Abstract
Glutamate transporters regulate normal synaptic network interactions and prevent neurotoxicity by rapidly clearing extracellular glutamate. GLT-1, the dominant glutamate transporter in the cerebral cortex and hippocampus, is significantly reduced in Alzheimer's disease (AD). However, the role GLT-1 loss plays in the cognitive dysfunction and pathology of AD is unknown. To determine the significance of GLT-1 dysfunction on AD-related pathological processes, mice lacking one allele for GLT-1(+/−) were crossed with transgenic mice expressing mutations of the amyloid-β protein precursor and presenilin-1 (AβPPswe/PS1ΔE9) and investigated at 6 or 9 months of age. Partial loss of GLT-1 unmasked spatial memory deficits in 6-month-old mice expressing AβPPswe/PS1ΔE9, with these mice also exhibiting an increase in the ratio of detergent-insoluble Aβ42/Aβ40. At 9 months both behavioral performance and insoluble Aβ42/Aβ40 ratios among GLT-1(+/+)/AβPPswe/PS1 E9 and GLT-1(+/−)/AβPPswe/PS1ΔE9 mice were comparable. These results suggest that deficits in glutamate transporter function compound the effects of familial AD AβPP/PS1 mutant transgenes in younger animals and thus may contribute to early occurring pathogenic processes associated with AD.
Keywords: Amyloid-β, dementia, excitatory, excitotoxicity, neurotransmission
INTRODUCTION
A family of five glutamate transporters called GLAST, GLT-1, EAAC1, EAAT4, and EAAT5 (also known as EAAT1-5) are responsible for maintaining extracellular glutamate concentrations within a range that permits normal excitatory neurotransmission [1]. Of these transporters, GLT-1 clears most of the glutamate released in the cortex and hippocampus. For example, approximately 80% of the glutamate transporters expressed in hippocampus are GLT-1 [2]. Although expressed primarily by astrocytes, GLT-1 is also expressed on neuronal axon terminals [3]. In addition to playing a critical role in preventing glutamate-mediated excitotoxicity [4], GLT-1 modulates normal synaptic interactions and neural plasticity [5, 6].
Alzheimer's disease (AD) is thought to reflect synaptic dysfunction [7]. Aberrant glutamate stimulation can cause synaptic dysfunction, which has been proposed as one of several mechanisms by which synapses are damaged in AD [8, 9]. Memantine, a drug thought to temper the excitatory properties of glutamate on NMDA receptors, has efficacy in treating AD [10], thus lending clinically based support to the hypothesis that aberrant glutamatergic activity may underlie pathogenic aspects of the disease process [11].
A number of studies have found that GLT-1 is significantly reduced or damaged in AD [12–17]. Considering the critical neuroprotective functions carried out by GLT-1, such findings raise the possibility that reduced GLT-1 levels may play a significant role in AD pathogenesis. Consequently, it is not yet clear whether GLT-1 dysfunction plays a pathogenic or bystander role in AD. In this regard progress has been limited by the lack of an appropriate animal model. Accordingly we generated a novel mouse model with partial loss of GLT-1 that also harbors two familial AD mutations (AβPPswe/PS1ΔE9) to test the hypothesis that reduced GLT-1 expression increases susceptibility to the cognitive and biochemical consequences of mutations associated with familial AD (FAD).
MATERIALS AND METHODS
Animals
AβPPswe/PS1ΔE9 hemizygous mice (line 85) maintained on a B6C3F1/J background [18] and GLT-1(+/–) mice maintained on a C57BL/6 background [4] were mated in order to generate F1 offspring littermates with the genotypes, GLT-1(+/+)/ non-transgenic, GLT-1(+/– )/non-transgenic, GLT-1 (+/+)/AβPPswe/PS1ΔE9, and GLT-1 (+/– )/AβPPswe /PS1ΔE9. All experiments were performed in accordance with procedures approved by the VAPSHCS Institutional Animal Care and Use Committee (IACUC).
