Abstract
In conventional ΜΙC tests, fungi are exposed to constant drug concentrations, whereas in vivo, fungi are exposed to changing drug concentrations. Therefore, we developed a new in vitro pharmacokinetic/pharmacodynamic model where human plasma pharmacokinetics of standard doses of 1 mg/kg amphotericin B, 4 mg/kg voriconazole, and 1 mg/kg caspofungin were simulated and their pharmacodynamic characteristics were determined against three clinical isolates of Aspergillus fumigatus, Aspergillus flavus, and Aspergillus terreus with identical MICs (1 mg/liter for amphotericin B, 0.5 mg/liter for voriconazole) and minimum effective concentrations (0.5 mg/liter for caspofungin). This new model consists of an internal compartment (a 10-ml dialysis tube made out of a semipermeable cellulose membrane allowing the free diffusion of antifungals but not galactomannan) inoculated with Aspergillus conidia and placed inside an external compartment (a 700-ml glass beaker) whose content is diluted after the addition of antifungal drugs by a peristaltic pump at the same rate as the clearance of the antifungal drugs in human plasma. Fungal growth was assessed by galactomannan production. Despite demonstrating the same MICs, amphotericin B completely inhibited (100%) A. fumigatus but not A. flavus and A. terreus, whose growth was delayed for 7.53 and 22.8 h, respectively. Voriconazole partially inhibited A. fumigatus (49.5%) and Α. flavus (27.9%) but not Α. terreus; it delayed their growth by 3.99 h (A. fumigatus) and 5.37 h (Α. terreus). Caspofungin did not alter galactomannan production in all of the species but A. terreus. The new model simulated human pharmacokinetics of antifungal drugs and revealed important pharmacodynamic differences in their activity.
INTRODUCTION
Invasive aspergillosis is associated with high morbidity and mortality, particularly in patients with hematologic malignancies or undergoing bone marrow transplantation. Among the most common Aspergillus species implicated in these severe infections are Aspergillus fumigatus, Aspergillus flavus, and Aspergillus terreus (19, 24). In vivo experimental and clinical data show that the efficacy of various antifungal agents differs for these species (31, 35). However, standardized in vitro susceptibility testing of these three species against amphotericin B, voriconazole, and caspofungin demonstrates comparable/similar activities of these agents against the vast majority of isolates, making in vitro-in vivo activity correlation difficult (18, 20).
The in vitro activities of antifungal agents against Aspergillus spp. are determined by standardized broth microdilution assays where the MIC (for azoles and amphotericin B) or minimum effective concentration (MEC; for echinocandins) is defined as the lowest drug concentration that completely inhibits fungal growth or causes typical morphological changes in the fungus, respectively (5). These endpoints are obtained using assays where the drug concentration remains constant over time (4). However, fungi are exposed in vivo to fluctuating drug concentrations over time because of absorption, elimination, excretion, metabolism, and distribution processes (9). The impact of fluctuating drug concentrations on Aspergillus growth is unknown and might be different from the effect of constant drug concentrations over time (16). Furthermore, a variety of important pharmacokinetic/pharmacodynamic (PK/PD) indices cannot be studied in vitro against Aspergillus spp. since MIC testing by standard methodology does not produce clinically relevant drug exposures (1).
In vitro models that simulate the human plasma pharmacokinetics of antifungal drugs have been developed for Candida species, enabling the study of the effects of clinically relevant drug concentrations on different Candida species (13, 15). Furthermore, they have proved to be very useful in investigating the efficacy of antifungal drug combinations against Candida spp. (14, 17). The development of similar models for Aspergillus spp. has been hampered so far by their filamentous growth, which makes it difficult to control drug exposure and determine fungal growth.
The purposes of this study were to develop an in vitro model that simulates the pharmacokinetics of amphotericin B, voriconazole, and caspofungin in humans after the intravenous administration of standard doses and to study the effects of these antifungal agents on clinical isolates of A. fumigatus, A. flavus, and A. terreus.
MATERIALS AND METHODS
Isolates.
Three isolates of A. fumigatus, A. flavus, and A. terreus obtained from patients with invasive pulmonary aspergillosis were used. By the CLSI M38A2 reference method (5), the MICs were 1 mg/liter for amphotericin B and 0.5 mg/liter for voriconazole, whereas the caspofungin MEC was 0.5 mg/liter tested in triplicate. The isolates were maintained at −70°C in 10% glycerol and were subcultured twice in Sabouraud dextrose agar at 30°C for 5 to 7 days before testing. Conidial suspensions were prepared in normal saline with 1% Tween 20. The inoculum concentration (1 × 105 CFU/ml) was determined with a Neubauer chamber and by culturing serial dilutions on Sabouraud dextrose agar.
