Abstract
Objective
Chronic fatigue syndrome (CFS) and multiple sclerosis (MS) are characterized by debilitating fatigue, yet evaluation of this symptom is subjective. We examined metabolite-detecting, adrenergic, and immune gene expression (mRNA) in patients with CFS (n=22) vs. MS (n=20) vs. healthy controls (n=23) and determined their relationship to fatigue and pain before and after exercise.
Methods
Blood samples and fatigue and pain ratings were obtained at baseline and 0.5, 8, 24, and 48 hours following sustained moderate exercise. Leukocyte mRNA of 4 metabolite-detecting receptors (ASIC3, P2X4, P2X5, TRPV1), 4 adrenergic (α-2a, β-1, β-2 receptors, COMT) and 5 immune markers (CD14, TLR4, IL-6, IL-10, LTa) was examined using quantitative PCR.
Results
CFS patients had greater post-exercise increases in fatigue and pain (10–29 pts above baseline, p<.001) and greater mRNA increases in P2X4, TRPV1, CD14 and all adrenergic receptors than controls (1.3 ± .14 to 3.4 ± .90 fold increase above baseline, p=.04 – .005). CFS patients with co-morbid fibromyalgia (n=18) also showed greater increases in ASIC3 and P2X5 (p<.05). MS patients had greater post-exercise increases than controls in β-1 and β-2 adrenergic receptor expression (1.4 ± .27 and 1.3 ± .06 fold increase, respectively, p=.02 and <.001) and greater decreases in TLR4 (p=.02). In MS, IL-10 and TLR4 decreases correlated with higher fatigue scores.
Conclusion
Post-exercise mRNA increases in metabolite-detecting receptors were unique to CFS patients while both MS and CFS showed abnormal increases in adrenergic receptors. Among MS patients, greater fatigue was correlated with blunted immune marker expression.
Keywords: Fatigue, real-time PCR, fatigue impact scale
Introduction
Pathological fatigue expressed as an overwhelming sense of tiredness is often the key presenting symptom for both multiple sclerosis (MS) and chronic fatigue syndrome (CFS), and no specific underlying pathology has been found to explain this debilitating chronic fatigue (1,2). For MS, fatigue adversely affects mental and physical functioning even when there is no apparent motor deficit and when few or no active lesions can be discerned. CFS diagnosis requires fatigue not relieved by rest to be present for at least 6 months and requires the presence of at least 4 of 8 additional symptoms, but several of these symptoms such as unrefreshing sleep, difficulty with memory or concentration, muscle or joint pain, and new or worsened headaches are also common in MS. In order to devise effective treatments for pathological fatigue, it is important to clarify which physiological pathways may be producing the fatigue. It is likely that some of these pathways may be the same for CFS and MS while others may differ.
Recent reports on CFS have focused on one of the 8 central CDC-defined symptoms, postexertional malaise (which is the second primary symptom of CFS according to the Canadian guidelines); this is defined as an increase in mental or physical fatigue, pain or general unwellness that lasts for 24–48 hours or longer following even moderate exercise (3–7). Postexertional malaise has been reported to occur in greater than 90% of CFS patients and helps to differentiate CFS patients from patients with major depression and other disorders (5,6,8–11). The predominant symptoms reported by CFS patients at 24 and 48 hrs after exercise were exaggerated physical fatigue/tiredness, increased muscle or joint pain, mental fatigue/cognitive dysfunction, and general weakness/shakiness (5). In MS, postexertional worsening of symptoms is not as frequent, severe, or long-lasting (12–14). Nevertheless, since some MS patients do experience increased fatigue following exercise, using self report of post-exercise symptom severity by itself may not be sufficient to clearly separate MS from CFS patients. Use of objective post-exercise biomarkers together with self reported symptoms could potentially provide a better test of discrimination (4). One of the latest tools to examine dysregulation in multiple physiological pathways concurrently in human patients is to use gene expression markers on leukocytes; exercise–induced changes in gene expression thus may be viable biomarkers to compare in MS and CFS. Leukocyte gene expression (mRNA) changes in a response to a common life experience like moderate exercise are valuable since they reveal how environmental factors influence genetic susceptibility (DNA) to result in up-regulation or down-regulation of key receptors and other proteins, and also allow tests of the magnitude and duration of any expression changes through simple repeated blood sampling.
Previously, our research group (Light et al. 2009) found that after 25 minutes of moderate exercise, CFS patients but not healthy controls showed increases in expression of several genes that lasted for 48 hours and that correlated significantly with postexertional increases in severity of mental fatigue, physical fatigue and pain (15). CFS patients showed increased expression of purinergic 2 X (P2X4 and P2X5) receptors and acid sensing ion channel 3 (ASIC3) receptors that detect muscle metabolites. In mouse, these receptors act in concert with TRPV1 receptors (transient receptor potential vanilloid type 1) activated by heat, acid, or endocannabinoids to detect metabolites that cause muscle pain and fatigue (16). In addition to greater increases in metabolite-detecting receptors, CFS patients also had greater increases in both alpha- and beta-adrenergic receptors that help regulate blood flow to working muscles. Although this same study also examined gene expression of several immune markers, only the anti-inflammatory cytokine interleukin-10 (IL-10) had greater expression in CFS vs. the control group. When comparing with MS patients, however, the immune genes may be more informative. Pro-inflammatory cytokines have been implicated in the pathogenesis of MS (17–20) and are associated with higher levels of daily fatigue (21–22).
This investigation examined whether post-exercise changes in leukocyte gene expression profiles (including the same metabolite-detecting receptors, adrenergic receptors, and immune markers used previously for CFS) could differentiate MS patients from CFS patients and/or from healthy individuals (15). We employed a 25 min moderate exercise task to test age- and gender-matched patients with MS and CFS along with healthy controls. We compared gene expression in these three groups together with subjective ratings of mental fatigue, physical fatigue and pain at pre-exercise baseline and 4 post-exercise time points: 0.5 hr, 8 hr, 24 hr and 48 hr.