Behavioral studies
Spatial reference memory was tested using a Morris water maze as previously described with minor modifications [19, 20]. Male mice at 6 or 9 months of age were trained for three consecutive days (4 trials per day, 90 s maximum trial duration; Training Trials), rested one day, and then followed by five testing trial days (4 trials per day, 90 s maximum trial duration; Testing Trials). On testing days 2 and 5 the hidden platform (a 10 cm × 10 cm square) was removed for the first trial of the day (Probe Trials). All mice were tested in genotype-blinded sets of 15–25 mice during the light phase starting at one of four randomized locations. An ANYmaze® imaging/data acquisition system (Stoelting, Wood Dale, IL) recorded swim duration, distance and speed during Training and Testing Trials. For Probe Trials, the number of crosses over the 14 cm diameter circular platform area was also recorded. Analysis of the Probe Trials data also included evaluation of the recorded parameters according to the quadrants.
Brain tissue processing ELISAs, and immunoblotting
Frozen cortical brain tissue was homogenized in TBS buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA) supplemented with protease inhibitor cocktail (Sigma, St. Louis, MO), sonicated, and differential ultracentrifugation was used to isolate distinct fractions with the resulting pellet further resuspended in 70% formic acid, ultracentrifuged and neutralized with 1 M Tris Base. The fractions were stored at –80°C. Triton-X 100-soluble and -insoluble Aβ levels were analyzed by ELISA as previously described [21, 22]. Western blots evaluated secreted and membrane-associated amyloid-β protein precursor (AβPP) levels, using the following AβPP/Aβ antibodies: 22C11 (Roche Molecular Biochemicals, Indianapolis, IN); 6E10 (Signet Laboratories, Dedham, MA); anti-AβPP C-terminus (Zymed Laboratories, Inc., San Francisco, CA). Additional antibodies used: actin (Sigma, St. Louis, MO), AB12 (GLT-1 polyclonal antibody provided by David Pow, Brisbaine, Australia), EAAC1, and GLAST (Alpha Diagnostics, San Antonio, TX). Densitometry analyses were performed using ImageJ 1.39u (NIH, Bethesda, MD). To visualize amyloid plaques, 20 μM frozen brain sections (n = 4 per genotype) were stained with 1% Thioflavin-S (Sigma, St. Louis, MO), evaluated using a Nikon, Eclipse TE300 fluorescent microscope with IPLAB-Scientific Image processing software (Scanlytics, Fairfax, VA) and analyzed using Image J software (NIH, Bethesda, MD). Samples were unblinded (age and genotype) after analysis.
Statistic
Data are reported as the mean ± standard error of the mean (SEM). For behavioral and biochemical studies analyses of variance (ANOVA) were used with training or test days as a within-subjects factor and genotype and/or age as between-subjects factors. Standard single factor contrasts accompanying ANOVA [23, 24] tested the following groups: (i) GLT-1(+/–)/AβPPswe/PS1ΔE9 versus other genotypes; (ii) GLT-1(+/+)/non-transgenic versus other genotypes; (iii) GLT-1(+/–)/non-transgenic and GLT-1(+/+)/non-transgenic versus GLT-1(+/–)/AβPP/PS1 and GLT-1(+/+)/AβPP/PS1. ELISAs and thioflavin-S staining data was assessed by one-way ANOVA with genotype as a between subjects factor. Values were considered significant when p ≤ 0.05. SPSS software (SPSS, Chicago, IL) was used for statistical analyses.
RESULTS
Spatial memory
Four experimental groups were studied: GLT-1 (+/+)/non-transgenic, GLT-1(+/–)/non-transgenic, GLT-1(+/+)/AβPPswe/PS1ΔE9, and GLT-1(+/–)/AβPPswe/PS1ΔE9 (abbreviated as GLTwt/nTg,GLThet/nTg, GLTwt/AβPP/PS1, and GLThet/AβPP/ PS1, respectively). All subjects were F1 littermate-matched males derived from the cross of AβPPswe/ PS1ΔE9 and GLT-1(+/–) mice. Among these geno-types the F1 offspring appeared indistinguishable with no statistically significant differences in body weight at 6 or 9 months of age (F[3,36] = 0.924, n.s. and F[3,38] = 0.751, n.s., respectively).