Antifungal drugs.
Amphotericin B (Fungizone; molecular mass, 0.92 kDa; Bristol-Myers, Athens, Greece), voriconazole (Vfend; molecular mass, 0.35 kDa; Pfizer, Athens, Greece), and caspofungin (Cancidas; molecular mass, 1.1 kDa; Merck, Athens, Greece) were reconstituted at 5,000 mg/liter, 10,000 mg/liter, and 5,000 mg/liter, respectively, according to the manufacturers' instructions and stored at −70°C. Preliminary studies with pure drugs did not show any differences in fungal growth inhibition compared to commercial formulations. Therefore, all further studies were performed with commercial formulations.
Medium.
The medium used throughout this study contained 10.4 g/liter RPMI 1640 with glutamine without sodium bicarbonate (Sigma-Aldrich, St. Louis, MO) and 0.165 M morpholinepropanesulfonic acid buffer (Invitrogen, Carlsbad, CA), pH 7.0, with 100 mg/liter chloramphenicol (Sigma-Aldrich, St. Louis, MO).
In vitro PK/PD model.
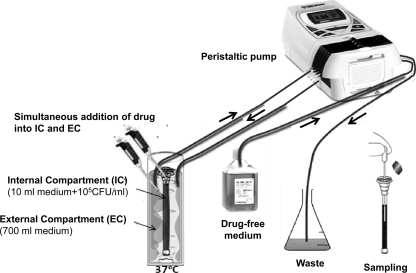
The in vitro PK/PD simulation model used in this study is shown in Fig. 1. It consists of a 10-ml-volume dialysis tube (internal compartment [IC]) made of a semipermeable cellulose membrane (Float-A-Lyzer; Spectrum Europe B.V., Breda, The Netherlands) allowing the free diffusion of small molecules (molecular mass, <20 kDa). This is placed in a glass beaker containing 700 ml medium (external compartment [EC]) the content of which is continuously diluted by a peristaltic pump (Minipuls Evolution; Gilson Inc., Villiers le Bel, France) removing drug-containing medium from the EC and adding drug-free medium to the EC at a rate equivalent to drug clearance from human plasma.
Fig 1.
Development of an in vitro PK/PD model that simulates the pharmacokinetics of antifungal drugs in human plasma and determines a drug's effect on Aspergillus growth. The model consists of an EC, a glass beaker containing 700 ml broth medium, and an IC, a dialysis tube containing 10 ml broth medium; the tube is made of a semipermeable cellulose membrane that allows free passage of small molecules (<20 kDa) like antifungals, but not galactomannan. The EC is placed on a heated magnetic stirrer (37°C and 2 rpm). A peristaltic pump introduces drug-free medium into the EC and removes its content concurrently in order to maintain a constant volume. The flow rate is adjusted to achieve drug concentrations corresponding to the drug's clearance from human plasma. At time zero, 105 CFU/ml of Aspergillus conidia are inoculated into the IC, while the drug is introduced into both the EC and the IC for rapid concentration equilibration. Subsequently, the drug concentration declines over time. Galactomannan levels in the IC medium are measured at regular time points.
At time zero, the IC was inoculated with 10 ml medium containing 1 × 105 CFU/ml of Aspergillus conidia. The cellulose membrane of the IC allowed the free diffusion of nutrients and drugs until an equilibrium with the EC was reached, while at the same time it retained the conidia, hyphae, and macromolecular products such as galactomannan (molecular mass of 25 to 75 kDa). Thus, galactomannan was concentrated inside the IC and was used as a biomarker of fungal growth. In order to ensure that galactomannan did not diffuse into the EC, galactomannan levels were determined in the EC.
Also at time zero, the drug was injected into the EC and the IC simultaneously in order to achieve rapid equilibration of the drug concentrations in the two compartments (preliminary experiments showed that when the drug was added only to the EC, there was a lag of 4 h until an equilibrium was reached). The drug-containing medium in the EC was then continuously diluted with drug-free medium by the peristaltic pump adjusted to a specific flow rate in order to reproduce average drug half-lives (t1/2s) observed in human plasma after the intravenous administration of amphotericin B (19 h), caspofungin (12 h), and voriconazole (6 h) in accordance with previous clinical studies (2, 28, 34). The EC is then placed on a heated magnetic stirrer adjusted to 37°C and 2 rpm. The temperature and flow rate were checked regularly throughout the experiment using a thermometer and by measuring the volume of medium pumped out of the EC in 1 min, respectively.
Bioassays for determination of drug levels.