Methods
Participants
This research was approved by the University of Utah Institutional Review Board, and all participants provided written informed consent. CFS and MS patients were recruited from local clinical practices that specialized in these populations and were tested between July 2006 and November 2009. Twenty-two CFS patients (6 of these are the same patients from our previous publication (15)) who met the CDC criteria for CFS (1), 20 relapsing-remitting MS patients with self-reported fatigue and definite MS (23), and 23 healthy controls participated. MS patients completed the 40 item Fatigue Impact Scale (FIS) to confirm their classification as “fatigued” (24). For the CFS patients, symptom onset was reported as sudden by 18 patients (82%) and gradual by 4 patients (18%). Fifteen (68%) reported onset was associated with one or several flu-like, mononucleosis or other viral illnesses or infections. Two patients (9%) associated onset with traumatic injuries, and five (23%) associated onset with surgery, life stress, or no memorable event. The average length of illness for the CFS group was 11 yrs (range of 3–21 yrs). Eighteen CFS patients (82%) had widespread muscle and joint pain on Tender Point exam, meeting American College of Rheumatology criteria for fibromyalgia (FM). Half of these CFS+FM patients were tested on FM medications, including either pregabalin (Lyrica, n=3), gabapentin (Neurontin, n=3) or duloxetine (Cymbalta, n=3). Because sensory ion channel receptors and β-adrenergic receptors can contribute to inflammatory hyperalgesia (25–27), we performed secondary analyses specifically examining responses in this CFS+FM subgroup. Sixteen MS patients (80%) were receiving immune modulating medications (beta interferon [IFN-β] or glatiramer acetate [GA]; n=12), or immune suppressing medication (natalizumab; n=4). The average time since diagnosis for the MS group was 7 yrs (range of 1–24 yrs). The patients reflected the local population with 94% being Caucasian, 6% being minority; thus our results may not apply to minorities with CFS or MS.
Protocol Overview
All participants refrained from exercise for 4 days, beginning 48 hours before the exercise task and until after the final (48 hr) blood sample was taken. Venous blood samples were obtained at baseline and at 0.5, 8, 24, and 48 hours after exercise. Acute ratings of physical fatigue (PF), described as bodily tiredness; mental fatigue (MF), described as mental fogginess or problems with memory, attention, or organization of thoughts; and overall body pain (Pain) were obtained in conjunction with all blood draws and also at the midpoint and end of the exercise task. These ratings were based on a 0 to 100 scale, where 100 was defined as the greatest level of fatigue or pain the subject could ever imagine experiencing. Immediately after the baseline blood draw, participants began the exercise protocol, described below.
Exercise Protocol
The exercise task was designed such that each subject exercised at the same relative intensity; 70% of age-predicted maximal heart rate. Combined arm and leg exercise was performed on a Schwinn Air-Dyne ergometer. Target heart rate was reached during the first five minutes of exercise. Thereafter, work rate was adjusted to maintain this submaximal steady state for the remaining 20 minutes of exercise. Ratings of perceived exertion (RPE) were obtained on a scale of 1 to 10 every 5 minutes, heart rate was recorded each minute, and blood pressure was measured at baseline, every 10 minutes during exercise, and on completion of the exercise.
mRNA Extraction and Analysis
Standardized extraction of mRNA and real-time quantitative PCR methods were followed, as we have previously published (15). Briefly, blood samples were collected in EDTA tubes and centrifuged for 12 minutes at 3200 rpm. The white cell layer was very carefully removed and placed in RLT+bME solution (Qiagen) and immediately quick frozen in dry-ice methanol slurry, then transferred to storage in a −80 freezer. Contamination with erythrocytes was avoided as this greatly altered the quantity and integrity of the mRNA. Total time between drawing the blood sample to placement in disruption buffer (RLT+bME) and freezing was 20–25 minutes for all samples. Integrity of RNA as assessed by Agilent 2100 bioanalyzer was greater than 9.2 RIN. Total RNA was extracted using RNeasy kits and converted to a cDNA library using the ABI High Capacity cDNA Archive Kit (Applied Biosystems, Inc., Foster City, CA).
The cDNA was loaded by robot into 384 well plates and analyzed on the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Inc., Foster City, CA), using ABI TaqMan Master Mix (Applied Biosystems, Inc., Foster City, CA). Each sample was run in duplicate with standards run in quadruplicates, and with one “no template” control. Primer probes (all from TaqMan Gene Expression Assays, Applied Biosystems, Inc., Foster City, CA) were: ASIC3 - Hs00245097_m1; P2X4 - Hs00175706_m1; P2X5 - Hs00175712_m1; TRPV1 - Hs00218912_m1; Adrenergic α-2A - Hs00265081_s1; Adrenergic β-1 - Hs02330048_s1; Adrenergic β-2 - Hs00240532_s1; COMT - Hs00241349_m1; IL6 - Hs00174131_m1; IL10 - Hs00174086_m1; Lymphotoxin-α -Hs00236874_m1; TLR4 - Hs00152937_m1; CD14 - Hs00169122_g1. Control primer probes included TF2B - Hs00155321_m1; β-Actin - Hs99999903_m1; PSMB6 - Hs00382586_m1; 18S - 4333760T; and GAPDH - Hs99999905_m1.). Real-time PCR results were analyzed with SDS 2.1 (Applied Biosystems, Inc. [ABI], Foster City, CA) and analyzed according to the ddCT method described in ABI User Bulletin #2. Baseline levels for each gene were computed relative to TF2B, and these baselines were used as the comparator for all measures taken after the exercise period.
Statistical Analysis
The ddCT method for mRNA analysis creates a skewed distribution; therefore, gene expression data were log-transformed. Baseline differences between groups for mRNA, descriptive, and exercise variables were determined with 1-way ANOVA. To decrease the likelihood of obtaining false-positive results due to multiple comparisons, we pooled post-exercise responses into a variable labeled area under the curve (AUC). AUC variables were grouped into 3 categories (metabolite-detecting, adrenergic, and immune). Multivariate analyses of variance (MANOVAs) were used to examine group differences in each category. Significant group differences were noted for metabolite-detecting and adrenergic markers (p=.006; η2=.28 and p=.048; η2=.13, respectively). For immune markers, the directionality of responses varied within and between groups, and the MANOVA yielded a p-value of 0.17; η2=.12. For secondary analyses of the CFS+FM group, the AUC MANOVAs for sensory and adrenergic markers both remained significant (p=.002 and p<.047, respectively).
Three (group) by five (time) repeated measures (RM) ANOVA were used to determine differences for mRNA data, as well as fatigue and pain scores. Planned contrasts were built into the model to evaluate post-exercise responses relative to baseline. Group differences were detected using post hoc Dunnett analyses. When significant group effects or group by time interactions were observed, separate within group RM ANOVAs were used to determine significant post-exercise changes. Pearson r correlations were used to examine relationships between changes in fatigue and pain ratings, exercise variables, and gene expression measures. All data are presented as means and standard errors, with significance set at p<.05.