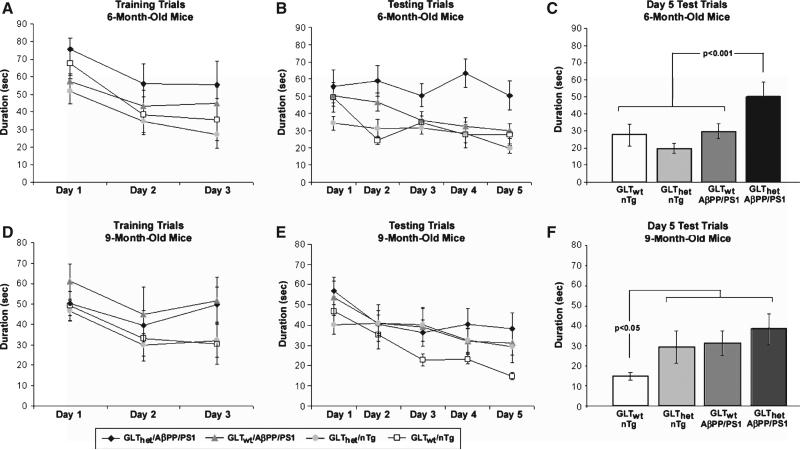
During the Training Trials the mice were required to locate a submerged platform in the center of the maze that was flagged above the waterline. Figure 1A shows that for 6-month-old mice performance differences among the four groups during training were not statistically significant (F[3,36] = 1.469, n.s.). There was a statistically significant reduction in the time required to find the platform during Training Trials (Training Trials factor, F[2,72] = 18.912, p < 0.0001). Unless otherwise indicated statistically non-significant interaction terms are not reported. In addition, there were no statistically significant genotype differences in swim speed (F[3,36] = 0.788, n.s.). These data indicate that mice in all groups learned comparably to locate the marked platform, thus arguing that geno-type differences did not alter visual abilities or motor performance.
Fig. 1.
Partial loss of GLT-1 increases spatial memory deficits in a Morris water maze task. A) The performance of 6-month-old mice to find the platform (Training Trials). All groups performed comparably. B) The performance of 6-month-old mice to find the hidden platform (Testing Trials). GLThet/AβPP/PS1 mice took longer to find the hidden platform than the other groups. C) Day 5 Testing Trial results for 6-month-old mice. The GLThet/AβPP/PS1 group performed significantly worse than the other groups (p < 0.001). D) The performance of 9-month-old mice to find the platform (Training Trials). All groups performed comparably. E) The performance of 9-month-old mice to find the hidden platform (Testing Trials). The GLTwt/nTg mice appeared better at finding the hidden platform in comparison to the other groups. F) Day 5 Testing Trial results for 9-month-old mice. The GLTwt/nTg mice were significantly better at locating the hidden platform than the other groups (p < 0.05). The data are presented as mean ± standard error of the mean (SEM), n = 8–14 mice per group.
In the Testing Trials the mice were required to find the hidden platform that was moved to a new location, which remained constant during Test Trials. In contrast to the training results, analysis of the time required to find the hidden platform during testing revealed a statistically significant difference among genotypes (F[3,36] = 5.610, p < 0.003) (Fig. 1B). The within-subjects test days factor and the genotype by test days interaction were also statistically significant (F[4,144] = 6.639, p < 0.001 and F[12, 144] = 1.978, p < 0.030, respectively). Inspection of Fig. 1B shows that the GLThet/AβPP/PS1 group performed markedly worse than the other groups, while the GLTwt/AβPP/PS1, GLThet/nTg, and GLTwt/nTg mice appeared to perform similarly. This outcome was most pronounced by the final day of testing, which is re-plotted in Fig. 1C. An ANOVA confirmed that performance differences among genotypes on the final Testing Trial were statistically significant (F[3,36] = 5.004, p < 0.005). A contrast analysis confirmed the a priori hypothesis that GLThet/AβPP/PS1 mice performed worse than the other three groups (p < 0.001) (Fig. 1C).
These findings suggest that GLT-1 augments the pathogenic effects of expressing mutant AβPP/PS1. There are at least two ways to view such results. One possibility is that GLT-1 loss amplifies mutant AβPP/PS1-related pathology. Under such circumstances one could expect that differences between GLThet/AβPP/PS1 and GLTwt/AβPP/PS1 mice would be maintained or possibly increase with age. Another interesting possibility is that GLT-1 loss is a vulnerability factor that shifts the onset of cognitive deficits to an age earlier in the pathogenic process before high rates of Aβ accumulation reach possibly saturating levels. Under such circumstances one could expect that differences between GLThet/AβPP/PS1 and GLTwt/AβPP/PS1 mice might be less pronounced in mice older than 6 months. Support for this latter possibility comes from analyses of a separate cohort of 9-month-old mice that were trained and tested at the same time and under identical conditions as the 6-month-old cohort.
