mutant strain CK1001 (22). The resulting strain (MAR-1; Table 1) was verified by both PCR (DNA) and real-time RT-PCR (RNA) (Fig. 3). Mutant MAR-1 (CB5011 ΔmprF::cat) was complemented either with pMPRF-1 containing the wild-type full-length mprF gene cloned in pPV72-2 (CB5011 ΔmprF::cat plus pMPRF-1, strain MAR-2; Table 1) or with pMPRF-2 harboring the full-length mprF gene containing the L826F site-directed mutation (CB5011ΔmprF::cat plus pMPRF-2, strain MAR-3; Table 1). mprF expression was verified following complementation in RNA samples prepared from cells collected at the exponential phase of growth (OD600, 0.6); mprF transcomplementation (strains MAR-2 and MAR-3; Table 1) resulted in mprF transcription levels similar to those observed in parental strain CB5011 (Fig. 3). Similar results of mprF expression were obtained in mprF mutants (MAR-14 and MAR-19; data not shown). Phenotypic analysis was then performed between the mprF mutant (MAR-1) and complemented MAR-2 and MAR-3 strains. MIC values showed a decrease in DAP MIC in MAR-1 compared to wild-type CB5011 (MICs, 0.094 μg/ml and 0.5 μg/ml, respectively; Table 4). Complementation of the MAR-1 strain with wild-type mprF (MAR-2) restored the DAP MIC to a level equivalent to that of CB5011 (0.5 μg/ml); however, full-length mprF containing the L826F mutation resulted in an increased DAP MIC, up to 2 μg/ml (MAR-3; Table 4), despite levels of transcription similar to those existing in the wild-type strain (Fig. 3).
Fig 3.
Quantitation of mprF mRNA by real-time RT-PCR. RNA was prepared from cells of DS CB5011, mprF-null mutant MAR-1, MAR-1 mprF-complemented MAR-2 and MAR-3, DR CB5012, mprF-null mutant MAR-4, and MAR-4 mprF-complemented MAR-5 and MAR-6 strains collected at exponential phase of growth, as described in Materials and Methods. Relative fold change values of specific mprF mRNA are shown on the vertical axis; 16S rRNA was used as an internal control. *, significantly less than control (P < 0.001).
Table 4.
Daptomycin MICs of mprF-null mutant and mprF-complemented strains
| Strain | Genotype | MIC (μg/ml) |
|---|---|---|
| CB5011 | DS | 0.5 |
| MAR-1 | CB5011 ΔmprF::cat | 0.094 |
| MAR-2 | MAR-1 + pMPRF-1 | 0.5 |
| MAR-3 | MAR-1 + pMPRF-2 | 2 |
| CB5012 | DR | 4 |
| MAR-4 | CB5012 + ΔmprF::cat | 0.5 |
| MAR-5 | MAR-4 + pMPRF-1 | 0.75 |
| MAR-6 | MAR-4 + pMPRF-2 | 3 |
| CB1631 | DS | 0.25 |
| MAR-14 | CB1631 ΔmprF::cat | 0.064 |
| MAR-15 | MAR-14 + pMPRF-1 | 0.5 |
| MAR-16 | MAR-14 + pMPRF-3 | 2 |
| CB1634 | DR | 4 |
| MAR-17 | CB1634 ΔmprF::cat | 0.5 |
| MAR-18 | MAR-17 + pMPRF-1 | 0.5 |
| MAR-18 | MAR-17 + pMPRF-3 | 3 |
Inactivation of mprF was then performed in the DR CB5012 strain by transduction with phage lysates from mprF-null mutant CK1001 (22), and lack of expression of mprF was verified by real-time RT-PCR (MAR-4; Fig. 3). Phenotypic analysis of MAR-4 revealed that inactivation of mprF increased susceptibility to DAP (MICs, 4 μg/ml and 0.5 μg/ml for CB5012 and MAR-4, respectively; Table 4). As with the DS CB5011 strain, complementation of the mprF mutant MAR-4 strain with wild-type full-length mprF (MAR-5) resulted in a DAP MIC of 0.75 μg/ml (Table 4), while complementation of MAR-4 with mprF L826F conferred higher resistance to DAP (MAR-6; MIC, 3 μg/ml; Table 4). To further investigate whether the L826F mprF mutation was responsible for the observed phenotype and was not due to a secondary effect related to the plasmid expression, a similar approach was performed in the pair CB1631 (DS) and CB1634 (DR) containing the same L826F mprF mutation. Results shown in Table 4 confirmed the observations established in pair CB5011/CB5012. Together, in agreement with previous observations (17, 26, 42), these results indicate that mutations observed in mprF may contribute to decreased susceptibility to DAP in DR strains. However, they also suggest that the introduction of only these mutations may not be sufficient to achieve the level of resistance observed in DR strains, as suggested by studies performed using the DS strain background. Further studies designed to establish the functional significance of mprF mutations detected in other pairs are ongoing.