Results
Patient Characteristics and Exercise Task Performance
MS patients had mild to moderate disability as reflected by Expanded Disability Status Scale (EDSS) scores (mean = 2.0; range = 1.0 to 3.0) and disease duration (mean = 7 ± 1.4 yrs). High baseline levels of fatigue as assessed by the FIS (mean = 71.7 ± 6.3) were confirmed in this group. CFS patients had high underlying fatigue by definition, which was verified by baseline physical fatigue scores (mean = 35 ± 3.9). Descriptive characteristics and exercise task variables are presented in Table 1. All subjects completed the exercise task, and no differences in relative heart rate (percentage of age-predicted heart rate) were observed. However, the relative work rate necessary to achieve 70% age-predicted maximal heart rate was significantly lower in CFS patients compared to controls (p<.001). On average, CFS patients had a significantly lower absolute heart rate compared to controls (p=.04), although this difference was primarily due to 3 subjects who had blunted heart rate responses. In MS patients relative work rate was lower than controls, but not significantly (p=.095). Despite lower absolute work rates, both patient groups perceived the exercise to be significantly more difficult (‘moderate’ to ‘hard’) compared to controls’ (‘moderate’), as indicated by their RPEs (p<.001 and .017 for CFS and MS vs controls, respectively; Table 1).
Table 1.
Descriptive characteristics for control subjects, CFS patients, and MS patients.
| Controls (n=23; 19 F) | CFS Patients (n=22; 19F) | MS Patients (n=20; 18F) | P1 | η2 | |
|---|---|---|---|---|---|
| Age (y) | 38.7 ± 2.4 | 40.8 ± 2.0 | 41.5 ± 2.0 | 0.70 | .01 |
| Body mass index (kg/m2) | 24.3 ± 0.8 | 25.0 ± 1.2 | 27.6 ± 1.3 | 0.09 | .08 |
| Resting MAP (mm Hg) | 91 ± 2.0 | 94 ± 2.7 | 98 ± 3.2 | 0.17 | .06 |
| Exercise MAP (mm Hg) | 105 ± 2.5 | 108 ± 3.4 | 111 ± 3.3 | 0.50 | .03 |
| Exercise HR (bpm) | 130 ± 1.6 | 123 ± 3.1* | 127 ± 1.7 | 0.07 | .08 |
| Exercise HR (%PMHR) | 71.7 ± 0.7 | 68.5 ± 1.5 | 71.3 ± 0.8 | 0.07 | .08 |
| Exercise WR (kcal/kg/min) | 6.6 ± 0.4 | 4.6 ± 0.3* | 5.5 ± 0.4 | 0.001 | .21 |
| Exercise RPE (0–10 scale) | 3.4 ± 0.2 | 4.7 ± 0.3* | 4.4 ± 0.3* | 0.002 | .19 |
Values are mean ± SE
MAP = mean arterial pressure; HR = heart rate; PMHR = percent maximal heart rate; WR = work rate; RPE = rating of perceived exertion
p values calculated using oneway ANOVA
p<.05 vs controls, using post hoc Dunnett test
Acute Fatigue and Pain Ratings
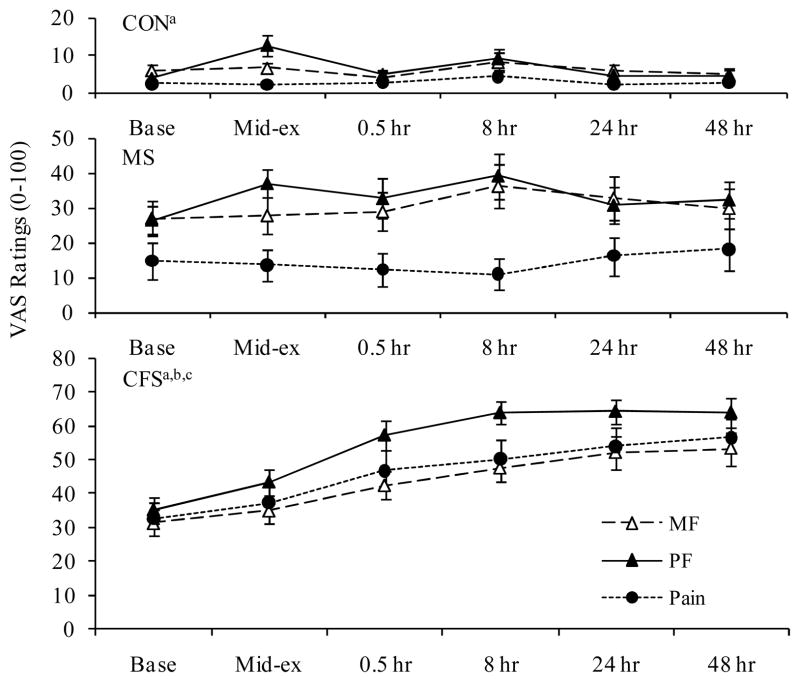
Ratings of mental fatigue (MF), physical fatigue (PF), and pain before, during, and following exercise are presented in Figure 1. At baseline, controls had significantly lower ratings for MF, PF, and pain than both CFS and MS patient groups (p<.001, except for controls vs MS patients for MF, p=.02). Additionally, baseline pain was significantly higher in CFS compared to MS (p=.02).
Figure 1.
Significant group by time interactions and time effects were observed for mental and physical fatigue and pain (p<.001). Analysis of the interactions revealed that both CFS and MS groups had significantly higher ratings of post-exercise MF, PF, and pain than controls (p<.001). Additionally, CFS patients had significantly higher post-exercise PF and pain ratings than MS patients (p<.002).
Within group time effects showed significant increases over time (RM ANOVA) for MF, PF, and Pain in CFS patients at all post-exercise time points, except for MF at mid-exercise. In controls, a significant within group time effect was noted for PF (p=.006) due to significant increases at mid-exercise. Within group time effects were not significant in MS patients; however, contrasts indicated significant increases in PF at mid-exercise (p=.001) and both MF and PF at 8 hrs post-exercise (p=.05 and .04, respectively). Although pain was always higher in the MS group compared to controls, neither group showed post-exercise increases in pain ratings.
Baseline Gene Expression
Baseline levels of metabolite-detecting, adrenergic, and immune genes (Table 2) were similar in all groups with the exception of adrenergic receptor α-2a. For this marker, MS patients had significantly higher baseline levels compared to CFS patients and controls (p=.04).
Table 2.