As with the 6-month-old mice, training differences among genotypes in the 9-month-old cohort were not significant (F[3,35] = 0.970, n.s.) and there was a significant decrease in the time required to find the flagged platform across trials (F[2,70] = 9.335, p < 0.0003) (Fig. 1D). There were no genotype differences in swim speed (F[3,35] = 1.509, n.s.). These results indicate that visual and motor abilities in the 9-month-old mice were comparable among genotypes.
Figure 1E shows that during Testing Trials the 9-month-old cohort demonstrated a different behavioral profile from the 6-month-old mice. The performance of the 9-month-old mice did not differ significantly among genotypes (F[3,35] = 1.162, n.s.), but they did exhibit improved performance during Training Trials (F[4,140] = 11.89, p < 0.0001). Nine-month-old GLThet/AβPP/PS1, GLTwt/AβPP/PS1, and GLThet/nTg mice performed similarly, while the 9-month-old GLTwt/nTg mice appeared to perform better than the others, particularly on test day 5 (Fig. 1F). Although overall differences among geno-types on test day 5 were not statistically significant (F[3,35] = 1.1.901, n.s.), a contrast analyzing the difference between the GLTwt/nTg mice versus the other groups was significant (p < 0.05).
The results in Fig. 1F suggest that by 9 months GLT-1 loss and AβPPswe/PS1ΔE9 expression may independently mediate modest effects on Testing Trials performance that were similar to the performance of GLThet/AβPP/PS1 mice. In this regard the outcome was different in the 6-month-old cohort where GLT-1 loss and AβPPswe/PS1ΔE9 expression alone had no effect, but when combined significantly impaired Testing Trials performance (Fig. 1 C). Nonetheless, even at 9 months of age the GLThet/AβPP/PS1 mice still performed somewhat worse than the other mice. These observations are consistent with the results of an overall ANOVA including both the 6- and 9-month-old mice (Genotype by Age by Test-Days) that confirmed differences among geno-types were statistically significant (F[3,71] = 5.185, p < 0.003). Performance differences between ages was not significant (F[1,71] = 0.426, n.s.), while the test days factor and the genotype by test days interaction were statistically significant (F[4,284] = 18.107, p < 0.0001 and F[12,284] = 1.815, p < 0.045, respectively). In this ANOVA a contrast performed on the genotype factor, which tested the prediction that the GLThet/AβPP/PS1 mice performed worse than the other mice, was also statistically significant (p < 0.001).
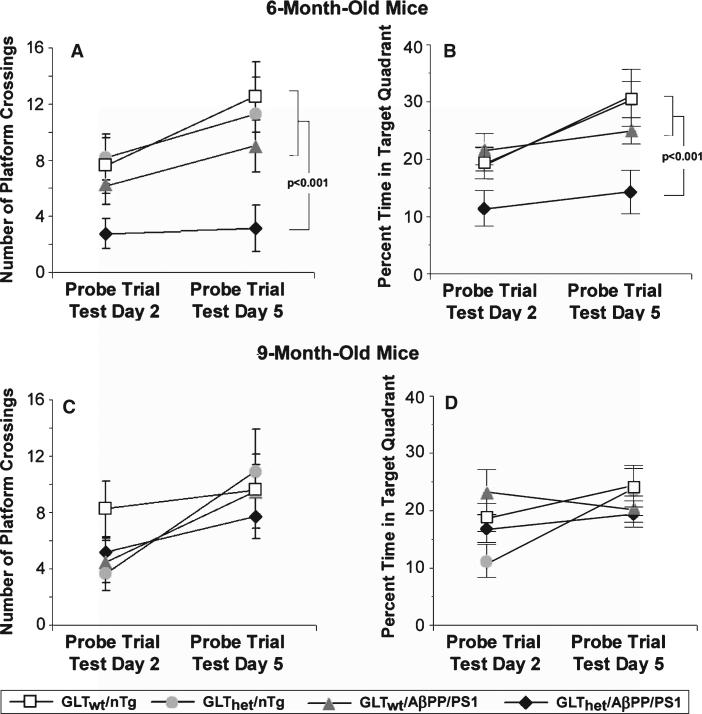
Probe Trials carried out on testing days 2 and 5 further confirmed performance distinctions between the 6- and 9-month-old mice (Fig. 2). Figure 2A shows that the 6 month old GLThet/AβPP/PS1 mice crossed the expected platform area fewer times than mice of other genotypes. With the exception of the GLThet/AβPP/PS1 mice, all mice showed an increase in the number of platform area crossings on day 5 compared to day 2, indicating improved retention of the platform location across days of testing. Analysis of these data revealed a statistically significant difference among genotypes (F[3,36] = 4.515, p < 0.01). The within-subjects Probe Trials factor was also statistically