Abstract
Background
Cardiosphere-derived cells (CDCs) are an attractive cell type for tissue regeneration, and autologous CDCs are being tested clinically. However, autologous therapy necessitates patient-specific tissue harvesting and cell processing, with delays to therapy and possible variations in cell potency. The use of allogeneic CDCs, if safe and effective, would obviate such limitations. We compared syngeneic and allogeneic CDC transplantation in rats from immunologically-mismatched inbred strains.
Methods and Results
In vitro, CDCs expressed MHC class I but not class II antigens or B7 costimulatory molecules. In mixed lymphocyte co-cultures, allogeneic CDCs elicited negligible lymphocyte proliferation and inflammatory cytokine secretion. In vivo, syngeneic and allogeneic CDCs survived at similar levels in the infarcted rat heart 1 week after delivery, but few syngeneic (and even fewer allogeneic) CDCs remained at 3 weeks. Allogeneic CDCs induced a transient, mild, local immune reaction in the heart, without histologically-evident rejection or systemic immunogenicity. Improvements in cardiac structure and function, sustained for 6 months, were comparable with syngeneic and allogeneic CDCs. Allogeneic CDCs stimulated endogenous regenerative mechanisms (cardiomyocyte cycling, recruitment of c-kit+ cells, angiogenesis) and increased myocardial VEGF, IGF-1 and HGF equally with syngeneic CDCs.
Conclusions
Allogeneic CDC transplantation without immunosuppression is safe, promotes cardiac regeneration and improves heart function in a rat myocardial infarction model, mainly through stimulation of endogenous repair mechanisms. This indirect mechanism of action rationalizes the persistence of benefit despite the evanescence of transplanted cell survival. This work motivates the testing of allogeneic human CDCs as a potential off-the-shelf product for cellular cardiomyoplasty.
Keywords: cardiac stem cells, allogeneic cell therapy, myocardial regeneration, paracrine effects
Cell transplantation has emerged as a promising therapeutic strategy for acute or chronic ischemic cardiomyopathy1,2. Multiple candidate cell types have been used in humans in efforts to repair or regenerate the injured heart either directly (through formation of new transplanted tissue) or indirectly, including skeletal myoblasts, bone marrow derived cells and, more recently, heart-derived cells2,3. During the first decade of cell therapy for heart disease, the vast majority of clinical trials were conducted using autologous cells. This approach avoids immunologic rejection, but necessitates patient-specific tissue harvesting, cell processing and quality control, imposing significant logistic, economic, and timing constraints. In addition, cell efficacy may be undermined by donor age and comorbidities4. The use of allogeneic cells, if safe and effective, would obviate such limitations, enabling the generation of highly-standardized “off the shelf” cell products. The obvious disadvantage is the risk of immune rejection, which may limit effectiveness whether or not it poses safety hazards. Nevertheless, since the vast majority of the observed functional benefit is attributable to indirect pathways even with heart-derived cells5,6, rejection of allogeneic cells may not be an issue if it occurs after the cells have exerted their beneficial paracrine effects and if the resulting benefits are durable.
Here we tested the hypothesis that allogeneic cardiosphere-derived cells (CDCs) are hypoimmunogenic and mobilize pathways of endogenous repair and regeneration, resulting in sustained functional benefit. For the first time, we: a) characterize the in vitro immunologic properties of heart-derived stem cells and b) monitor host immune system kinetics (leukocyte infiltration, inflammatory cytokine secretion, development of cellular/humoral memory response), and transplanted cell survival, and c) quantify functional effects post-myocardial infarction (MI) in an immunologically-mismatched rat model of allogeneic CDC transplantation.
Methods
An expanded Methods section is available in the Online Data Supplement
Experimental animals
To create a stringent model of allogeneic cell transplantation, we used rats from highly-inbred, immunologically-divergent strains, characterized by complete mismatch of major histocompatibility complex (MHC) antigens. Male Wistar Kyoto (WKY) rats (MHC haplotype: RTIl) were used as CDC donors, while female WKY and Brown Norway (BN) rats (MHC haplotype; RTIn) were used as syngeneic and allogeneic recipients respectively. In a model of xenogeneic transplantation, used as a positive control for immune rejection, human CDCs were transplanted into BN rats. Sample sizes for each experiment are listed in Supplemental Table 1. All experimental protocols were approved by the Institutional Animal Care and Use Committee.
Cell culture
Rat CDCs (rCDCs) were expanded from 8-week old male WKY rat hearts. Human CDCs (hCDCs) were expanded from endomyocardial biopsies or myocardial samples, obtained from adult male patients during clinically-indicated procedures after informed consent. Patient characteristics are presented in Supplemental Table 2. CDCs were cultured as described7,8. All experiments were performed with CDCs at passage 1. In a subset of experiments, CDCs were lentivirally-transduced to express green fluorescent protein (GFP), to track transplanted cell fate by histology.
Flow cytometry
Flow cytometry was performed to evaluate surface expression of MHC class I, class II and costimulatory molecules (CD80, CD86) in hCDCs and rCDCs, under baseline conditions and after stimulation with interferon-γ. In addition, we characterized the general phenotype of CDCs (expression of CD105, c-Kit, CD90, CD31, CD45, CD140b, discoidin domain-containing receptor 2 [DDR2] and α-smooth muscle actin [αSMA]; antibodies listed in Supplemental Table 3).