Baseline Mean ± SEM for All mRNAs Relative to TF2B
| Controls (n=23; 19 F) | CFS Patients (n=22; 19F) | MS Patients (n=20; 18F) | P1 | η2 | |
|---|---|---|---|---|---|
| Metabolite Detecting | |||||
| ASIC3 | 9.54E-03 ± 8.94E-04 | 8.26E-03 ± 1.19E-03 | 9.46E-03 ± 8.27E-04 | 0.59 | .01 |
| P2X4 | 1.99E-01 ± 1.84E-02 | 1.97E-01 ± 2.08E-02 | 1.70E-01 ± 1.08E-02 | 0.45 | .03 |
| P2X5 | 2.85E-01 ± 3.48E-02 | 2.68E-01 ± 3.07E-02 | 3.06E-01 ± 3.75E-02 | 0.74 | .02 |
| TRPV1 | 1.42E-02 ± 1.52E-03 | 1.28E-02 ± 1.41E-03 | 1.36E-02 ± 7.28E-04 | 0.74 | .01 |
| Adrenergic | |||||
| α-2a | 3.51E-03 ± 3.82E-04 | 3.11E-03 ± 5.28E-04 | 6.47E-03 ± 1.68E-03* | 0.04 | .12 |
| β-1 | 9.22E-03 ± 2.47E-03 | 2.31E-02 ± 1.17E-02 | 8.96E-03 ± 2.70E-03 | 0.30 | .04 |
| β-2 | 1.15E+00 ± 1.79E-01 | 8.85E-01 ± 7.92E-02 | 8.99E-01 ± 6.43E-02 | 0.22 | .04 |
| COMT | 1.89E-01 ± 1.36E-02 | 1.86E-01 ± 2.34E-02 | 2.19E-01 ± 2.05E-02 | 0.44 | .04 |
| Immune | |||||
| IL-6 | 5.58E-03 ± 2.55E-03 | 4.28E-03 ± 6.33E-04 | 4.98E-03 ± 9.28E-04 | 0.85 | .01 |
| IL-10 | 4.90E-03 ± 6.14E-04 | 3.56E-02 ± 2.84E-02 | 5.77E-03 ± 6.60E-04 | 0.34 | .03 |
| Lymphotoxin-α | 9.51E-02 ± 1.47E-02 | 9.62E-02 ± 2.31E-02 | 1.54E-01 ± 3.59E-02 | 0.20 | .05 |
| TLR4 | 4.84E-01 ± 1.27E-01 | 4.94E-01 ± 6.62E-02 | 3.98E-01 ± 5.59E-02 | 0.72 | .01 |
| CD14 | 1.74E+00 ± 1.46E-01 | 2.23E+00 ± 2.91E-01 | 2.09E+00 ± 2.32E-01 | 0.30 | .04 |
Table values are mRNA counts relative to control gene TF2B (mean ± SE)
p values calculated using oneway ANOVA
p<.05 vs controls, using post hoc Dunnett test
Gene Expression Responses to Exercise
Metabolite-detecting gene expression
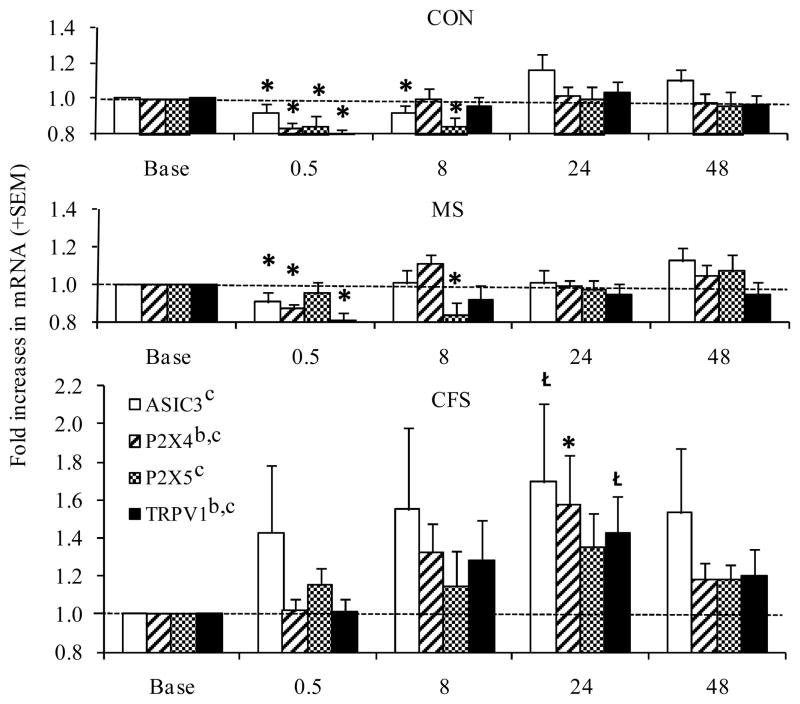
For all metabolite-detecting genes, the overall 3 by 5 RM ANOVAs indicated significant time effects (p=.044–.002; η2 = .01–.17). Additionally, significant group effects were observed for P2X4, in which CFS patients differed from both MS patients and controls, and for TRPV1, in which CFS patients differed from MS patients (p=.023–.009; η2 = .21–.14, see Figure 2). Within group analyses indicated decreases in ASIC3 (p=.05 and .02), P2X4 (p<.001), and TRPV1 (p=.001) at 30 min post-exercise and in P2X5 (p=.004 and .03) at 8 hrs post-exercise were significant for both controls and MS patients, respectively, while controls also had significant decreases in P2X5 at 30 min post-exercise (p=.005). In contrast, CFS patients showed a significant increase in expression of P2X4 (p=.03) and a trend toward increasing ASIC 3 (p=.06) and TRPV1 (p=.08) at 24 hrs post-exercise.
Figure 2.
Adrenergic receptor gene expression
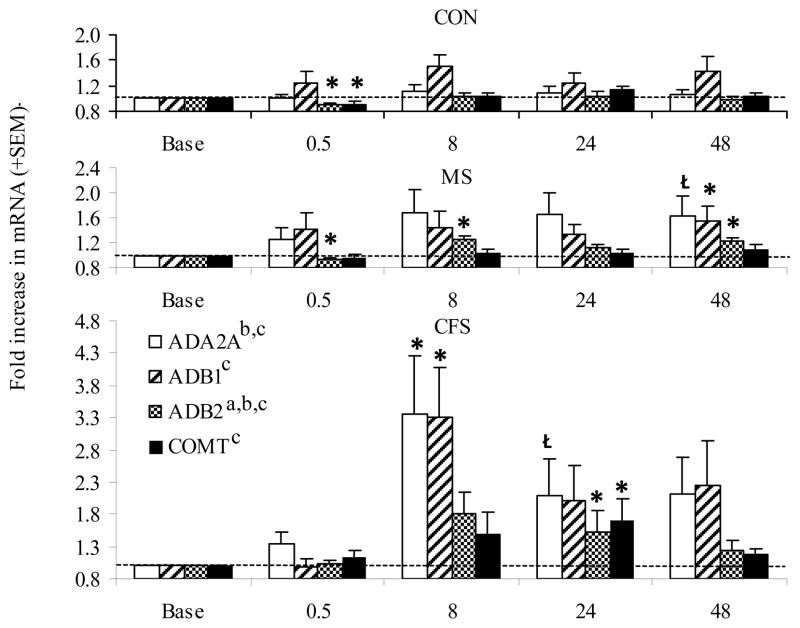
The overall 3 by 5 RM ANOVA analyses indicated significant group by time, time, and group effects for β-2 receptor expression (p=.02–.001; η2 = .10–.16, see Figure 3). In addition, significant overall time effects were observed for all adrenergic receptors (p=.035–.001; η2 = .04–.16). CFS and MS patients demonstrated post-exercise increases in adrenergic markers beginning at 8 hrs post-exercise and continuing through 24–48 hrs post-exercise. For MS, these included increases in β-2 expression at 8 and 48 hrs (p=.001 and .014, respectively) and increased β-1 at 48 hrs post-exercise (p=.02). In CFS, α-2a, β-1, and β-2 expression increased at 8 hrs post-exercise and remained elevated through 24 hrs post-exercise (p=.007–.04). COMT expression was also significantly increased at 24 hrs post-exercise in this group (p=.04). The only significant changes in adrenergic expression for controls were decreases in β-2 and COMT at 30 min post-exercise (p=.005 and .023, respectively).