significant (F[1,36] = 4.296, p < 0.045). A contrast analysis of the genotype factor included in this ANOVA testing the prediction that the GLThet/AβPP/PS1 mice would perform more poorly than the other mice was statistically significant (p < 0.001). In the 9-month-old cohort, differences in Probe Trial performance among genotypes were not significant (F[3,35] = 0.502, n.s.) (Fig. 2C). Among the 9-month-old mice, all increased in the number of platform crosses from day 2 to day 5, a finding consistent with a statistically significant difference in the within-subjects probe trials factor (F[1,35] = 11.678, p < 0.002).
Fig. 2.
Partial GLT-1 loss increases spatial memory deficits during Probe Trials. A) Number of times 6-month-old mice crossed the platform area during Probe Trials on testing days 2 and 5. Six-month-old GLThet/AβPP/PS1 mice crossed the platform area significantly fewer times than the other groups (p < 0.001). B) Percent time 6-month-old mice were in the target quadrant. Compared to the other groups, 6-month-old GLThet/AβPP/PS1 mice were in the target quadrant significantly less (p < 0.001). C) Number of times 9-month-old mice crossed the platform area during Probe Trials on testing days 2 and 5. D) Percent time 9-month-old mice were in the target quadrant. The data are presented as mean ± SEM, n = 8–14 mice per group.
Probe Trials performance was also examined by measuring percent time spent in the target quadrant (Fig. 2B and 2D). This analysis revealed similar results as the platform crossing data, further confirming the impaired performance of the GLThet/AβPP/PS1 mice. The effect of genotype in 6-month-old mice was statistically significant (F[3,36] = 4.908, p < 0.006). The percent of time 6-month-old GLThet/AβPP/PS1 mice were in the target quadrant (Fig. 2B) was significantly less compared to the other mice during Probe Trial 5 (p < 0.001). Nine-month-old mice exhibited no statistically significant difference among geno-types in the percent time spent in the target quadrant (F[3,35] = 0.494, n.s.).
Aβ and AβPP biochemistry
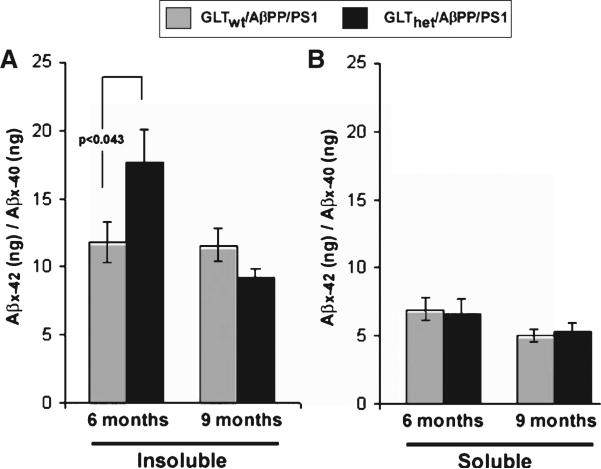
Triton-X-100 soluble and Triton-X-100-insoluble (70% formic acid soluble) Aβx–40 and Aβx–42 were measured in cortex samples from 6- and 9-month- old GLTwt/AβPP/PS1 and GLThet/AβPP/PS1 mice. As shown in Fig. 3A, at 6 months of age partial GLT-1 loss produced a statistically significant increase in the ratio of insoluble Aβx–42/Aβx–40 (F[1,19] = 4.697, p < 0.043), while at 9 months of age the ratio of insoluble Aβx–42/Aβx–40 in GLTwt/AβPP/PS1 and GLThet/AβPP/PS1 mice was not significantly different (F[1,14] = 3.699, n.s.). At both ages the ratio of detergent-soluble Aβx–42/Aβx–40 was comparable (F[1,19] = 0.055, n.s., F[1,14] = 0.120, n.s., 6- and 9-month-old mice respectively) (Figure 3B). Additionally, Thioflavin-S staining of GLThet/AβPP/PS1 and GLTwt/AβPP/PS1 mice cortex revealed that plaque number was not distinguishable by genotype either at 6 months (F[1,6] = 0.916, n.s.) or 9 months (F[1,6] = 2.59, n.s.) (data not shown). Similarly, differences in plaque area among these groups was not significantly different at 6 months (F[1,6] = 0.03, n.s.) or at 9 months (F[1,6] = 0.12, n.s.) (data not shown).