The drug levels in the EC and the IC were determined by microbiological methods using Candida kefyr strain NCPF 3234 for voriconazole and caspofungin and Paecilomyces variotii strain ATCC 22319 for amphotericin B as described previously for amphotericin B and voriconazole (25, 30). Briefly, 3 × 105 CFU/ml C. kefyr or 5 × 104 CFU/ml P. variotii were inoculated into prewarmed medium (54°C) containing 15 g/liter agar (Difco Bacto Agar; BD Hellas SA, Athens, Greece). The medium was poured into square (10 by 10 cm) plastic petri dishes, and after solidification, 2-mm-diameter holes were opened. One-hundred-microliter serial 2-fold drug dilutions (range, 0.25 to 16 mg/liter) and 100-μl samples obtained from the EC or IC medium were added to the holes. The plates were incubated at 37°C for 24 h, and the diameters of the inhibition zones around the holes were measured with a ruler. A standard curve of drug concentrations versus diameters of inhibition zones was constructed and subjected to linear regression analysis. Based on this standard curve, the drug concentrations of the EC and the IC were determined at any time point. In order to ensure that the drug concentration within the IC was uniform, samples from the center and periphery of the top, middle, and bottom parts of the IC were tested.
Pharmacokinetic analysis.
The following standard doses were simulated in the in vitro model: 1 mg/kg amphotericin B, 1 mg/kg caspofungin, and 4 mg/kg voriconazole with average peak total concentrations in human plasma of 2.8, 10, and 3.6 mg/liter and average t1/2s of 19 h, 12 h, and 6 h, respectively, after intravenous administration at steady state (2, 28, 34). The levels of antifungal drugs at 0, 4, 6, 8, 20, and 24 h after the introduction of the drug were determined in the IC and EC with the bioassays. The data were subjected to nonlinear regression analysis based on the one-compartment pharmacokinetic model, which is described by the equation Ct = C0e−k/t, where Ct (dependent variable) is the drug concentration at a given time (independent variable), C0 the initial drug concentration at 0 h, e is the physical constant 2.18, and k is the rate of drug removal. The t1/2 was calculated using the equation t1/2 = k/0.693 for the EC and the IC separately and compared with the respective values obtained in human plasma (2, 28, 34) using Student's t test. Finally, the area under the concentration-time curve from 0 to 24 h after dose administration (AUC0-24) was calculated by the trapezoidal rule.
Galactomannan levels for determination of fungal growth.
Fungal growth in the IC was monitored by galactomannan production. Galactomannan levels were measured by enzyme-linked immunosorbent assay (Platelia; Bio-Rad Laboratories, Athens, Greece), and results were expressed as a galactomannan index (GI) according to the manufacturer's instructions. In order to correlate GIs with fungal growth, different IC tubes were inoculated with 103, 104, 105, or 106 CFU/ml A. fumigatus and incubated without a drug for 24 h at 37°C in the in vitro PK/PD model and GIs were determined at regular time intervals. The kinetics of the GIs were subjected to nonlinear regression analysis based on the Emax model described by the equation E = Emax · Tγ/(Tγ + T50), where E is the GI (dependent variable), Emax is the maximum GI, T is the incubation time (independent variable), T50 is the time corresponding to 50% of Emax, and γ is the slope of the curve. The T50 parameter was then correlated with the initial concentration of the suspension of conidia by linear regression analysis.
Galactomannan levels were also correlated with real-time PCR results. IC tubes were inoculated with increasing inocula of A. fumigatus, and DNA was extracted from 100-μl samples with a Maxwell 16-cell DNA purification kit according to the manufacturer's instructions (Promega, Madison, WI) after incubation for 2, 4, 8, and 24 h at 37°C in the in vitro PK/PD model. Real-time PCR was performed with the MycAssay Aspergillus kit according to the manufacturer's instructions (Mycognostica, Manchester, United Kingdom). The threshold cycle (CT) of each sample, which identifies the cycle number during PCR when fluorescence exceeds a threshold value determined by the software, was converted to conidial equivalent (CE) A. fumigatus DNA by interpolation from a 4-point standard curve of CT values obtained with 103 to 106 Aspergillus CFU/ml. The area under the GI-versus-time curve was then correlated with the area under the PCR CE-versus-time curve by linear regression analysis.
Pharmacodynamic analysis.
The GIs in the IC inoculated with 105 CFU/ml A. fumigatus, A. flavus, or A. terreus were measured at regular time intervals up to 72 h. The pharmacodynamic data were subjected to nonlinear regression analysis based on the Emax model as described above. The goodness of fit of the Emax model was assessed by R2 analysis of the residuals and visual inspection of the curves. The parameters T50 and Emax of GI-versus-time curves in the presence of drugs (T50,D and Emax,D) were compared with the corresponding parameters of drug-free growth controls (T50,GC and Emax,GC). The percent reduction was calculated as 100% × (Emax,D − Emax,GC)/Emax,GC, and the difference, T50,GC − T50,D, was calculated for each drug and species.