Analysis of differentially expressed genes by spotted microarrays in isogenic DS/DR MRSA clinical strains.
We performed differential gene expression analysis by spotted DNA microarray to identify additional mechanisms that may be involved in establishing resistance to DAP. This analysis was determined in three of the pairs used in the present investigation, i.e., pair A (CB5011 [DS] versus CB5012 [DR]), pair E (CB5035 [DS] versus CB5036 [DR]), and pair F (CB182 [DS] versus CB183 [DR]) (Table 5). As shown in Table 5, overall changes in expression levels of most genes were moderate, with ratios in the range of 2- to 5-fold. On the basis of a cutoff value arbitrarily set at a 2-fold change, genes upregulated in DR versus DS strains in the above-mentioned pairs included genes related to cell wall metabolism, mecA (SA0038_N315) and penicillin-binding protein 2, pbp2 (SA1283_N315); two-component sensor histidine kinase vraS (SA1701_N315) and two-component response regulator vraR (SA1700_N315), which play a major role in sensing and reacting to cell wall stress; and the peptidoglycan synthesis (elongation) gene sgtB (SA1691_N315). Other upregulated genes were vraF (ABC transporter ATP-binding protein, SA0616_N315) and vraG (ABC transporter permease, SA0617_N315), known to be involved in mechanisms of transport (7); ddh (2-hydroxyacid dehydrogenase, SA2312_N315); opp-1B (SA2254_N315, oligopeptide transporter putative membrane-permease domain); and opp-2F (SA1211_N315, oligopeptide transporter putative ATPase domain). Among downregulated genes, the analysis revealed lrgB (antiholin-like protein LrgB, SA0253_N315), a genetic locus reported to influence autolysis, with LrgB proteins acting as antiholins that regulate the activity of the holin CidA and CidB proteins, and a group involved in the metabolism of glycerol, including glpF (glycerol uptake facilitator, SA1140_N315), pgsA (phosphatidylglycerophosphate synthase, SA1126_N315), and dehydrogenases (alanine dehydrogenase, SA1272_N315; glycine dehydrogenase, SA1365_N315) (Table 5). These results indicate that genes involved in both cell wall and membrane synthesis may play a role during the acquisition of DAP resistance in the DR strains described herein.
Table 5.