Mixed-lymphocyte reactions
The in vitro immunogenicity of CDCs was assessed by one-way mixed lymphocyte reactions (MLRs), Mitomycin-inactivated stimulating rCDCs and hCDCs were cocultured with responder lymphocytes for 5 days. Responder cell proliferation was assessed by BrdU incorporation. The following experimental conditions were tested: a) rCDCs cocultured with WKY lymphocytes (syngeneic coculture); b) rCDCs cocultured with BN lymphocytes (allogeneic coculture); c) hCDCs cocultured with BN lymphocytes (xenogeneic coculture). Alloreactive and xenoreactive lymphocyte proliferation is presented as relative proliferative response, normalized to syngeneic lymphocyte proliferation (stimulation index). The cell-free supernatant of the cocultures was collected and the levels of secreted IFN-g, IL-1b, IL-13, IL-4, IL-5, KC/GRO, TNF-a and IL-2 were measured by electrochemiluminescence and enzyme-linked immunosorbent assay (ELISA).
Myocardial infarction and cell injection
Female WKY and BN rats (8–10 week old) underwent permanent ligation of the left anterior descending coronary artery. CDCs (2 million, suspended in 120 μl of phosphate-buffered saline [PBS]) or vehicle were intramyocardially injected at 4 sites along the periphery of the infarct. Five permutations were investigated: a) rCDCs injected into WKY hearts (syngeneic group); b) rCDCs injected into BN hearts (allogeneic group); c) hCDCs injected into BN hearts (xenogeneic group); d) vehicle injected into WKY hearts (control group a); e) vehicle injected into BN hearts (control group b). Two control groups were used in order to confirm that both rat strains respond similarly to MI. Data for peri-operative and longer-term mortality are presented in Supplemental Table 4. To monitor proliferation of both transplanted and endogenous cells, a subset of animals was intraperitoneally-injected with BrdU daily for either the first week or the second and third week post-MI.
Echocardiography
Echocardiography was performed to assess global cardiac function 6 hours (baseline), 3 weeks, 3 months and 6 months after surgery. Fractional area change (FAC), left ventricular ejection fraction (LVEF) and fractional shortening (FS) were measured.
Quantification of engraftment by real time PCR
To monitor transplanted cell survival 1 and 3 weeks post-MI, male cells were injected into female rats and absolute cell engraftment was quantified using species-specific SRY gene primers.
Histology
Rats were sacrificed 1 week, 3 weeks and 6 months after treatment. Hearts were cryo-sectioned and fixed with 4% paraformaldehyde. Quantitative morphometric analysis with Masson’s trichrome staining was performed to quantify scar size, infarcted wall thickness and LV remodeling. To evaluate immune rejection, sections stained with hematoxylin and eosin were evaluated in a blinded manner by a cardiac pathologist (D.L); in addition, immunostaining against immune cell markers was performed. Differentiation of CDCs, incidence of cycling host myocytes, recruitment of endogenous progenitors and vessel density in the border zone were evaluated by immunohistochemistry (antibodies listed in supplemental table 3).
Assessment of systemic immunogenicity and development of memory immune response
To assess systemic immunogenicity, levels of circulating inflammatory cytokines (IFN-g, IL-1b, IL-13, IL-4, IL-5, KC/GRO and TNF-α) were quantified by electrochemiluminescence in rat sera from recipients of syngeneic, allogeneic, xenogeneic CDCs and controls.
To assess humoral memory immune response, recipient rat sera were isolated 1 and 3 weeks post-transplantation and levels of circulating alloreactive and xenoreactive anti-donor IgG and IgM antibodies were quantified by flow cytometry.
To evaluate cellular memory immune response, spleens from allogeneic recipients were harvested 3 weeks post-transplantation. Lymphocytes were isolated and their reactivity against allogeneic donor cells by one-way MLRs was compared to that of naïve lymphocytes. The cell-free supernatant of the cocultures was collected and the levels of secreted IFN-g, IL-1b, IL-13, IL-4, IL-5, KC/GRO, TNF-α and IL-2 were measured by electrochemiluminescence and ELISA.
Western Blotting
Western blot analysis was performed to compare myocardial levels of VEGF, IGF-1 and HGF at various time points post-MI in rat hearts from syngeneic, allogeneic, xenogeneic and control groups. Myocardial samples from the peri-infarct area were collected 5 minutes, 1 day, 4 days, 7 days and 21 days post-MI. Protein was extracted and western blots performed as described5 with antibodies listed in supplementary table 3.
Statistical analysis
Results are presented as means ± SEM. Normality of data was tested using the Shapiro-Wilk Test and equality of variances was tested using Levene’s Test. If normality of data and equality of variances were established, statistical significance was determined by one-way ANOVA followed by the Bonferroni post hoc test. If normality of data or equality of variances could not be confirmed, statistical significance was determined by the Kruskal-Wallis test followed by Dunns post hoc test. Linear mixed effects models were used to compare the repeated measurements of cardiac function across groups. The outcome was the dependent variable, treatment group and time were the fixed effects and an unstructured trend in time was assumed. Correlation in data from the same animal was taken into account by a random effect at the rat level. Categorical data were tested using Fisher’s exact test. Differences between 2 groups were tested using the Mann-Whitney U Test. Differences were considered significant when p<0.05.