All of the experiments were repeated at least twice. All statistical analysis was performed with the software Prism 5.01 (GraphPad Inc., La Jolla, CA).
RESULTS
Validation of drug concentration determination by the bioassays.
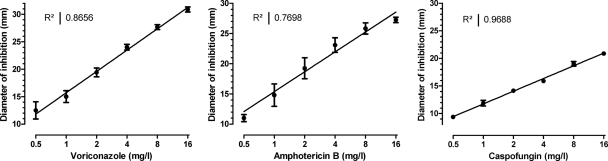
The standard curves of inhibition zone diameters versus drug concentrations are shown in Fig. 2. The drug concentrations determined with the bioassay ranged from 0.5 mg/liter to 16 mg/liter. The diameter of the inhibition zone correlated linearly with the log10 drug concentration (r2, >0.77). The intra- and interexperimental coefficients of variation ranged from 5% to 15%, with an average of 8%.
Fig 2.
Validation of bioassays used for the determination of amphotericin B, voriconazole, and caspofungin concentrations. The correlation between diameter of inhibition zones and drug concentrations is shown together with the respective correlation coefficients of linear regression analysis. Results were obtained from eight experiments for each drug on different days. Error bars represent standard deviations.
Validation of fungal growth determination by measuring galactomannan production.
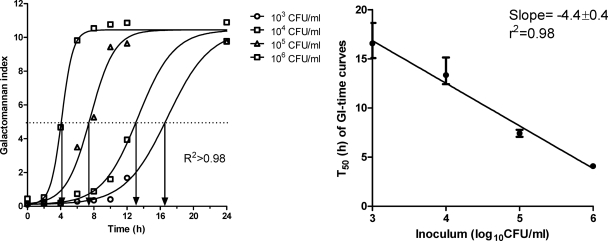
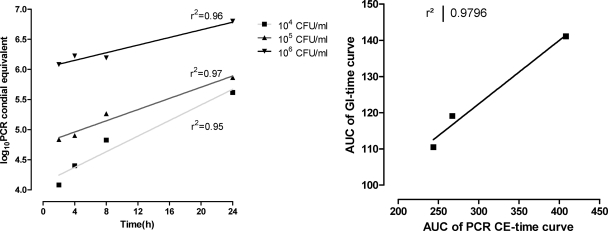
The production of galactomannan by A. fumigatus followed a sigmoid curve described very well by the Emax model (r2, >0.98) (Fig. 3). The parameter T50 was inversely associated with the initial concentration of conidia (r2, 0.98; slope, −4.4 ± 0.4) and was therefore considered valid for quantifying fungal growth. Real-time PCR of Aspergillus DNA confirmed that the total increase in PCR CEs was associated with the total increase in galactomannan over time as assessed with the areas under the corresponding curves (r2, 0.98) (Fig. 4). Although we did not assess the validity of using galactomannan to estimate the fungal burdens of other species/strains, any species/strain-dependent difference in galactomannan production will affect both drug-treated and drug-free control samples, hence without altering the net effect.
Fig 3.
Kinetics of galactomannan production by increasing inocula of A. fumigatus (left graph) and correlation of the T50 parameter of the Emax model with the initial inoculum (right graph). The arrows on the left graph show the T50 parameter (the time corresponding to 50% of Emax) for each curve. Error bars represent 95% confidence intervals.
Fig 4.
Kinetics of real-time PCR CEs for three increasing inocula of A. fumigatus (left graph) and correlation between the area under the PCR CE-versus-time curve and the area under the GI-versus-time curve as in Fig. 2 (left graph). Results from experiments with a starting inoculum of 103 CFU/ml were excluded from the analysis because the PCR signal was very close to the lower limit of detection, resulting in highly variable results.
Pharmacokinetic analysis.
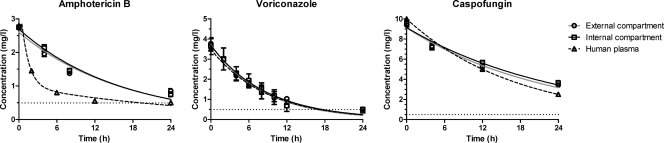
The drug concentration-time profiles of the three antifungal drugs in both the EC and the IC corresponded to those previously detected in human plasma after single-dose administration of 4 mg/kg voriconazole, 1 mg/kg caspofungin, and 1 mg/kg amphotericin B (2, 28, 34) (Fig. 5). The intra- and interexperimental variations in drug concentrations were <10% overall and <5% within the IC.
Fig 5.