Differential gene expression between DS and DR strainsa
| ORF | Gene | Product or putative function | Fold change |
|---|---|---|---|
| SA0616_N315 | vraF | ABC transporter ATP-binding protein | +3.8 |
| SA0617_N315 | vraG | ABC transporter permease | +4.3 |
| SA2312_N315 | ddh | 2-Hydroxyacid dehydrogenase | +3.1 |
| SA2254_N315 | opp-1B | Oligopeptide transporter putative membrane permease domain | +3.5 |
| SA1211_N315 | opp-2F | Oligopeptide transporter putative ATPase domain | +3.5 |
| SA0038_N315 | mecA | PBP 2a | +4.2 |
| SA1283_N315 | pbp2 | PBP 2 | +4.5 |
| SA1691_N315 | sgtB | Elongation of peptidoglycan synthesis | +3.2 |
| SA1023_N315 | ftsL | Cell division protein | +4.4 |
| SA1700_N315 | vraR | Two-component response regulator | +5.5 |
| SA1701_N315 | vraS | Two-component sensor histidine kinase | +4.7 |
| SA0661_N315 | saeR | Response regulator | +3.1 |
| SA0760_N315 | Glycine cleavage system protein H | +4.1 | |
| SA1458_N315 | lytH | N-Acetylmuramoyl-l-alanine amidase | +3.8 |
| SA0149_N315 | capF | Capsular polysaccharide synthesis enzyme Cap5F | +2.8 |
| SACOL2509 | fnbB | Fibronectin binding protein | −3.3 |
| SA0747_N315 | cspC | Cold shock protein | −4.3 |
| SAR2790 | cspB | Cold shock protein | −3.8 |
| SA1141_N315 | glpK | Glycerol kinase | −3.5 |
| SA1140_N315 | glpF | Glycerol uptake facilitator | −4.6 |
| SA1126_N315 | pgsA | Phosphatidylglycerophosphate synthase | −2.6 |
| SA1272_N315 | Alanine dehydrogenase | −4.5 | |
| SA1365-N315 | Glycine dehydrogenase subunit 2 | −3.2 | |
| SA1047-N315 | pryF | Orotidine-5-phospate decarboxylase | −3.1 |
| SA2425_N315 | arcC | Carbamate kinase | −4.2 |
| SA0253_N315 | lrgB | Antiholin-like protein LrgB | −4.8 |
| SA2499_N315 | gidB | Glucose-inhibited division protein B | −2.7 |
Analysis of strain pairs A (CB5011 [DS] versus CB5012 [DR]), E (CB5035 [DS] versus CB5036 [DR]), and F (CB182 [DS] versus CB183 [DR]); analysis of each pair was performed in triplicate. The data were normalized by applying the LOWESS algorithm (block mode; smooth parameter, 0.33) and using MIDAS software (http://www.jcvi.org/cms/research/software/), and significant changes were identified with SAM software. Values are averages of the three pairs analyzed.
Daptomycin resistance is functionally associated with increased expression of the vraSR regulon in clinical MRSA strains.
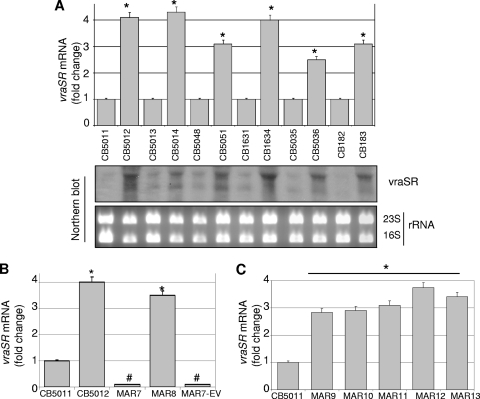
Taking into account results from gene expression analysis described above (genes involved in cell wall synthesis and turnover) and previous studies showing that the two-component regulator and cell wall stress stimulon VraSR is involved in the action of DAP (7, 35) prompted us to characterize the role of vraSR in our strains. Changes in the expression of vraSR were further analyzed by RT-PCR and Northern blot analysis using RNA from DS and DR cells corresponding to pairs A through E. As shown in Fig. 4A, increased expression of vraSR was observed in all DR strains compared to their DS counterparts. To define the mechanistic significance of vraSR upregulation, the vraSR operon was inactivated in DR strains CB5012 (pair A, CB5012 ΔvraSR::cat [MAR-7]; Table 1) and CB5014 (pair B, CB5014 ΔvraSR::cat) by phage 11-mediated transduction from the vraSR-null mutant KVR (28). Lack of expression of vraSR in mutant strains was monitored by real-time RT-PCR. Phenotypic analysis of vraSR-null mutant MAR-7 by DAP Etest showed an increase in DAP susceptibility compared to the parental CB5012 strain (MICs, 0.25 μg/ml versus MIC 4 μg/ml); similar results were observed in vraSR-null mutant strain CB5014 ΔvraSR::cat (data not shown). vraSR-null mutant MAR-7 was then complemented with a cloned full-length vraSR (6) (MAR-8; Table 1). vraSR complementation was verified by RT-PCR and resulted in vraSR transcription levels similar to those corresponding to DR CB5012 (Fig. 4B); as expected, no changes in vraSR expression were observed in MAR-7 complemented with an empty vector (Fig. 3B). Phenotypic analysis of the MAR-8 strain showed that vraSR complementation restored DAP resistance with MICs of 0.25 μg/ml versus 3 μg/ml for MAR-7 and MAR-8, respectively, values comparable to those observed in CB5012 (pair A; Table 3).