Results
Characterization of CDC antigens including MHC and costimulatory molecules
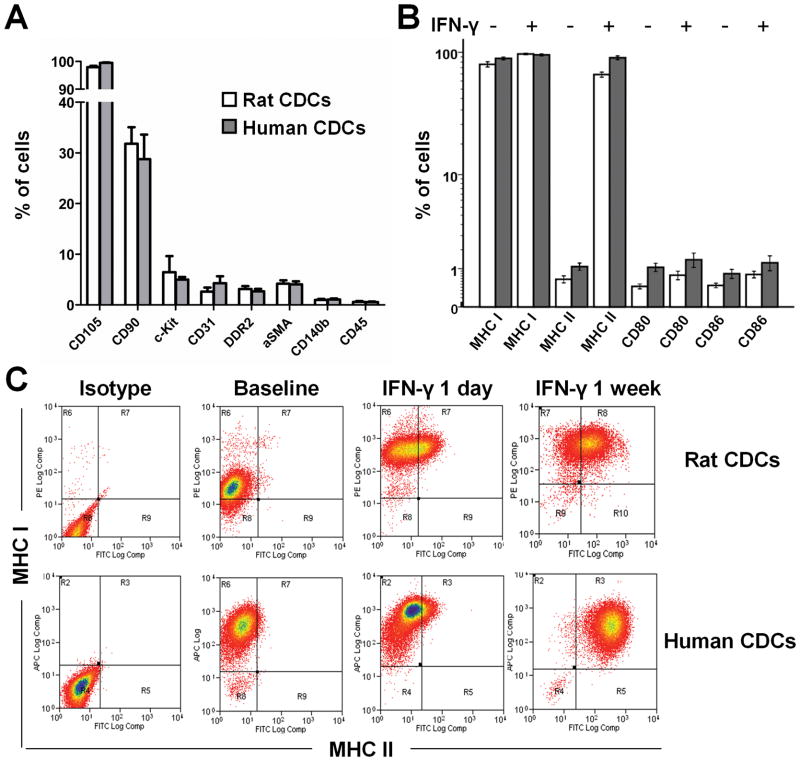
Consistent with previous characterizations7,9, flow cytometry revealed that both rCDCs and hCDCs are naturally-heterogeneous cell populations of non-hematological origin (CD45−), positive for CD105; subgroups positive for c-kit or CD90 are consistent with cardiac progenitor and cardiac mesenchymal fractions, respectively, while <4% of cells are positive for fibroblast (DDR2) or myofibroblast (αSMA) markers (Fig. 1A). With regard to immune antigens, both rCDCs and hCDCs express MHC I but not MHC class II surface antigens, or CD80/CD86 costimulatory molecules, under baseline conditions (Fib 1B). Incubation with interferon-γ upregulated MHC I and MHC II expression (but not costimulatory molecule expression) in a time-dependent manner (Fig. 1B, 1C). The observed baseline immunophenotype of CDCs renders them attractive for allogeneic applications. Expression of MHC class I antigens is important because it protects cells from natural-killer cell-mediated deletion10, while lack of expression of MHC class II antigens allows CDCs to escape direct recognition from CD4+ T helper cells. MHC class I antigens may activate effector T cells, but, in the absence of costimulatory molecules, a secondary signal would not engage, theoretically leaving T cells anergic11.
Figure 1.
Phenotypic characterization of rat and human CDCs by flow cytometry. (A) Antigenic profiles of CDCs (n=4–5/group). (B) Immunophenotype of CDCs under baseline conditions and after IFN-γ stimulation (n=4–5/group). (C) CDCs at baseline express MHC class I but not MHC class II antigens. Incubation with interferon-γ upregulates expression of MHC class I and class II antigens in a time-dependent manner.