Pharmacokinetic analysis of simulated doses of 1 mg/kg amphotericin B, 1 mg/kg caspofungin, and 4 mg/kg voriconazole in the new in vitro PK/PD model and comparison to results previously obtained by testing human plasma (2, 28, 34). Data represent drug levels in the EC (circles), IC (squares), and human plasma (triangles), and lines represent the regression lines obtained with the one-compartment pharmacokinetic intravenous model. Error bars represent standard deviations. The horizontal dotted lines represent the lower detection limits of the bioassays.
In particular, the t1/2 of voriconazole in the in vitro model was 5.9 h with a maximum concentration of 3.7 mg/liter (Table 1). The corresponding values for caspofungin were 14 h and 9.3 mg/liter. These results are comparable to those for human plasma pharmacokinetic parameters, namely, 6.5 h and 3.62 mg/liter for voriconazole (2, 28, 34) and 12.2 h and 10.0 mg/liter for caspofungin (2, 28, 34). For amphotericin B, the maximum concentration (2.6 mg/liter) was similar to that in human plasma (2.83 mg/liter) whereas the t1/2 was shorter than the average value (11 h and 19.65 h, respectively) (2, 28, 34). For all antifungal drugs, the AUC0-24 values in the in vitro model were similar to the corresponding measurements in humans (Table 1).
Table 1.
Pharmacokinetic parameters of amphotericin B, caspofungin, and voriconazole in humans and in the in vitro PK/PD model
| Drug (simulated dose [mg/kg]) and pharmacokinetic parametera | Avg value in human plasmab | Mean value in ICc ± SEM |
|---|---|---|
| Amphotericin Β (1) | ||
| Cmax (mg/liter) | 2.83 | 2.6 ± 0.1 |
| t1/2 (h) | 19.65 | 11 ± 1.5 |
| AUC0-24 (mg · h/liter) | 28.98 | 34.52 |
| Voriconazole (4) | ||
| Cmax (mg/liter) | 3.62 | 3.7 ± 0.17 |
| t1/2 (h) | 6.5 | 5.9 ± 0.6 |
| AUC0-24 (mg · h/liter) | 22.7 | 30.37 |
| Caspofungin (1) | ||
| Cmax (mg/liter) | 10 | 9.3 ± 0.25 |
| t1/2 (h) | 12.2 | 14 ± 1.25 |
| AUC0-24 (mg · h/liter) | 97.20 | 120.31 |
Pharmacodynamic analysis.
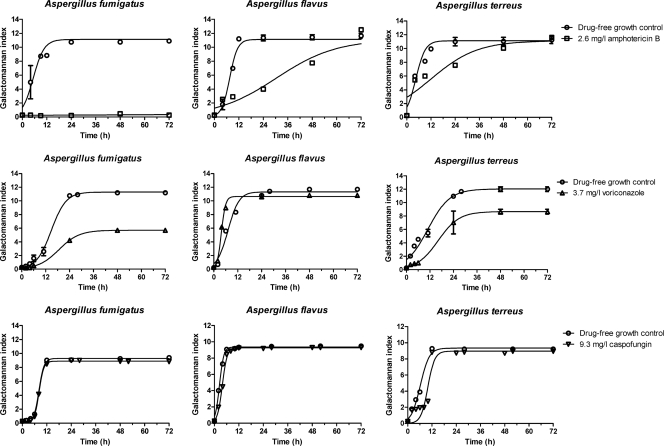
The pharmacodynamic data from the simulated doses of antifungal agents against the three isolates are shown in Fig. 6. The Emax model described the data well, as demonstrated by an R2 value of >0.86 and the normal distribution of residuals around 0. After 72 h of incubation and according to the Emax parameter, amphotericin B completely inhibited the growth of A. fumigatus but not the growth of the other two strains (Table 2). According to the T50 parameter, fungal growth was delayed for 7.53 h for A. terreus, 22.8 h for A. flavus, and >66.7 h for A. fumigatus (Table 2). Voriconazole inhibited the growth of A. fumigatus by 49.5% and that of A. terreus by 27.9% but had no effect on A. flavus growth. In contrast to A. flavus, for which voriconazole accelerated the production of galactomannan for 3.3 h, galactomannan production by A. fumigatus and A. terreus was delayed for 3.99 h and 5.37 h, respectively. Caspofungin did not have any effect on galactomannan production by A. fumigatus and A. flavus; whereas a delay of 2.8 h in galactomannan production was observed for A. terreus. When Aspergillus DNA was measured by real-time PCR, 2.2-, 0.9-, and 1.6-log reductions in the CEs of A. fumigatus, A. flavus, and A. terreus, respectively, were observed after 6 h of incubation in the in vitro PK/PD model in the presence of caspofungin compared to those of the drug-free controls (data not shown).
Fig 6.