Fig 4.
Quantitation of vraSR mRNA by real-time RT-PCR and Northern blot analysis. RNA was prepared from DS and DR cells (Table 1) grown in the absence of DAP and collected at exponential phase of growth, as described in Materials and Methods. (A, top) relative fold change values of specific vraSR mRNA are shown on the vertical axis; 16S rRNA was used as an internal control. *, significantly greater than control (P < 0.001). (A, bottom) Increase of VraSR transcription in DR strains compared to DS strains examined in the same group of strains for which fold change results are shown in the top panel. Ethidium bromide-stained 23S and 16S rRNA bands are shown below the Northern blots as loading controls. (B) Quantitation of vraSR mRNA by real-time RT-PCR in DS/DR CB5011/CB5012 strains (negative and positive vraSR controls, respectively), vraSR-null mutant MAR-7, and vraSR- or empty vector-complemented MAR-8 and MAR-7EV strains, respectively. RNA was prepared from cells grown and collected at exponential phase of growth. Relative values of specific vraSR mRNA are shown in the vertical axis; 16S rRNA was used as an internal control. *, significantly greater than control (P < 0.001); #, significantly less than DR CB5012 (P < 0.01). (C) Quantitation of vraSR mRNA by real-time RT-PCR in DS vraSR-overexpressing MAR-9 to MAR-13 strains. CB5011 (DS) was included as a control. Relative fold change values of specific vraSR mRNA are shown on the vertical axis; 16S rRNA was used as an internal control.*, significantly greater than sample CB5011 (DS) (P < 0.001). In all cases, three independent cultures were sampled in triplicate to minimize error caused by inter- and intrasample variation.
To determine the mechanistic role of increased vraSR expression in daptomycin resistance, additional studies were performed by overexpressing vraSR in DS CB5011, CB5013, CB1631, CB5048, and CB5035 strains; complementation resulted in expression levels similar to those observed in the corresponding DR strains determined by RT-PCR (Fig. 4C). Phenotypic differences were determined by DAP MIC analysis; an increase in DAP MIC was consistently observed in vraSR-complemented DS strains, with values ranging from 2 μg/ml to 6 μg/ml (Table 6). Interestingly, increases in DAP resistance after vraSR overexpression in DS strains were associated with a decrease in oxacillin resistance (e.g., oxacillin MICs, 0.5 μg/ml versus 32 μg/ml for MAR-9 and DS CB5011, respectively; Table 6), reflecting relationships between DAP and oxacillin phenotypes similar to those described in the original DS/DR strains (Table 3).
Table 6.
MICs to DAP and oxacillin determined in DS and DS vraSR-overexpressing derivative strains
| Strain | Genotype | MIC (μg/ml) |
|
|---|---|---|---|
| DAP | OXAa | ||
| MAR-9 | CB5011 (DS) | 0.5 | 32 |
| CB5011 + pVRASR-2 | 4 | 0.5 | |
| MAR-10 | CB5013 (DS) | 0.5 | 512 |
| CB5013 + pVRASR-2 | 6 | 256 | |
| MAR-11 | CB1631 (DS) | 0.5 | 32 |
| CB1631 + pVRASR-2 | 6 | 0.75 | |
| MAR-12 | CB5048 (DS) | 0.25 | 32 |
| CB5048 + pVRASR-2 | 2 | 2 | |
| MAR-13 | CB5035 (DS) | 0.25 | 512 |
| CB5035 + pVRASR-2 | 2 | 256 | |
OXA, oxacillin.