Figure 3.
Immune marker gene expression
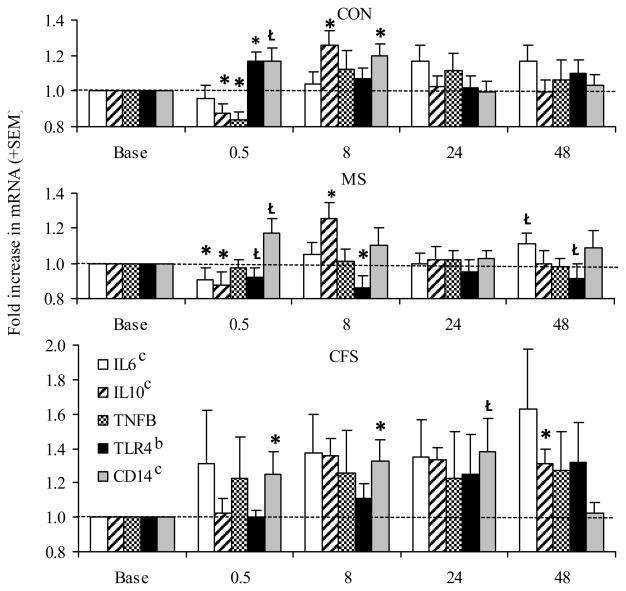
Significant overall time effects were observed for IL-6, IL-10, and CD14 (p=.025–.002; η2 = .05–.06), and a significant group effect was noted for TLR4 (p=.015; η2 = .14, see Figure 4). At 30 min post-exercise, controls showed significant decreases in IL-10 and Lymphotoxin-α and increases in CD14 (p=007–.001). At this time point, both patient groups differed from controls, with MS patients showing decreases in IL-6 (p=.04) and CFS patients showing a significant increase in CD14 (p=.04). Controls showed significant increases in IL-10 and CD14 at 8 hrs, mirrored by similar trends in the CFS and MS groups. Post-exercise TLR4 expression differed between MS vs. the CFS and control groups, with MS patients decreasing at all post-exercise time points while control and CFS responses were unchanged or increased (group effect, p=.015).
Figure 4.
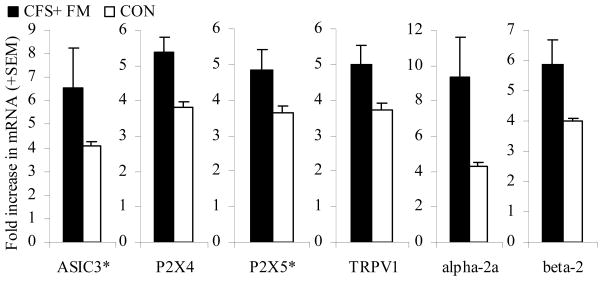
Secondary Analyses Focusing on the CFS+FM Subgroup
In the 18 CFS+FM patients, none of the original group differences were lost, and several additional differences attained or approached significance. Post-exercise increases in P2X4 AUC and TRPV1 AUC were significant (p < .0001 and p< .029) and approached significance for ASIC3 AUC and P2X4 AUC (p < .074 and .10, see Figure 5). Likewise, overall increases in post-exercise α-2a and β-2 receptor AUC were significant (p < .02 and .043). For ASIC3 and P2X5, differences from the MS group and from controls achieved formal significance at the 30 min and 24 hr time points (p<.05).
Figure 5.
Gene Expression Markers Linked to Greater Fatigue and Pain
The patient groups showed different relationships between subjective ratings of fatigue and pain relative to gene expression. Because fatigue and pain levels in controls were so low, these relationships were not examined separately in this group. Within the CFS group, greater pain ratings at 0.5, 8, and 24 hrs post-exercise were correlated with greater post-exercise P2X4 and α-2a adrenergic receptor expression (r = .45 to .47, p<.05), and greater MF at 8 hrs post-exercise was also associated with higher post-exercise P2X4 receptor expression (r = .49, p<.05).
Although MS patients had higher baseline α-2a expression than other groups, those with lower levels of this marker than other MS patients had greater increases in PF, MF, and pain at mid-exercise and at 0.5 and 8 hours post-exercise, and greater increases in pain at 24 hours post-exercise (r = −.46 to −.78, p <.05). For the immune measures, MS patients with lower post-exercise TLR4 AUC reported higher daily fatigue as determined by the FIS (r = −.52, p<.05), and those with lower IL-10 AUC reported greater fatigue at 24 hours post-exercise (r = −.74, p< .01). AUC IL-10 was also directly correlated with TLR4 (r = .52, p<.05). Immune responses differed as a function of treatment, with MS patients on immune-modulating and immune-suppressing treatments showing significantly lower post-exercise TLR4 expression than other patients.
Discussion
The main results of this study were that 1) CFS and MS patients had significantly higher baseline and post-exercise ratings of fatigue and pain compared to controls, 2) CFS patients demonstrated greater and longer lasting post-exercise increases in fatigue and pain (post-exertional malaise) compared to MS patients as well as controls, and 3) post-exercise gene expression patterns differentiated between CFS patients, MS patients, and controls, with increases in adrenergic receptors evident in both MS and CFS patients but not controls, while increases in metabolite-detecting sensory receptors were evident only in the CFS group.
This study confirmed that CFS patients experience greater and longer lasting postexertional malaise, reflected by increased PF, MF, and pain at all post-exercise times through 48 hrs, than MS patients or controls, supporting previous research (5,7,11,28). MS patients, who showed greater fatigue and pain than controls at baseline, experienced no increase in pain after exercise and reported increases in PF and MF only at the 8 hr post-exercise time.