Allogeneic CDCs exhibit negligible in vitro immunogenicity
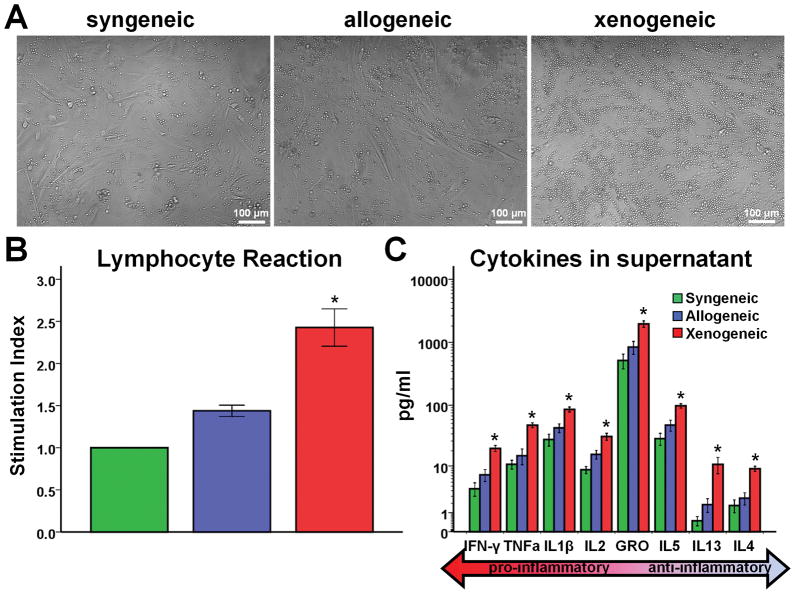
One-way MLR experiments revealed that allogeneic rCDCs elicit negligible lymphocyte proliferation, comparable to that seen with syngeneic CDCs. On the other hand, xenogeneic hCDCs induce a strong proliferative response (Fig 2A, 2B). Levels of pro-inflammatory (IFN-γ, TNF-a, IL1b, IL2, KC/GRO) and anti-inflammatory (IL5, IL13, IL4) cytokines were comparable in syngeneic and allogeneic coculture supernatants. Conversely, in the xenogeneic setting, secretion of all inflammatory cytokines was markedly increased, indicating significant activation of responder lymphocytes (Fig 2C).
Figure 2.
Assessment of immunogenicity of CDCs in vitro. (A) Representative images of syngeneic, allogeneic and xenogeneic cocultures. Significant lymphocyte proliferation can be observed in the xenogeneic setting. Quantitative analyses of (B) responder cell proliferation (n= 6–8/group) and (C) inflammatory cytokine secretion (n=21–26/group) demonstrate that allogeneic CDCs, contrary to xenogeneic, exhibit negligible functional immunogenicity in vitro. (* p<0.05 vs. syngeneic, allogeneic groups)
Limited survival of allogeneic and syngeneic CDCs post-transplantation
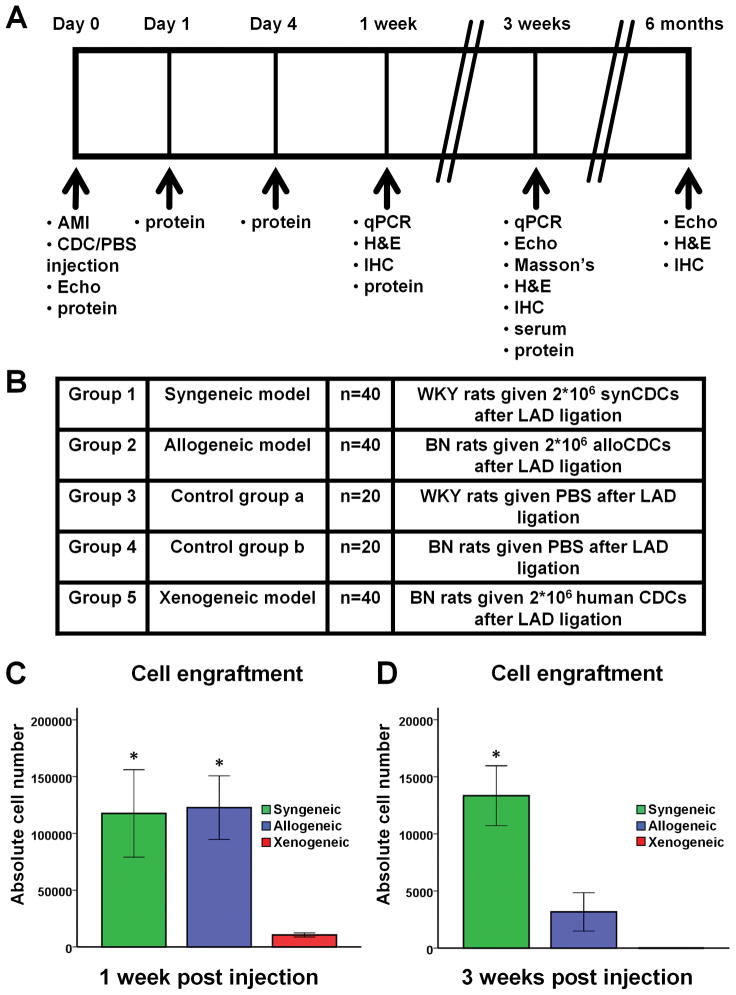
Two million male syngeneic, allogeneic or xenogeneic CDCs were implanted into the ischemic myocardium of female rats, immediately after LAD ligation. Quantitative PCR using the male SRY gene as target revealed that engraftment of allogeneic and syngeneic CDCs is similar 1 week post MI (Fig. 3C). Three weeks post MI, cell survival decreases markedly (to <1% of cells transplanted) in both groups, but the residual number of surviving cells is higher after syngeneic transplantation (Fig. 3D). These results indicate that allogeneic CDCs are cleared more rapidly than syngeneic CDCs between days 8 and 21 post-delivery. On the other hand, the vast majority of xenogeneic CDCs are rejected within 1 week of transplantation (Fig. 3C), with no surviving cells detectable 3 weeks post MI (Fig. 3D). The observed prompt rejection of xenogeneic CDCs in immunocompetent hosts echoes previous findings12.