As mentioned before, the two-component regulator VraSR is actively involved in the regulation of cell wall stress induced by treatment with DAP (7, 35). To investigate the phenotypes associated with reduced susceptibility to DAP, we analyzed cell wall thickness in pair CB5011/CB5012 and derivative mutants. Electron microscopy analysis showed that DR CB5012 displayed a thicker cell wall (33.5 ± 1.2 nm) than DS CB5011 (Table 7). To determine the contribution of mprF and vraSR to cell thickness, mutants and complemented mprF and vraSR strains were analyzed. Inactivation of mprF in CB5012 (MAR-4) resulted in a significantly thinner cell wall (25.4 ± 1.1 nm) compared with that of CB5012 (33.2 ± 1.5 nm). Complementation with wild-type mprF (MAR-5) did not alter MAR-4 cell wall thickness; by contrast, complementation with mutated mprF (MAR-6) did restore it to the CB5012 original thickness, emphasizing the functional role of the mprF L826F mutation. On the other hand, CB5012 vraSR inactivation (MAR-7) also reduced cell wall thickness, an effect that was reversed by vraSR complementation (MAR-8). Thus, these results support the mechanistic involvement of both mutated mprF and increased expression of vraSR in DAP-resistant S. aureus. The data also suggest that the mutual presence of both genes is required to confer a maximal DAP-resistant phenotype.
Table 7.
Cell wall thickness of pair CB5011/CB5012 and mutant derivatives
| Strain | Cell wall thickness (nm) | DAP MIC (μg/ml) | Relevant genotype and phenotype |
|---|---|---|---|
| CB5011 | 23.1 ± 1.1 | 0.5 | DS |
| CB5012 | 33.2 ± 1.5a | 4.0 | DR |
| MAR-4 | 25.4 ± 1.1b | 0.5 | CB5012 + ΔmprF::cat |
| MAR-5 | 25.3 ± 1.3b | 0.75 | MAR-4 + pMPRF-1 (wild type) |
| MAR-6 | 35.5 ± 1.4 | 2.0 | MAR-4 + pMPRF-2 (mutant) |
| MAR-7 | 24.5 ± 1.1b | 0.25 | CB5012 ΔvraSR::cat |
| MAR-8 | 31.3 ± 1.1 | 3.0 | MAR-7 + pVRASR-2 |
Significantly higher than DS CB5011 (P < 0.01).
Significantly lower than DR CB5012 (P < 0.01).
DISCUSSION
Previous studies performed with in vitro-generated DAP-resistant strains demonstrated that four open reading frames representing three distinct proteins (i.e., MprF, an LPG synthetase; YycG, a sensor histidine kinase; and RpoB and RpoC, β and β′ subunits of RNA polymerase) were implicated in DAP MIC increases (17). In support of these observations, several groups have reported an association between increased S. aureus DAP MICs and single point mutations in the mprF gene that are acquired during either in vitro or in vivo exposure to DAP (17, 20, 42). A gain-in-function mutation has been postulated to impact DAP nonsusceptibility via a charge-repulsive mechanism (25, 40). In a recent study performed on an isogenic set of clinical bloodstream S. aureus isolates, it was also observed that a DAP-nonsusceptible strain showed an increased expression of mprF associated with mprF point mutations (48). However, other investigations showed that neither the transcriptional profile of mprF nor the results of membrane phospholipid analyses were compatible with an mprF gain-in-function phenotype. In this case, the DAP-resistant phenotype appeared to be related to enhanced dlt expression coincident with an increased positive surface charge and reduced DAP binding (46). In the present study, which was conducted in isogenic DS/DR MRSA strains obtained from DAP-treated patients, we first attempted to determine whether similar events regarding mprF may account for the resistant phenotype. Although no changes in mprF expression levels were found, we determined the existence of point mutations that, as we were able to demonstrate, contributed to the mechanisms of DAP resistance. Furthermore, we found that, independently of their domain localization (i.e., putative synthase domain [CB5012] and N-terminal translocase domain [CB5036, CB183]), none of the identified mprF mutations produced significant changes in net cell surface charge in DR CB5012, CB5036, and CB183 strains compared to their corresponding susceptible DS counterparts, CB5011, CB5035, CB182, respectively. In agreement with these premises, the present group of strains displayed no changes in their susceptibility to cationic molecules such as gentamicin, indicating that surface charge may not represent the primary factor dictating DAP