Fig. 3.
Partial loss of GLT-1 increases insoluble Aβ42/40 ratio. A) Ratios of insoluble Aβx–42 to Aβx–40 in cortex of 6- or 9-month-old mice. Six-month-old GLThet/AβPP/PS1 mice have significantly highly ratios of insoluble Aβx–42/Aβx–40 compared to 6-month-old GLTwt/AβPP/PS1 mice (p < 0.043). B)Ratios of soluble Aβx–42/Aβx–40 in cortex of 6- or 9-month-old mice. The data are presented as mean ± SEM, n = 13 mice per group.
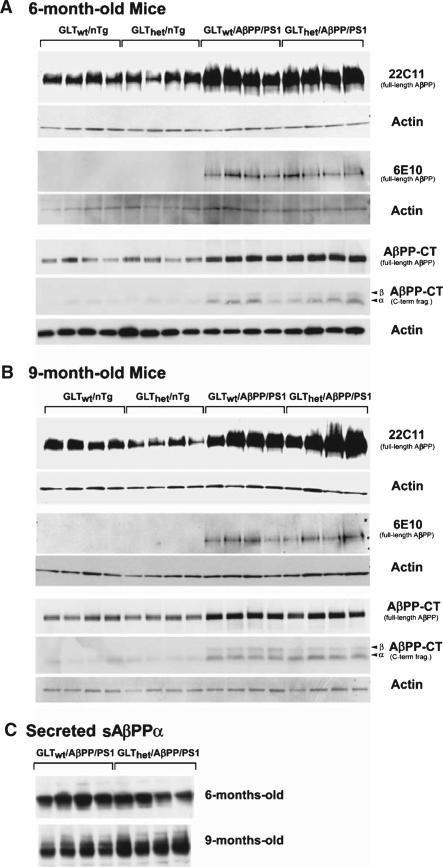
To address the question of whether partial GLT-1 loss changed overall AβPP expression levels or altered AβPP processing, the levels of full-length membrane-bound AβPP were measured by Western blot using antibodies that recognize the AβPP N-terminal ectodomain (22C11), human-specific Aβ domain (6E10), and the intracellular AβPP C-terminus (C-term). Figure 4A and 4B show that at both 6 and 9 months of age total membrane-associated full-length AβPP levels were not significantly altered by GLT-1 heterozygousity as measured by 22C11 (6-month-old mice: F[1,12] = 0.517, n.s.; 9-month-old mice: F[1,12] = 0.898, n.s.), 6E10 (6-month-old mice : F[1,6] = 1.168, n.s.; 9-month-old mice: F[1,6] = 0.011, n.s.), and AβPPCT antisera (6-month-old mice : F[1,12] = 0.044, n.s.; 9-month-old mice: F[1–12] = 0.148, n.s.). Secreted AβPPα measured using 6E10 was unaffected by GLT-1 genotype (F[1,6] = 0.989, n.s.). Comparison of the sAβPPα in 9-month-old GLThet/AβPP/PS1 mice to GLTwt/AβPP/PS1 mice (mean relative intensities 128.1 and 104.7, respectively) showed GLThet/AβPP/PS1 mice levels to be 22% elevated (F[1,6] = 11.057, p < 0.016). Overall, while the sAβPPα was modestly elevated in the GLThet/AβPP/PS1 mice, the pattern of AβPP expression/processing was comparable on the basis of GLT-1 genotype. Similarly, at both 6 and 9 months of age AβPP β- and α- secretase carboxyl terminus fragments (AβPP-CTFs) expression (Figure 4A, B) appeared unaffected by partial GLT-1 loss (6-month-old mice β-CTF: F[1,12] = 1.085, n.s.; 6-month-old mice α-CTF: F[1,12] = 0.130, n.s.; 9-month-old mice β-CTF: F[1,12] = 1.281, n.s.; 9-month-old mice α-CTF: F[1,12] = 0.554, n.s.). In close accordance with previous findings [4, 25], Western blot analysis indicated that GLT-1 expression in heterozygous mice was roughly half that of wild-type mice (6-month-old mice: F[1,12] = 22.616, p < 0.0005; 9-month-old mice: F[1,12] = 9.697, p < 0.009). Additionally, the other primary glutamate transporters, EAAC1 (6-month-old mice: F[1,12] = 2.515, n.s.; 9-month-old mice: F[1,12] = 3.597, n.s.) and GLAST (6-month-old mice: F[1,12] = 0.018, n.s.; 9-month-old mice: F[1,12] = 1.984, n.s.) were not altered by GLT-1 loss (Western blots not shown).
Fig. 4.
AβPP expression was not affected by GLT-1 heterozygousity. Western blot analysis of protein from the cortex of 4 mice of each genotype at 6 months (A) or 9 months (B). The upper panels represent levels of membrane-associated AβPP. Full-length AβPP was detected using 22C11, 6E10, and anti-AβPP C-terminus (AβPP-CT) antibodies. AβPP-CT also detected AβPP C-terminal fragments (α and β AβPP-CTFs). C) Levels of secreted sAβPPα in 6- or 9-month-old mice were detected using the 6E10 antibody. Western blots were stripped and re-probed with an antibody recognizing actin.