Figure 3.
Study outline, experimental groups and CDC engraftment. (A) Study outline. (B) Experimental groups. (C) Cell engraftment by quantitative PCR 1 week (n=5–6/group) and (D) 3 weeks (n=5–6/group) post-MI and cell transplantation. Syngeneic and allogeneic CDCs demonstrated similar survival rates 1 week after transplantation, while the vast majority of xenogeneic cells had already been rejected. Three weeks post-transplantation, cell survival was poor in both syngeneic and allogeneic groups, but significantly higher after transplantation of syngeneic cells. No xenogeneic cells were detectable at 3 weeks. (* p<0.05 vs. xenogeneic group)
Allogeneic and syngeneic CDCs exert comparable and sustained beneficial effects on infarcted heart structure and function
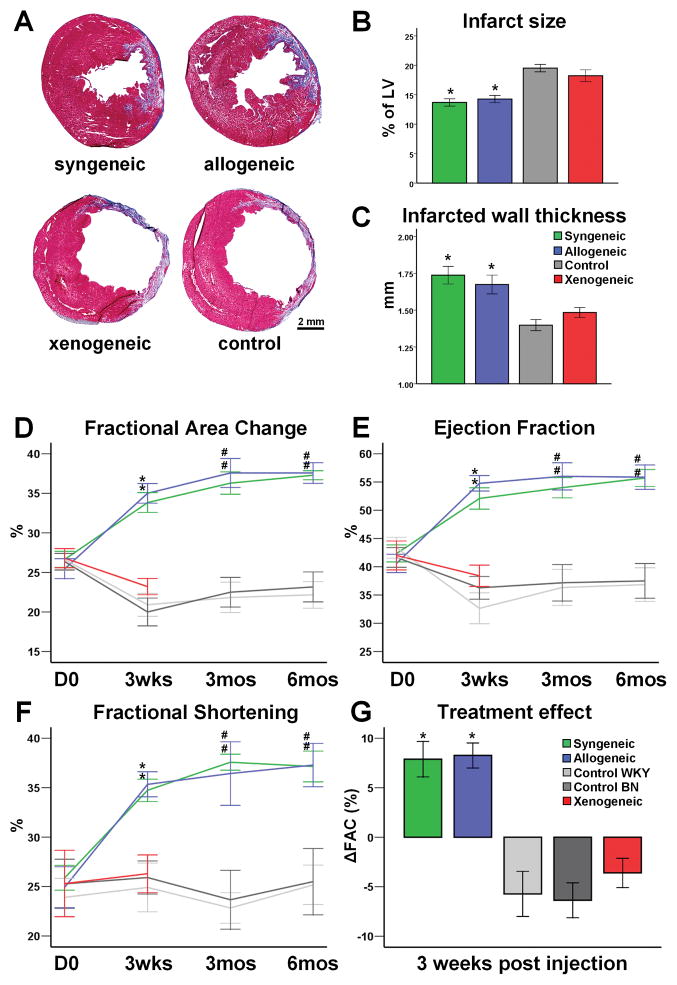
Morphometric analysis of explanted hearts 3 weeks post-MI showed severe LV chamber dilatation and infarct wall thinning in animals in the xenogeneic and control groups (Fig 4A). In contrast, the syngeneic and allogeneic groups exhibited smaller scar size, increased infarcted wall thickness and attenuation of LV remodeling (Fig 4A–C). Scar size and infarcted wall thickness did not differ among animals treated with syngeneic or allogeneic CDCs, suggesting similar favorable treatment effects in these 2 groups.
Figure 4.
Structural and functional benefits following syngeneic and allogeneic CDC transplantation. (A) Representative images of Masson’s Trichrome staining of infarcted rat hearts 3 weeks post-MI. Both syngeneic and allogeneic transplantation reduced infarct size (B) and increased infarcted wall thickness (C), compared to xenogeneic or control groups (n=5–8/group). Echocardiographic assessment of LV function revealed that both syngeneic and allogeneic CDC transplantation resulted in a robust and sustained improvement of fractional area change (D), ejection fraction (E) and fractional shortening (F). The treatment effect was similar in syngeneic and allogeneic groups (G) and was sustained at least for 6 months. (* p<0.05 vs xenogeneic, control groups; # p<0.05 vs control groups; sample sizes for D–G listed in Supplemental Table 1)
To investigate whether allogeneic cell transplantation offers functional benefit, global cardiac function was assessed by echocardiography. At baseline, FAC, LVEF and FS did not differ among treatment groups, indicating similar degrees of initial injury. Over the first 3 weeks post-MI, indices of function did not improve in the xenogeneic and control groups, whereas FAC, LVEF and FS all rose significantly, and to similar degrees, in the syngeneic and allogeneic groups. Notably, the functional benefit observed at 3 weeks persisted at 6 months (Fig 4D–G). Thus, despite lower engraftment at 3 weeks, allogeneic CDCs pack the same punch functionally and structurally as do syngeneic CDCs.