resistance in these strains. In support of these results, a recent study performed in in vitro-selected DAP-resistant S. aureus strains showed a reduction of cytochrome c binding and reduced cell membrane depolarization without associated mprF mutations (39), suggesting that in the absence of any changes in mprF or dltA expression or in the MprF sequence, additional loci may be involved in modulating the relative surface charge of S. aureus (39). Taking into account these observations, our results suggested that in addition to the L826F mprF mutation, other factors may be involved in DAP resistance. In fact, one of the most consistent observations among the different strains was that DAP resistance was accompanied by an increase in expression of the two-component regulator vraSR, which positively modulates cell wall biosynthesis (28). Previous observations showing that a change in the cell wall thickness is associated with DAP resistance (7) prompted us to look more closely at the function of vraSR. Furthermore, a number of genes differentially expressed when comparing DS and DR strains were well-known vraSR-regulated targets, including pbp2 and sgtB. Our findings are in line with those of recent studies showing that DAP is able to induce the cell wall stress stimulon (16) and genes responsive to membrane depolarization in methicillin-susceptible S. aureus (35). Upregulation of vraSR has also been observed in in vitro-developed DAP-resistant S. aureus (7) and by transcriptome studies performed in Bacillus subtilis, where DAP strongly induced the transcription of the two-component system liaRS, a vraSR homolog (21). However, until now, no functional studies have been performed in clinical MRSA strains to address the VraSR contribution to the resistant phenotype. Our results clearly demonstrated that inactivation of vraSR in DR strains resulted in a significant decrease in DAP resistance. Moreover, overexpression of vraSR in DS strains significantly decreased susceptibility to DAP, indicating a functional role of increased vraSR expression as a factor associated with DAP resistance. The fact that a number of genes showing differential expression are under vraSR regulation further supports this notion. A group of vraSR-regulated genes was closely involved in cell wall synthesis, and our results consistently demonstrated that inactivation of vraSR in DR cells resulted in a thinner cell wall comparable to that of DS cells, suggesting that DAP resistance-mediated increased expression of vraSR may contribute to the DR phenotype by modulating components of cell wall synthesis. In the same context, results from microarray analyses showed upregulation of vraF and vraG in DR strains. These transporter proteins have been shown to be associated with vancomycin resistance as important components of activated cell wall peptidoglycan synthesis (33). The thickness of the cell wall has been reported to be associated with DAP-resistant strains (5, 7, 32), although not in all cases. Some of these studies were demonstrated in DAP-resistant strains that concomitantly displayed a vancomycin-intermediate S. aureus (VISA) phenotype, making it difficult to discern whether vancomycin can induce similar phenotypic perturbations as DAP in terms of cell wall thickness. In our investigation, DR strains did show a thickened cell wall with no parallel decrease in susceptibilities to vancomycin. Recently, it was also shown that the thickness of the cell wall in DAP-resistant strains was correlated with an increased capacity of these isolates to synthesize d-alanylate WTA, an effect mediated in part by the concomitant upregulation of WTA genes tagA and dlt (5). Converse to these observations, we did not observe changes in expression of these genes by either microarray analysis or real-time RT-PCR. One of the interesting aspects of our investigation is that the L826F mutation in the mprF gene had a mechanistic impact on cell wall thickness, as demonstrated by mprF inactivation and transcomplementation of either full-length wild-type or mutated mprF in DR strains. These results suggested that although no direct effect at the cell net surface charge change was observed, the mprF L826F mutation contributed to cell wall thickness through a mechanism yet to be fully determined.