Taken together these data argue that the cognitive deficits and the corresponding increased insoluble Aβx–42/Aβx–40 ratio in the 6-month-old GLThet/AβPP/PS1 mice were not related to overt changes in overall AβPP expression or AβPP processing, and were not associated with altered expression of either neuron-specific EAAC1 or the other major astrocytic glutamate transporter, GLAST.
DISCUSSION
It has been estimated that as much as 80% of the metabolic energy consumed by the brain under basal conditions is devoted to glutamate cycling [26]. Thus, even at rest the brain functions via highly dynamic processes that regulate the excitatory properties of glutamate. This sustained metabolic burden may render the CNS vulnerable to the progressive impact of AD pathology on systems associated with neurotransmitter cycling. Consistent with this idea are data showing that seizure activity is increased in AD patients [27–29] and reports that mice harboring FAD-related AβPP mutations also display aberrant neuronal network excitability [30, 31]. Additionally, recent findings show that proximity to amyloid deposits is associated with neuron hyperactivity [32], which suggests that aberrant neuronal excitability, a process likely preceding overt synaptic loss, may be an important feature of AD pathogenesis.
GLT-1 plays a dominant role in maintaining extra-cellular glutamate in the low nanomolar range [33]. GLT-1 also regulates normal synaptic cooperativity by regulating the spread of synaptically released glutamate to neighboring synapses [5]. This idea is supported by data showing that GLT-1 regulates stimulus-specific synaptic plasticity [6]. Considering the importance of these processes in maintaining normal cognitive activity, it is potentially significant that GLT-1 is reduced in AD [12]. More recent studies further confirm that both GLT-1 mRNA and protein levels are reduced in AD [14–17]. In addition, GLT-1 is oxidatively damaged in AD patients and in Aβ-treated synaptosomes [13]. Such findings suggest that partial GLT-1 loss is associated with AD pathology. However, whether GLT-1 loss influences the cognitive manifestations of AD or amyloid-related pathology has not been addressed previously.
This report shows that impaired performance on a spatial reference memory task and a corresponding increase in the ratio of insoluble Aβ42/Aβ40 was associated with partial GLT-1 loss in 6-month-old AβPPswe/PS1ΔE9 mice, with no difference in total Aβ levels. These findings are similar to previous work showing that early-onset behavioral impairments in Tg2576 (AβPP695 Swe) mice are more closely associated with an increase in the Aβ42/Aβ40 ratio than with total Aβ levels [34]. This is further supported by studies showing that human cerebral spinal fluid (CSF) Aβ42/Aβ40 ratios increase early, but not later, in the course of the disease [35].