Allogeneic CDCs are hypoimmunogenic in vivo
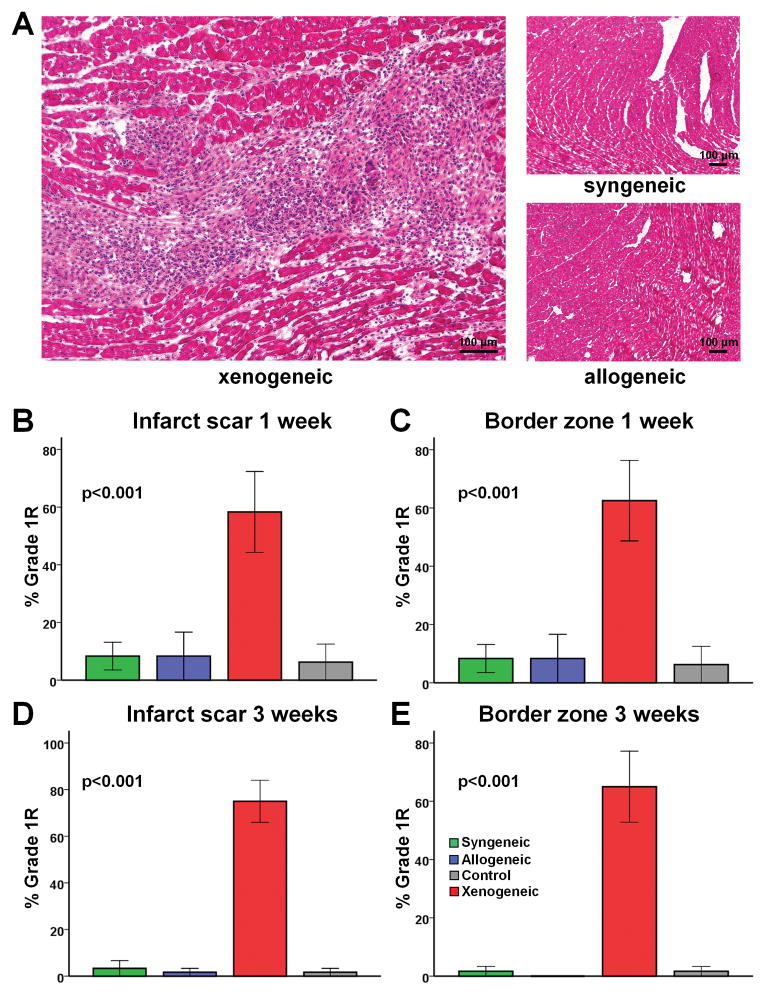
In order to evaluate the spatiotemporal development of immune rejection in the scar, border zone and remote myocardium, H&E-stained sections obtained at 1 week, 3 weeks and 6 months after treatment were evaluated using the International Society of Heart and Lung Tranplantation (ISHLT) grading system (used in clinical practice to diagnose rejection) (Fig 5) and a “homemade”, more descriptive grading system (Supplemental tables 5–7). No clearcut immune rejection could be detected in the allogeneic setting at any time point. In contrast, xenogeneic cell transplantation resulted in Grade 1R rejection, with significant mononuclear infiltration in the infarct scar and border zone 1 week (Fig 5B, 5C, Suppl Table 5) and 3 weeks (Fig 5D, 5E, Suppl Table 6) post-MI. The infiltrating cells were localized within interstitial and perivascular spaces (Fig 5A), but no foci of myocyte damage could be detected, even with xenogeneic CDCs. The remote myocardium was consistently clear of rejection, consistent with previously-observed homing of transplanted CDCs to the infarct and peri-infarct areas5,7.
Figure 5.
Assessment of local immune rejection by H&E staining. (A) Representative images of H&E stained heart sections. No immune reaction can be detected in the allogeneic setting, while perivascular and interstitial mononuclear infiltration with no foci of myocyte damage can be observed in the xenogeneic setting (Grade 1R rejection). (B–E) Quantitative analysis of immune rejection based on the ISHLT grading system demonstrated that no significant immune rejection could be detected in the infarct scar and border zone 1 or 3 weeks after allogeneic cell transplantation. In contrast, xenogeneic cell transplantation resulted in Grade 1R rejection (n=4–5/group at each timepoint).