Pharmacodynamic data analysis according to the in vitro PK/PD model. Simulated human dosing with 1 mg/kg amphotericin B, 4 mg/kg voriconazole, or 1 mg/kg caspofungin was used against A. fumigatus, A. flavus, and A. terreus clinical isolates. The circles and squares represent galactomannan indices obtained in experiments without and with the drugs, respectively, while the lines represent the regression lines obtained with the Emax model.
Table 2.
Pharmacodynamic parameters of simulated doses of amphotericin B, voriconazole, and caspofungin against A. fumigatus, A. flavus, and A. terreus isolates in the in vitro PK/PD model
| Drug (simulated dose [mg/kg]) and species | Mean Emaxa (GI) ± SD |
% Change | Mean T50b (h) ± SD |
|||
|---|---|---|---|---|---|---|
| With drug | Without drug | With drug | Without drug | Difference | ||
| Amphotericin Β (1) | ||||||
| A. fumigatus | 10.22 ± 0.65 | 0 | −100c | 5.3 ± 0.7 | >72 | >−66.7c |
| A. flavus | 11.54 ± 0.21 | 11.12 ± 0.26 | −3.6 | 7.5 ± 0.67 | 30.31 ± 4.24 | −22.8c |
| A. terreus | 10.72 ± 0.36 | 10.96 ± 1.73 | 2.2 | 4.45 ± 0.61 | 11.98 ± 2.84 | −7.53c |
| Voriconazole (4) | ||||||
| A. fumigatus | 11.29 ± 0.18 | 5.70 ± 0.03 | −49.5c | 14.81 ± 0.61 | 18.80 ± 0.25 | −3.99c |
| A. flavus | 11.35 ± 0.43 | 10.63 ± 0.14 | −6.3 | 7.13 ± 0.76 | 3.82 ± 0.09 | 3.31c |
| A. terreus | 12.02 ± 0.34 | 8.66 ± 0.29 | −27.9c | 10.92 ± 0.93 | 16.29 ± 1.33 | −5.37c |
| Caspofungin (1) | ||||||
| A. fumigatus | 9.24 ± 0.08 | 8.97 ± 0.08 | −2.9 | 8.28 ± 0.30 | 8.22 ± 0.07 | −0.02 |
| A. flavus | 9.38 ± 0.06 | 9.21 ± 0.04 | −1.8 | 3.26 ± 2.1 | 4.41 ± 0.45 | 1.15 |
| A. terreus | 9.27 ± 0.48 | 8.83 ± 0.06 | −4.7 | 7.68 ± 0.49 | 10.48 ± 0.52 | 2.8c |
Emax, maximum GI.
T50, time required to obtain 50% of Emax.
Statistically significantly different (P < 0.05).
DISCUSSION
Here we report for the first time, to our knowledge, a new in vitro pharmacokinetic model for Aspergillus species that simulates the human plasma pharmacokinetics of three major antimold agents (amphotericin B, caspofungin, and voriconazole) after the intravenous administration of standard doses (1 mg/kg, 1 mg/kg, and 4 mg/kg, respectively). For the pharmacodynamic analysis of these regimens, galactomannan production was determined as a surrogate marker of fungal growth. Three Aspergillus strains representing the most clinically significant species (A. fumigatus, A. flavus, and A. terreus) were tested by this model. The isolate of each species was deliberately selected for identical MICs of a given antifungal drug. Thus, the MICs were the same for amphotericin B (1 mg/liter) and voriconazole (0.5 mg/liter) and the MECs were also the same (0.5 mg/liter) in order to elucidate the species-specific in vitro PK/PD effects of a given antifungal drug that were not necessarily reflected by the MIC.
The in vitro pharmacokinetic parameters were similar to those observed in humans for all the three drugs except amphotericin B, for which the t1/2 in the in vitro model was around 11 h, as opposed to 19 h, which represents the mean residence time of amphotericin B in plasma after noncompartmental analysis. Compartmental analysis showed a rapid distribution A phase with a t1/2 of <1 h observed at 0 to 6 h and a slower elimination B phase with a t1/2 of 15 h observed 6 to 24 h after intravenous administration (2, 3). This divergence has also been observed in previous in vitro models developed for the study of Candida species and has been attributed to amphotericin B degradation during incubation at 37°C (13). This difference is unlikely to affect the drug's pharmacodynamics, as our in vitro PK/PD model accurately captures the peak concentration of amphotericin B, which is the critical pharmacodynamic variable of this polyene antifungal agent.