Gene expression changes likewise began at 0.5 hrs and persisted for 48 hrs after the exercise task in CFS patients. For metabolite-detecting gene expression, the CFS group showed greater overall increases in P2X4 and TRPV1 across the 48 hr period and greater increases in P2X5 at 24 hrs compared to the MS group and controls. The CFS+FM group also showed greater overall post-exercise increases in ASIC3 receptor expression. Greater P2X4 expression was also directly associated with severity of postexertional fatigue and pain only in the CFS group and may be important for sustaining post-exertional malaise and reduced pain threshold observed in CFS patients (28–30). MS patients showed no increases in any of these metabolite detecting genes, nor were they correlated with greater fatigue in the MS group. Instead, controls and MS patients demonstrated decreases in expression of ASIC3, P2X4, P2X5 and TRPV1 receptors at 30 min post-exercise with P2X5 decreases persisting at 8 hrs. These changes returned to pre-exercise levels by 24 hrs. These transitory decreases in expression of these receptors after exercise may represent adaptive down-regulation in response to enhanced receptor activation. The full return to pre-exercise levels for all 4 receptors that was evident in both MS and control groups by 24 hrs later is also consistent with a well-regulated sensory pathway.
Recent basic research has indicated that ASIC and P2X receptors are in direct contact with each other, functioning as an interactive receptor complex, which may be further modulated by adrenergic receptor activity (31, 32). Because only the CFS patients showed increases in these metabolite-detecting receptors, the sensory receptor elements of this gene profile seem particularly specific to CFS and may reflect dysregulated pathways that directly contribute to increased effort sense during exercise and postexertional malaise. Jones et al (3) recently observed that CFS patients showed abnormalities in post-exercise recovery of intramuscular pH. They suggest two possible sources of this abnormality, both of which involve altered adrenergic influences: 1) changes in the adrenergic pathway influencing the sodium-proton antiporter function; 2) alterations in the adrenergic pathway influencing local vasodilation during and after muscle activity which serves to remove built-up protons and other metabolites (3). These mechanisms have yet to be confirmed. It is also possible that even “normal” metabolite levels produced during exercise in CFS patients can lead to increased expression of metabolite detecting genes, producing amplified sensations of effort during exercise and post-exercise malaise. Indeed, investigators have shown that in fitness and activity-matched CFS patients and controls, CFS patients demonstrated significantly higher RPE in response to a given heart rate or workload during incremental exercise (33–35).
In contrast to metabolite detecting receptor responses, post-exercise adrenergic receptor responses differentiated MS and CFS groups from controls. Both CFS and MS patients showed exaggerated post-exercise increases in adrenergic receptors, suggesting that adrenergic dysregulation may be linked to the pathological fatigue in both of these disorders. The MS group showed increases in β-2 receptor expression at 8 hrs post-exercise that coincided with increases in mental and physical fatigue. Interestingly both β-2 and β-1 receptor expression were increased in the MS group at 48 hrs, when their self reported fatigue ratings had returned to baseline levels. Like the MS group, CFS patients showed increases in β-2 receptors at 8 hrs post-exercise, but they also showed increases in α-2a and β-1 receptors at this time. These increases persisted through the 24 hr time point, when COMT expression was also increased. A growing body of evidence suggests that β-adrenergic receptors present on muscle sensory afferents can enhance metabolite signals, thereby increasing fatigue and pain sensations (36). β-2 and α-2a receptors are important in directing blood flow to working muscles during exercise to provide O2 and to remove metabolites. They also may mediate SNS effects on the immune system (37, 38). Numerous prior investigations have indicated autonomic dysregulation in patients with CFS, FM, or both disorders together, indexed by abnormal responses to orthostatic challenges, decreased heart rate variability, or altered plasma catecholamine and cardiovascular responses to stressors (39–41). A genetic diplotype influencing COMT function has been related to greater delayed onset muscle pain following exercise (42). Also, increases in another biomarker from the noradrenergic pathway, neuropeptide Y, have previously been shown to differentiate CFS patients from controls (43). Autonomic dysregulation in MS is also commonly reported and has been attributed to lesions that affect brain and spinal cord areas that regulate autonomic function (44).
Immune pathways may also contribute to debilitating fatigue in both MS and CFS, but in different ways. In this study, the MS group showed abnormally reduced expression of TLR4 relative to controls both 0.5 and 8 hrs after exercise, suggesting decreased generalized immune activity, while the CFS group had abnormally high IL-10 increases at 48 hrs, indicating prolonged anti-inflammatory activity which could enhance vulnerability to viruses and opportunistic infections. Recent studies have indicated that exercise may induce complement activation in CFS linked to their post-exertion symptom severity (4), and that complement cascade-related proteins obtained from cerebrospinal fluid may be useful as diagnostic biomarkers of this disorder (45). This latter study also indicated that proteins from the cyclin dependent kinase 5 (CDK5) pathway are some of the most specific markers for CFS; this kinase may modulate sensory signaling of pain and possibly fatigue, and may be linked to modulation of TRPV1 and other sensory receptors (46). However, Nijs and colleagues (11) observed post-exertional malaise in a sample of CFS patients following submaximal exercise without complement activation. The discrepancy in results may be related to the intensity and duration of exercise or to the location and timing of sample acquisition. For example, Yoshiuchi (47) and colleagues report that following a graded maximal exercise test, CFS patients do not show symptom worsening until 5 days following the stressor, illustrating that maximal and submaximal exercise may produce different responses.
Although a primary intent of disease modifying treatments in MS is to reduce pro-inflammatory activity, there were no significant baseline differences between MS patients and controls on any specific pro-inflammatory marker. However, MS patients demonstrated lower overall post-exercise immune activity reflected by lower TLR4 expression. The observation that lower TLR4 expression was associated with higher baseline fatigue was surprising, because TLR signaling controls innate immune responses which ultimately promote proinflammatory activity, and TLRs have been implicated in MS (48). Further, researchers have shown that nonresponders to the immune-modulating drug IFN-β have overexpression in toll-like receptor signaling pathways (49). Post hoc analyses of MS treatment subgroups showed that MS patients receiving immune-modulating or immune-suppressing treatments had significantly lower TLR4 and IL-10 expression compared to both controls and MS patients not receiving treatment. As noted by Marta (50), TLR4-dependent production of proinflammatory cytokines is regulated by mechanisms that dampen the pathway and prevent excess damage in animal models of MS. Treatments that decrease TLR4 may reduce pro-inflammatory T-helper (Th) 1 cell responses but could also interfere with these beneficial regulatory mechanisms or with anti-inflammatory Th2 immune responses. Thus, our data support previous observations that disease modifying treatments that primarily target and reduce pro-inflammatory responses may not necessarily ameliorate fatigue (51).