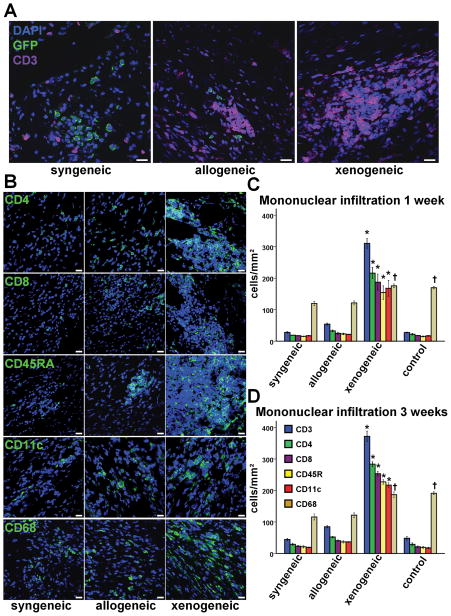
While clinically useful in the assessment of transplant rejection, detection of small foci of rejection by H&E staining is complicated in a post-MI setting by the natural inflammatory response to the ischemic insult. Since our quantitative PCR data revealed disproportionate loss of allogeneic CDCs at 3 weeks, we performed extensive immunostaining to define the identity of the infiltrating inflammatory cells (Fig 6). In the allogeneic setting, immunohistochemistry revealed rare events of rejection; a few small and sparse infiltrates (some around transplanted cells [Fig 6A]) were detected 3 weeks post-treatment in the infarct and peri-infarct areas, comprising mainly CD3+ T lymphocytes (with equal contributions of CD8+ T cytotoxic and CD4+ T helper subpopulations) and to a lesser extent CD45RA+ B lymphocytes and CD11c+ dendritic cells. The similar amounts of CD4+ and CD8+ T lymphocytes, as well as the presence of dendritic cells in the grafts, hint at a more prominent role of the indirect pathway of allorecognition in the immune rejection of transplanted cells. It is plausible that antigens shed by apoptotic donor CDCs are phagocytosed by host antigen-presenting cells (e.g., dendritic cells) and subsequently presented to CD4+ cells, thus activating the immune cascade; however, a role for the direct pathway of allorecognition cannot be ruled out13. Importantly, the increased lymphohistiocytic infiltration observed at 3 weeks was much lower than that seen with xenogeneic transplantation (Fig 6D), and had completely resided by 6 months (Suppl Figure 4, Suppl Table 7). The higher infiltration of macrophages (which did not localize within the infiltrates but were evenly dispersed along the infarct), detected at 1 and 3 weeks post MI in the xenogeneic and control groups, was consistent with the larger infarct size observed in those groups.
Figure 6.
Assessment of local immune rejection by immunohistochemistry. (A,B) Immunohistochemistry revealed small, sparse interstitial infiltrates in the proximity of some allogeneic CDCs 3 weeks post transplantation, while large infiltrates could be detected in the xenogeneic setting. Infiltrates comprised mainly CD3+ T lymphocytes (with equal contributions of CD8+ T cytotoxic and CD4+ T helper subpopulations) and to a lesser extent CD45RA+ B lymphocytes and CD11c+ dendritic cells. CD68+ macrophages did not localize within the infiltrates and were evenly dispersed along the infarct (scale bars: 20μm). Mononuclear infiltration was significantly higher in the xenogeneic group at 1 week (C) (n=4/group) and 3 weeks (D) (n=4/group) post transplantation. (* p<0.05 vs syngeneic, control groups; † p<0.05 vs syngeneic, allogeneic groups)
In order to assess the possibility of systemic immunogenicity of CDC transplantation, levels of circulating inflammatory cytokines were measured in rat serum samples obtained 3 weeks post-treatment. Quantification of inflammatory cytokines demonstrated comparable levels of circulating pro-inflammatory (IFN-γ, TNF-a, IL1b, KC/GRO) and anti-inflammatory (IL5, IL13, IL4) cytokines in syngeneic, allogeneic and control groups. Conversely, in the xenogeneic setting, the circulating levels of IFN-γ, IL1β, IL13 and IL4 were markedly increased (Suppl Fig 1). Taken together, these data indicate that the systemic inflammatory response observed after xenogeneic transplantation did not occur in the allogeneic setting.
Allogeneic CDCs elicit a cellular but not a humoral immune memory response
To assess the development of cellular memory immune response after allogeneic CDC transplantation, the alloreactivity of lymphocytes isolated from spleens of allogeneic recipients 3 weeks post-transplantation was assessed by one-way MLRs. Lymphocytes from sensitized animals exhibited higher proliferation after coculture with allogeneic CDCs, compared to naïve lymphocytes or syngeneic cocultures (Suppl Fig 3A, 3B). In addition, supernatant levels of inflammatory cytokines were markedly increased in the sensitized lymphocyte cocultures (Suppl Fig 3C). These findings, indicative of a T cell memory response, are in accordance with the immunohistochemistry data, showing a predominant role of T cells in the sparse mononuclear infiltrates observed 3 weeks post allogeneic transplantation (Fig 6C,D). We did not test whether the intensity of the cellular memory response diminishes with time, as reported in studies of allogeneic mesenchymal cell transplantation14.
To assess the development of a humoral memory response, recipient rat sera obtained 1 and 3 weeks post-transplantation were screened for circulating anti-donor antibodies. No alloreactive antibodies could be detected in any recipients of allogeneic CDCs at any timepoint. In contrast, in the xenogeneic setting, high titers of xenoreactive IgM antibodies were detected 1 and 3 weeks post transplantation, while a progressive increase of xenoreactive IgG antibodies was observed from week 1 to week 3 (Suppl Fig 2). The development of anti-donor antibodies in xenogeneic but not allogeneic recipients is consistent with the ~8 fold higher B cell myocardial infiltration observed in the xenogeneic setting (Fig 6C,D).