Previous data indicate that the AβPPswe/PS1ΔE9 transgene does not affect water maze performance significantly in 6-month-old mice [36]. In addition, the process of Aβ deposition in AβPPswe/PS1ΔE9 mice is comparatively modest at 6 months, with mice developing significant amyloid burden by 9 months [18]. If GLT-1 normally functions to protect the brain from the deleterious consequences of aberrant amyloid accumulation, partial loss of GLT-1 might be expected to unmask latent pathogenic processes capable of disrupting memory. Deficits in water maze performance and the increased Aβ42/Aβ40 ratio in the 6-month-old GLThet/AβPP/PS1 mice support this. Nine-month-old GLTwt/AβPP/PS1 and GLThet/AβPP/PS1 mice performed comparably, with correspondingly similar Aβ42/Aβ40 ratios. Altogether this suggests that the neuroprotective properties of GLT-1 may be restricted to earlier stages of the amyloid-related pathogenic process, rather than to more advanced stages where the deposition process has become more substantial.
In summary, these findings are consistent with the idea that partial loss of GLT-1 increases the vulnerability of the brain to cognitive insults associated with expressing mutant forms of AβPP/PS1. These findings also offer new evidence suggesting that glial dysfunction may be an important feature of AD pathology. Supporting this idea, a recent report shows that astrocyte networks may convey the impact of Aβ deposits well beyond anatomical regions immediately surrounding amyloid plaques [37]. It is possible that therapeutic strategies designed to enhance the natural neuroprotective properties of astrocytes may present novel opportunities to reduce or forestall the symptoms of AD.
ACKNOWLEDGMENTS
This work was supported by the Veteran's Affairs Office of Research and Development Medical Research Serve and by NIH institutional fellowships to M.A.M (T32 AG000258), JSM (T32 AG000258), and P.M. (T32 AG000258).
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=857).
REFERENCES
- 1.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 2.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 5.Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- 6.Tsvetkov E, Shin RM, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 8.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 10.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 11.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 12.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 13.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1- 42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grunblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer's disease. J Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 15.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, Kraner SD, Norris CM. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR. Glutamate transporter variants reduce glutamate uptake in Alzheimer's disease. Neurobiol Aging. 2011;32:553.553.e1–553.e11. doi: 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Wotjer RL, Duerson K, Fullmer JM, Mookherjee P, Ryan AM, Montine TJ, Kaye JA, Quinn JF, Silbert L, Erten-Lyons D, Leverenz JB, Bird TD, Pow DV, Tanaka K, Watson GS, Cook DG. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:667–676. doi: 10.1097/NEN.0b013e3181e24adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 19.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green PS, Bales K, Paul S, Bu G. Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer's disease. Endocrinology. 2005;146:2774–2781. doi: 10.1210/en.2004-1433. [DOI] [PubMed] [Google Scholar]
- 22.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 23.Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. McGraw-Hill Inc.; 1991. [Google Scholar]
- 24.Field A. Sage Publications; London: 2005. Discovering Statistics Using SPSS. [Google Scholar]
- 25.Pardo AC, Wong V, Benson LM, Dykes M, Tanaka K, Roth-stein JD, Maragakis NJ. Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp Neurol. 2006;201:120–130. doi: 10.1016/j.expneurol.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 27.Amatniek JC, Hauser WA, Del Castillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 28.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayadev S, Leverenz JB, Steinbart E, Stahl J, Klunk W, Yu C-E, Bird TD. Alzheimer's disease phenotypes and genotypes associated with mutations in presenilin 2. Brain. 2010;133:1143–1154. doi: 10.1093/brain/awq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyper-excitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 33.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen M, Schroder J, Blomberg M, Engvall B, Pantel J, Ida N, Basun H, Wahlund LO, Werle E, Jauss M, Beyreuther K, Lannfelt L, Hartmann T. Cerebrospinal fluid A beta42 is increased early in sporadic Alzheimer's disease and declines with disease progression. Ann Neurol. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercelllar calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]