Similar to findings reported by other groups (32, 47), we observed a correlation between increased resistance to DAP and a concomitant decrease in oxacillin resistance, an effect that we found was not related to deletion or excision of SCCmec/mecA. We were able to reproduce this observation when vraSR was overexpressed in DS cells, further emphasizing the role of VraSR in the DR phenotype. In fact, deletion of the VraSR operon is known to decrease levels of resistance to most of its inducing antibiotics, including β-lactam antibiotics (13, 18, 28, 35). However, the precise mechanism by which increased susceptibility to β-lactams occurs in MRSA isolates with VraSR mutations/deletion remains unknown (24, 49). On the other hand, it may be plausible to speculate that the paradoxical effect observed when VraSR was overexpressed in the present group of strains could be related to the presence of mprF mutations, which in turn may affect VraSR sensing signaling toward cell wall-targeting antibiotics. Together, the present results suggest that DAP has an impact on the proper assembly of the staphylococcal cell wall, thereby affecting the cell response not only to DAP but also to oxacillin.
In summary, a key aspect of this study was the demonstration by genetic analysis and manipulation of the functional role played by mprF mutations. Furthermore, our results strongly suggest that the mechanisms and pathways underlying the in vivo-selected clinical daptomycin-resistant strains used in this study may be determined by the mutual cooperation between mprF and the two-component regulator VraSR through a mechanism that may involve regulation of genes related to cell wall synthesis and turnover.
ACKNOWLEDGMENTS
This research was supported by grants from Cubist Pharmaceuticals, Lexington, MA. Microarray studies were funded in part through a PFGRC-NIAID grant (to A.E.R.).
We acknowledge Gordon Archer for his input and discussions. Electron microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy facility and at the Baylor College of Medicine, Houston, TX. We are very grateful to Keiichi Hiramatsu and Susan Boyle-Vavra for providing the KVR strain and pVRASR-2 plasmid, respectively. Special thanks go to Kathryn Stockbauer and Philip Randall, from the Office of Academic Development, TMHRI, for their assistance with manuscript editing.
Footnotes
Published ahead of print 10 October 2011
REFERENCES
- 1. Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673–1681 [DOI] [PubMed] [Google Scholar]
- 2. Baltz RH. 2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13:144–151 [DOI] [PubMed] [Google Scholar]
- 3. Baltz RH, Miao V, Wrigley SK. 2005. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 22:717–741 [DOI] [PubMed] [Google Scholar]
- 4. Bannerman TL, Hancock GA, Tenover FC, Miller JM. 1995. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J. Clin. Microbiol. 33:551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertsche U, et al. 2011. Correlation of daptomycin resistance in a clinical Staphylococcus aureus strain with increased cell wall teichoic acid production and d-alanylation. Antimicrob. Agents Chemother. 55:3922–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle-Vavra S, Yin S, Daum RS. 2006. The VraS/VraR two-component regulatory system required for oxacillin resistance in community-acquired methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 262:163–171 [DOI] [PubMed] [Google Scholar]
- 7. Camargo IL, Neoh HM, Cui L, Hiramatsu K. 2008. Serial daptomycin selection generates daptomycin-nonsusceptible Staphylococcus aureus strains with a heterogeneous vancomycin-intermediate phenotype. Antimicrob. Agents Chemother. 52:4289–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers HF. 1995. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for the penicillin-binding protein PBP 2a. Antimicrob. Agents Chemother. 39:462–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chambers HF. 2005. Community-associated MRSA—resistance and virulence converge. N. Engl. J. Med. 352:1485–1487 [DOI] [PubMed] [Google Scholar]
- 10. Chambers HF, Archer G, Matsuhashi M. 1989. Low-level methicillin resistance in strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 33:424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui L, Murakami H, Kuwahara-Arai K, Hanaki H, Hiramatsu K. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 44:2276–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuirolo A, Plata K, Rosato AE. 2009. Development of homogeneous expression of resistance in methicillin-resistant Staphylococcus aureus clinical strains is functionally associated with a beta-lactam-mediated SOS response. J. Antimicrob. Chemother. 64:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dengler V, Meier PS, Heusser R, Berger-Bachi B, McCallum N. 2011. Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics. BMC Microbiol. 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ernst CM, et al. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 5:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finan JE, Rosato AE, Dickinson TM, Ko D, Archer GL. 2002. Conversion of oxacillin-resistant staphylococci from heterotypic to homotypic resistance expression. Antimicrob. Agents Chemother. 46:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer A, et al. 2011. Daptomycin resistance mechanisms in clinically derived Staphylococcus aureus strains assessed by a combined transcriptomics and proteomics approach. J. Antimicrob. Chemother. 66:1696–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman L, Alder JD, Silverman JA. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gardete S, Wu SW, Gill S, Tomasz A. 2006. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:3424–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldstein F, et al. 2007. Identification and phenotypic characterization of a beta-lactam-dependent, methicillin-resistant Staphylococcus aureus strain. Antimicrob. Agents Chemother. 51:2514–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gqada Z, et al. 2008. Clinical rationale for treatment of endocarditis caused by methicillin-susceptible Staphylococcus aureus developing nonsusceptibility to daptomycin. J. Clin. Microbiol. 46:2471–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hachmann AB, Angert ER, Helmann JD. 2009. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 53:1598–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichihashi N, Kurokawa K, Matsuo M, Kaito C, Sekimizu K. 2003. Inhibitory effects of basic or neutral phospholipid on acidic phospholipid-mediated dissociation of adenine nucleotide bound to DnaA protein, the initiator of chromosomal DNA replication. J. Biol. Chem. 278:28778–28786 [DOI] [PubMed] [Google Scholar]
- 23. Ito T, et al. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jo DS, Montgomery CP, Yin S, Boyle-Vavra S, Daum RS. 2011. Improved oxacillin treatment outcomes in experimental skin and lung infection by a methicillin-resistant Staphylococcus aureus isolate with a vraSR operon deletion. Antimicrob. Agents Chemother. 55:2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones T, et al. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Julian K, et al. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kennedy AD, et al. 2008. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc. Natl. Acad. Sci. U. S. A. 105:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuroda M, et al. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49:807–821 [DOI] [PubMed] [Google Scholar]
- 29. Kuroda M, et al. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225–1240 [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Margolis PS, et al. 2000. Peptide deformylase in Staphylococcus aureus: resistance to inhibition is mediated by mutations in the formyltransferase gene. Antimicrob. Agents Chemother. 44:1825–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mishra NN, et al. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moe PC, Levin G, Blount P. 2000. Correlating a protein structure with function of a bacterial mechanosensitive channel. J. Biol. Chem. 275:31121–31127 [DOI] [PubMed] [Google Scholar]
- 34. Mohedano ML, et al. 2005. Evidence that the essential response regulator YycF in Streptococcus pneumoniae modulates expression of fatty acid biosynthesis genes and alters membrane composition. J. Bacteriol. 187:2357–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muthaiyan A, Silverman JA, Jayaswal RK, Wilkinson BJ. 2008. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob. Agents Chemother. 52:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. NCCLS/CLSI 2007. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A8, 8th ed NCCLS/CLSI, Wayne, PA [Google Scholar]
- 37. Novick R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166 [DOI] [PubMed] [Google Scholar]
- 38. Oliveira DC, de Lencastre H. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel D, et al. 2011. Mechanisms of in-vitro-selected daptomycin-non-susceptibility in Staphylococcus aureus. Int. J. Antimicrob. Agents 38:442–446 [DOI] [PubMed] [Google Scholar]
- 40. Peschel A, et al. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peschel A, et al. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405–8410 [DOI] [PubMed] [Google Scholar]
- 42. Pillai SK, et al. 2007. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in MprF. Antimicrob. Agents Chemother. 51:2223–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rohrer S, Maki H, Berger-Bachi B. 2003. What makes resistance to methicillin heterogeneous? J. Med. Microbiol. 52:605–607 [DOI] [PubMed] [Google Scholar]
- 44. Rosato AE, Craig WA, Archer GL. 2003. Quantitation of mecA transcription in oxacillin-resistant Staphylococcus aureus clinical isolates. J. Bacteriol. 185:3446–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tenover FC, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang SJ, et al. 2009. Enhanced expression of dltABCD is associated with the development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J. Infect. Dis. 200:1916–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang SJ, et al. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 54:3161–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang SJ, et al. 2009. Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 53:2636–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yin S, Daum RS, Boyle-Vavra S. 2006. VraSR two-component regulatory system and its role in induction of pbp2 and vraSR expression by cell wall antimicrobials in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]