Because our MS treatment subgroups were so small, any observations specific to treatments should be viewed with caution. Without a within subject comparison of gene expression responses before vs. after beginning disease modifying treatment, we cannot be certain that the treatment regimens contribute to the adrenergic or immune responses that were linked to greater fatigue in MS patients. It would be beneficial to examine patients longitudinally to determine whether these biomarkers are responsive to changes in fatigue levels and disease status.
This study suggests interventions to reduce pathological fatigue in CFS and MS. For both disorders, the sympathetic-immune interface may be a target for intervention. Alterations in β-adrenergic activity have been shown to affect production of both pro- and anti-inflammatory cytokines (37, 38), and may also modulate pain pathways (39). Use of adrenergic agonists and antagonists, or behavioral changes such as carefully graded exercise training may be useful. Light and colleagues (36) have shown that the β-adrenergic antagonist propranolol, when administered in a dose too low to alter mean arterial blood pressure, reduced clinical pain severity in patients with fibromyalgia including those who had comorbid CFS. For CFS patients, studies on the use of carefully prescribed graded exercise training are in progress, but early results indicate that post-exercise increases in pain and/or fatigue are not prevented (7, 30). Kop et al (52) observed that higher ambulatory ratings of pain and fatigue resulted in reduced subsequent physical activity in CFS patients; thus, post-exertional worsening of symptoms make it less likely that patients will engage in further exercise, and this may contribute to drop-out and failures in exercise interventions for this disorder. For individuals with MS, evidence indicates that regular exercise is beneficial for improving fitness and reducing fatigue (9–11, 53). In CFS, the metabolite–detecting ion channel receptors also provide a potential target for treatments, although safe and selective pharmacological interventions for these receptors still need to be developed.
Conclusion
Although both CFS and MS patients reported greater physical and mental fatigue than controls, the CFS group reported more fatigue and pain at pre-exercise than the MS group, and their postexertional symptoms increased more and were still increased after 48 hrs while the MS group recovered by 24 hrs. Gene expression of metabolite-detecting receptors increased following exercise only in the CFS group, and only in this group was there a correlation between P2X4 increases and severity of post-exercise fatigue and pain. Thus, the pathology of CFS may include a susceptibility to disproportionate fatigue in response to exercise stress that is uniquely expressed in this patient group. The pattern of gene expression may have potential for use as a biomarker for diagnosis and treatment responses. Abnormal increases in adrenergic receptors following exercise in both MS and CFS patients suggest that dysregulation in sympathetic pathways contributes to the exaggerated fatigue in both conditions. In MS, reduced expression of immune markers TLR4 and IL10 following exercise was also associated with greater fatigue, which may be an unintended effect of therapies targeting immune activity.
Acknowledgments
This investigation was supported by grants from the National Multiple Sclerosis Society (ATW) and the CFIDS Association (ARL), and by NIH Grant R21 NS057821 from NINDS and NIAMS (KCL).
Acronyms used
- MS
multiple sclerosis
- CFS
chronic fatigue syndrome
- ASIC3
acid sensing ion channel receptor-3
- P2X4
purinergic type 2X4 receptor
- P2X5
purinergic type 2X5 receptor
- TRPV1
transient receptor potential vanilloid type 1
- TLR4
toll-like receptor-4
- COMT
catechol-o-methyl-transferase
- IL
interleukin
- PCR
polymerase chain reaction
- AUC
area under the curve
- MAP
mean arterial pressure
- HR
heart rate
- WR
work rate
- RPE
rating of perceived exertion
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994 doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Kos D, Kerckhofs E, Nagels G, D’hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: Review of the literature. Neurorehabil Neural Repair. 2008;22:91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 3.Jones DE, Gray J, Frith J, Newton JL. Fatigue severity remains stable over time and independently associated with orthostatic symptoms in chronic fatigue syndrome: a longitudinal study. J Intern Med. 2010;267:394–401. doi: 10.1111/j.1365-2796.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen B, Jones JF, Vernon SD, Rajeevan MS. Transcriptional control of complement activation in an exercise model of chronic fatigue syndrome. Mol Med. 2009;15:34–42. doi: 10.2119/molmed.2008.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Ness JM, Stevens SR, Bateman L, Stiles TL, Snell CR. Postexertional malaise in women with chronic fatigue syndrome. J Women’s Health. 2010;19:239–244. doi: 10.1089/jwh.2009.1507. [DOI] [PubMed] [Google Scholar]
- 6.Van Oosterwijck J, Nijs J, Meeus M, Lefever I, Huybrechts L, Lambrecht L, Paul L. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: an experimental study. J Intern Med. 2010;268:265–278. doi: 10.1111/j.1365-2796.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 7.Twisk FN, Maes M. A review on cognitive behavioral therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuro Endocrinol Lett. 2009;30:284–99. [PubMed] [Google Scholar]
- 8.Hawk C, Jason LA, Torres-Harding S. Differential diagnosis of chronic fatigue syndrome and major depressive disorder. Int J Behav Med. 2006;13:244–251. doi: 10.1207/s15327558ijbm1303_8. [DOI] [PubMed] [Google Scholar]
- 9.Knoop H, Bleijenberg G, Gielissen MF, van der Meer JW, White PD. Is a full recovery possible after cognitive behavioural therapy for chronic fatigue syndrome? Psychother Psychosom. 2007;76:171–176. doi: 10.1159/000099844. [DOI] [PubMed] [Google Scholar]
- 10.Jason LA, Porter N, Hunnell J, Brown A, Rademaker A, Richman JA. A natural history study of chronic fatigue syndrome. Rehab Psychol. 2011;56:32–42. doi: 10.1037/a0022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nijs J, Van Oosterwijck J, Meeus M, Lambrecht L, Metzger K, Fremont M, Paul L. Unravelling the nature of postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: the role of elastase, complement C4a and interleukin-1beta. J Intern Med. 2010;267:418–35. doi: 10.1111/j.1365-2796.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 12.White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Med. 2004;34:1077–1100. doi: 10.2165/00007256-200434150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Mostert S, Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception, and activity levels of subjects with multiple sclerosis. Mult Scler. 2002;2:161–168. doi: 10.1191/1352458502ms779oa. [DOI] [PubMed] [Google Scholar]
- 14.Smith C, Hale L, Olson K, Schneiders AG. How does exercise influence fatigue in people with multiple sclerosis? Disabil Rehabil. 2009;31:685–692. doi: 10.1080/09638280802273473. [DOI] [PubMed] [Google Scholar]