Amphotericin B was for decades the treatment of choice for aspergillosis. Clinical and experimental data indicated differences in efficacy against infections caused by various Aspergillus species (6). Lack of clinical efficacy, however, was not associated with significantly increased MICs (12, 18, 21), which are similar for all three species, with slightly higher MICs of amphotericin B for A. terreus (20). In the present study, the Aspergillus isolates exhibited the same MIC (1 mg/liter), classified as susceptible (4), suggesting similar efficacies of amphotericin B. However, the new in vitro model revealed differences in the efficacy of amphotericin B against the three Aspergillus species, with the following order: A. fumigatus > A. flavus > A. terreus. These findings are in agreement with previous comparative animal studies where treatment with amphotericin B was more effective against infection cause by A. fumigatus than against infection with A. flavus and less effective against infection with A. terreus (23, 36). It seems that the new in vitro model described here may better characterize the pharmacodynamic characteristics of amphotericin B for the major Aspergillus species tested than conventional in vitro susceptibility systems can.
Currently, voriconazole is the drug of choice for the treatment of aspergillosis (10). In general, the MICs of voriconazole for most of the isolates of A. fumigatus, A. flavus, and A. terreus are similar (20). However, there are significant differences in the in vivo activities of voriconazole against infections caused by these three Aspergillus species (32, 37). Although each isolate of the three Aspergillus species used in the present study had the same MIC (0.5 mg/liter), the new in vitro PK/PD model revealed significant pharmacodynamic differences, with the greatest efficacy of voriconazole found against A. fumigatus and less against A. terreus; whereas no activity against A. flavus was detected. Voriconazole at a dose of 10 mg/kg, which results in a drug exposure in mice similar to that achieved with standard doses in humans, is active against A. fumigatus (22, 32) but not against A. flavus (37) infections in murine models of invasive aspergillosis. In clinical studies, the survival rate of patients with invasive aspergillosis caused by A. terreus is generally lower than that in infections with A. fumigatus after treatment with voriconazole (10, 31). Of note, voriconazole showed better efficacy than other antifungals against A. terreus infections (31), as in our study. These in vivo and clinical data suggest that voriconazole may be more active against A. fumigatus than against A. terreus and may have little activity against A. flavus, in agreement with the findings of the new in vitro model. Thus, the new in vitro PK/PD model revealed clinical and in vivo relevant pharmacodynamic differences in voriconazole efficacy against the three Aspergillus spp. tested.
Caspofungin is used as salvage therapy against invasive aspergillosis, although recent studies suggest its effectiveness as first-line therapy (11). In the new in vitro model, caspofungin did not demonstrate any effect on the growth of an Aspergillus species other than A. terreus, in which galactomannan production was simply delayed for 2.83 h. Caspofungin inhibits 1,3-β-d-glucan synthesis, which results in distortion of the cell wall and suppression of growth. Galactomannan may be released during the process of cell wall disruption. Consequently, galactomannan levels may remain high despite effective antifungal activity. Thus, for pharmacodynamic study of the actions of caspofungin and other echinocandins, measurement of galactomannan production appears not to be informative either in vitro and in vivo (26). Another biomarker is needed to monitor the pharmacodynamics of echinocandins. An alternative endpoint for determining the activity of antifungal drugs, and particularly of echinocandins, may be PCR CEs.
In conclusion, in the present study, we developed and for the first time reported an in vitro PK/PD model which simulates the pharmacokinetics of three major antifungal drugs in humans and assesses their efficacy against three clinically significant Aspergillus species. The species-dependent pharmacodynamic differences revealed with the in vitro PK/PD model are also reflected by the different epidemiological cutoff values found for these Aspergillus species (7), in line with the species-specific breakpoints for Candida spp. (27, 29), and may be attributed to different genetic background (33), particularly for genes involved in growth and drug transport (8). Because total drug concentrations were simulated in the in vitro PK/PD model, the drug that is available for microbiologic activity may be overestimated due to protein binding in vivo. Furthermore, this model should be validated with results of in vivo antifungal efficacy from animal experiments. This model can be adapted to incorporate serum, neutrophils, and other host factors that may affect antifungal pharmacodynamics. It can also be used for exploring more effective dosing regimens with dose escalation and fractionation studies, for PK/PD modeling and for studying the activity of drug combinations in order to improve the treatment and prognosis of Aspergillus and even other mold infections.
ACKNOWLEDGMENTS
This study was supported by Marie Curie Reintegration Grant MIRG-CT-2007-208796 from the European Commission, Bodosakis Foundation, FLOGA, and the University of Athens SARG 70/3/6915 for infrastructural subsidy.
Footnotes
Published ahead of print 7 November 2011
REFERENCES
- 1. Andes D. 2006. Pharmacokinetics and pharmacodynamics of antifungals. Infect. Dis. Clin. North Am. 20:679–697 [DOI] [PubMed] [Google Scholar]
- 2. Ayestarán A, et al. 1996. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob. Agents Chemother. 40:609–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bekersky I, et al. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cantón E, Espinel-Ingroff A, Peman J. 2009. Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev. Anti Infect. Ther. 7:107–119 [DOI] [PubMed] [Google Scholar]
- 5. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. Second edition CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Denning DW, et al. 1998. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J. Infect. 37:173–180 [DOI] [PubMed] [Google Scholar]
- 7. Espinel-Ingroff A, et al. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J. Clin. Microbiol. 48:3251–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fedorova ND, et al. 2008. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groll AH, Piscitelli SC, Walsh TJ. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343–500 [DOI] [PubMed] [Google Scholar]
- 10. Herbrecht R, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 11. Herbrecht R, et al. 2010. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant. 45:1227–1233 [DOI] [PubMed] [Google Scholar]
- 12. Johnson EM, et al. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85–93 [DOI] [PubMed] [Google Scholar]
- 13. Lewis RE, Kontoyiannis DP, Darouiche RO, Raad II, Prince RA. 2002. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 46:3499–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis RE, Lund BC, Klepser ME, Ernst EJ, Pfaller MA. 1998. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by using a dynamic in vitro mycotic infection model. Antimicrob. Agents Chemother. 42:1382–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis RE, Wiederhold NP, Prince RA, Kontoyiannis DP. 2006. In vitro pharmacodynamics of rapid versus continuous infusion of amphotericin B deoxycholate against Candida species in the presence of human serum albumin. J. Antimicrob. Chemother. 57:288–293 [DOI] [PubMed] [Google Scholar]
- 16. Li RC, Zhu ZY. 1997. In vitro models for prediction of antimicrobial activity: a pharmacokinetic and pharmacodynamic perspective. J. Chemother. 9(Suppl. 1):55–63 [PubMed] [Google Scholar]
- 17. Lignell A, Johansson A, Lowdin E, Cars O, Sjolin J. 2007. A new in-vitro kinetic model to study the pharmacodynamics of antifungal agents: inhibition of the fungicidal activity of amphotericin B against Candida albicans by voriconazole. Clin. Microbiol. Infect. 13:613–619 [DOI] [PubMed] [Google Scholar]
- 18. Lionakis MS, Lewis RE, Chamilos G, Kontoyiannis DP. 2005. Aspergillus susceptibility testing in patients with cancer and invasive aspergillosis: difficulties in establishing correlation between in vitro susceptibility data and the outcome of initial amphotericin B therapy. Pharmacotherapy 25:1174–1180 [DOI] [PubMed] [Google Scholar]
- 19. Marr KA, Patterson T, Denning D. 2002. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. North Am. 16:875–894 [DOI] [PubMed] [Google Scholar]
- 20. Meletiadis J, et al. 2007. Differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrob. Agents Chemother. 51:3329–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosquera J, et al. 2001. Susceptibility testing of Aspergillus flavus: inoculum dependence with itraconazole and lack of correlation between susceptibility to amphotericin B in vitro and outcome in vivo. Antimicrob. Agents Chemother. 45:1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murphy M, Bernard EM, Ishimaru T, Armstrong D. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Odds FC, et al. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patterson TF, et al. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore) 79:250–260 [DOI] [PubMed] [Google Scholar]
- 25. Perea S, et al. 2000. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petraitiene R, et al. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 13:180–195 [DOI] [PubMed] [Google Scholar]
- 28. Purkins L, et al. 2003. The pharmacokinetics and safety of intravenous voriconazole—a novel wide-spectrum antifungal agent. Br. J. Clin. Pharmacol. 56(Suppl. 1):2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez-Tudela JL, Arendrup MC, Cuenca-Estrella M, Donnelly JP, Lass-Florl C. 2010. EUCAST breakpoints for antifungals. Drug News Perspect. 23:93–97 [DOI] [PubMed] [Google Scholar]
- 30. Shadomy S, McCay JA, Schwartz SI. 1969. Bioassay for hamycin and amphotericin B in serum and other biological fluids. Appl. Microbiol. 17:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steinbach WJ, et al. 2004. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 39:192–198 [DOI] [PubMed] [Google Scholar]
- 32. Takemoto K, et al. 2009. Comparative study on the efficacy of liposomal amphotericin B and voriconazole in a murine pulmonary aspergillosis model. Chemotherapy 55:105–113 [DOI] [PubMed] [Google Scholar]
- 33. Tekaia F, Latge JP. 2005. Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol. 8:385–392 [DOI] [PubMed] [Google Scholar]
- 34. Walsh TJ, et al. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 49:4536–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh TJ, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 36. Walsh TJ, et al. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305–319 [DOI] [PubMed] [Google Scholar]
- 37. Warn PA, et al. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198–1207 [DOI] [PubMed] [Google Scholar]