- 15.Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10:1099–1112. doi: 10.1016/j.jpain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Light AR, Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2009;100(3):1184–201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heesen C, Nawrath L, Reich C, Bauer N, Schulz K-H, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77:34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez Filho JA, Vedeler CA, Myhr KM, Nyland H, Pandey JP. TNF-alpha and –beta gene polymorphisms in multiple sclerosis: a highly significant role for determinants in the first intron of the TNF-beta gene. Autoimmunity. 2002;35:377–80. doi: 10.1080/0891693021000021549. [DOI] [PubMed] [Google Scholar]
- 19.Glabinski AR, Bielecki B, Ransohoff RM. Chemokine upregulation follows cytokine expression in chronic relapsing experimental autoimmune encephalomyelitis. Scand J Immunol. 2003;58:81–88. doi: 10.1046/j.1365-3083.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 20.Lock C, Oksenberg J, Steinman L. The role of TNFa and lymphotoxin in demelinating disease. Ann Rheum Dis. 1999;58(Suppl I):I121–I128. doi: 10.1136/ard.58.2008.i121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombo B, Martinelli Boneschi F, Rossi P, Rovaris M, Maderna L, Filippi M, Comi G. MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol. 2000;247:506–9. doi: 10.1007/s004150070148. [DOI] [PubMed] [Google Scholar]
- 22.Flackenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler. 2004;10:165–169. doi: 10.1191/1352458504ms991oa. [DOI] [PubMed] [Google Scholar]
- 23.Poser CM. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 24.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 25.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Vierck CJ, Yezierski RP, Light AR. Long-lasting hyperalgesia and sympathetic dysregulation after formalin injection into the rat hind paw. Neruosci. 2008;153:501–506. doi: 10.1016/j.neuroscience.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Walder RY, Rasmussen LA, Ranier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injection. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Oosterwijck J, Nijs J, Meeus M, Lefever I, Huybrechts L, Lambrecht L, Paul L. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: an experimental study. J Intern Med. 2010;268:265–78. doi: 10.1111/j.1365-2796.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 29.Meeus M, Roussel NA, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehab Med. 2010;42:884–90. doi: 10.2340/16501977-0595. [DOI] [PubMed] [Google Scholar]
- 30.Nijs J, Almond F, De Becker P, Truijen S, Paul L. Can exercise limits prevent post-exertional malaise in chronic fatigue syndrome? An uncontrolled clinical trial. Clin Rehabil. 2008;22:426–35. doi: 10.1177/0269215507084410. [DOI] [PubMed] [Google Scholar]
- 31.Meisner JG, Reid AR, Sawynok J. Adrenergic regulation of P2X3 and TRPV1 receptors: differential effects of spared nerve injury. Neurosci Lett. 2008;444:172–5. doi: 10.1016/j.neulet.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–49. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook DB, Nagelkirk PR, Poluri A, Mores J, Natelson BH. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis Rheum. 2006;54:3351–62. doi: 10.1002/art.22124. [DOI] [PubMed] [Google Scholar]
- 34.Gibson H, Carroll N, Clague JE, Edwards RH. Exercise performance and fatiguability in patients with chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1993;56:993–8. doi: 10.1136/jnnp.56.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallman KE, Morton AR, Goodman C, Grove R. Physiological responses during a submaximal cycle test in chronic fatigue syndrome. Med Sci Sports Exerc. 2004;36:1682–8. doi: 10.1249/01.mss.0000142406.79093.90. [DOI] [PubMed] [Google Scholar]
- 36.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–552. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elenkov IJ. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 38.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve: An integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 39.Light KC, Vierck CJ. HPA Axis and sympathetic influences on pain and fatigue in fibromyalgia, chronic fatigue, and overlapping functional pain syndromes. In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes: Presentation and Pathophysiology. IASP Press; Seattle, WA: 2009. [Google Scholar]
- 40.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum. 2000;29:217–27. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 41.Furlan R, Colombo S, Perego F, Atzeni F, Diana A, Barbic F, Porta A, Pace F Mulliani A, Sarzi-Puttini P. Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J Rheumatol. 2005;32:1787–93. [PubMed] [Google Scholar]
- 42.George SZ, Dover GC, Wallace MR, Sack BK, Herbstman DM, Aydog E, Fillingim RB. Biopsychosocial influence on exercise-induced delayed onset muscle soreness: catatrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24:793–801. doi: 10.1097/AJP.0b013e31817bcb65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fletcher MA, Rosenthal M, Antoni M, Ironson G, Zeng XR, Barnes Z, Harvey JM, Hurwitz B, Levis S, Broderick G, Klimas NG. Behav Brain Functions. 2010;6:76. doi: 10.1186/1744-9081-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haensch CA, Jorg J. Autonomic dysfunction in multiple sclerosis. J Neurol. 2006;253(Suppl 1):I3–9. doi: 10.1007/s00415-006-1102-2. [DOI] [PubMed] [Google Scholar]
- 45.Schutzer SE, Angel TE, Liu T, Schepmoes AA, Claus TR, Adkins JN, Camp DG, II, Holland BK, Bergquist J, Coyle PK, Smith RD, Fallon BA, Natelson BH. Distinct cerebrospinal fluid proteomes differentiate post-treatment Lyme disease from chronic fatigue syndrome. PLoS One. 2011;6(2):e17287. doi: 10.1371/journal.pone.0017287. 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pareek TK, Keller J, Kesavapany S, Agarwal N, Kuner R, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2007;104:660–5. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marta M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann N Y Acad Sci. 2009;1173:458–62. doi: 10.1111/j.1749-6632.2009.04849.x. [DOI] [PubMed] [Google Scholar]
- 47.Yoshiuchi K, Cook DB, Ohashi K, Kumano H, Kuboki T, Yamamoto Y, Natelson BH. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiol Behav. 2007;92:963–8. doi: 10.1016/j.physbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Downer EJ, Clifford E, Gran B, Nel HJ, Fallon PG, Moynagh PN. Identification of the synthetic cannabinoid R(+)WIN55,212–2 as a novel regulator of IFN regulatory factor 3 activation and IFN-beta expression: relevance to therapeutic effects in models of multiple sclerosis. J Biol Chem. 2011;286:10316–28. doi: 10.1074/jbc.M110.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comabella M, Lunemann JD, Rio J, Sanchez A, Lopez C, Julia E, Fernandez M, Nonell L, Camina-Tato M, Deisenhammer F, Caballero E, Tortola MT, Prinz M, Montalban X, Martin R. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132:3353–65. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- 50.Marta M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann N Y Acad Sci. 2009;1173:458–62. doi: 10.1111/j.1749-6632.2009.04849.x. [DOI] [PubMed] [Google Scholar]
- 51.Yildiz M, Tettenborn B, Putzki N. Multiple sclerosis-associated fatigue during disease-modifying treatment with Natalizumab, Interferon-Beta and Glatiramer Acetate. Eur Neurol. 2011;65:231–232. doi: 10.1159/000324028. [DOI] [PubMed] [Google Scholar]
- 52.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 53.Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39(4):432–41. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]