Allogeneic CDCs promote endogenous cardiac regeneration
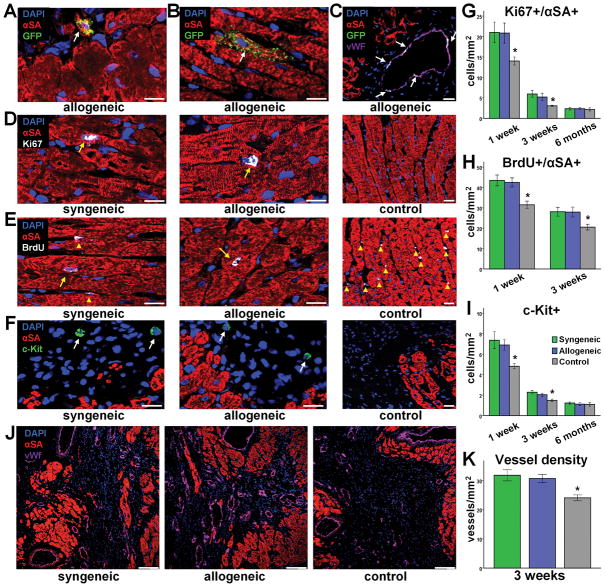
To investigate the mechanisms of benefit, we examined the fate of transplanted cells. Immunohistochemistry revealed that syngeneic and allogeneic CDCs primarily resided in the border zone and infarct scar; a subset of cells were found to be cycling in vivo 1 and 3 weeks post-MI, as indicated by Ki-67 positivity and BrdU incorporation (Suppl Fig 5). Rare events of cardiomyogenic (GFP+/αSA+ cells) and angiogenic (GFP+/vWf+ cells) differentiation of surviving CDCs could be detected in both the syngeneic and the allogeneic setting. While most GFP+/αSA+ cells were small and exhibited an immature cardiomyocyte phenotype (Fig 7A), mature GFP/αSA+ cells structurally integrated into the host myocardium were occasionally seen (Fig 7B). In addition, GFP+/vWf+ cells were found to be incorporated in microvessels in the risk region (Fig 7C). These observations, which confirm previous reports7,9,15, demonstrate the multilineage potential of CDCs. However, these “needle in haystack” instances of direct differentiation are likely too low to account for the observed robust functional benefit. We thus attempted to quantify endogenous cardiac regeneration. Possible mechanisms include upregulation of cycling cardiomyocytes (arising either from resident cardiomyocyte cell cycle reentry16 or from differentiation of endogenous stem cells17), recruitment of endogenous progenitor cells to the site of cell transplantation6,17,18, and enhanced angiogenesis19. We found that syngeneic and allogeneic CDC therapy markedly enhanced the number of cycling host cardiomyocytes (GFP−/αSA+/Ki67+ and GFP−/αSA+/BrdU+ cells; Fig 7D,E,G,H) 1 and 3 weeks post-MI. However, the number of cycling host cardiomyocytes significantly decreased from 1 week to 3 weeks, dropping to nearly undetectable levels at 6 months. Syngeneic and allogeneic CDC transplantation also recruited endogenous stem cells (Fig 7F,I); the number of GFP−/c-Kit+ cells was increased in CDC-treated hearts compared to controls at 1 week and 3 weeks post MI. As with resident cycling myocytes, the number of endogenous progenitors decreased as a function of time post-treatment.
Figure 7.
Direct and indirect contributions of allogeneic CDCs to myocardial repair. Rare events of long-term engraftment and cardiogenic (A,B) or angiogenic (C) differentiation of allogeneic CDCs could be detected. More importantly, syngeneic and allogeneic CDCs promoted endogenous mechanisms of regeneration by stimulating cardiomyocyte cycling (D,E,G,H) (n=5–8/group at each timepoint), host stem cell recruitment (F,I) (n=5–8/group at each timepoint) and angiogenesis (J,K) (n=8/group). (* p<0.05 vs syngeneic, allogeneic groups; arrows in D,E denote cardiomyocyte nuclei, while arrowheads denote non-cardiomyocyte nuclei; arrows in F denote c-Kit+ cells; A–F scale bars: 20 μm; J,K scale bars: 100 μm)
Finally, syngeneic and allogeneic CDC transplantation enhanced angiogenesis in the infarct border zone. Vessel density, identified by immunostaining for vWf, was markedly increased 3 weeks after cell therapy compared to controls (Fig 7J,K). It should be noted that these endogenous reparative mechanisms were also mobilized in the control hearts. However, their magnitude was amplified (to similar degrees) by syngeneic and allogeneic CDC therapy. Taken together, these data indicate that exogenous CDC administration stimulates activation of endogenous repair and regeneration pathways, confirming previous studies5,6 reporting that the majority of the observed benefit following cell therapy is attributable to indirect mechanisms, rather than differentiation of transplanted cells. We thus quantified myocardial levels of beneficial paracrine factors in the infarct border zone. Western blot analysis revealed increased secretion of VEGF, IGF-1 and HGF in hearts treated with syngeneic and allogeneic CDCs, compared to controls, at days 1, 4 and 7 post-MI (Fig 8A–D). On the contrary, rats treated with xenogeneic CDCs had increased myocardial levels of these cytokines only at 1 day post-MI, but not at later timepoints (Suppl. Fig 6). Three weeks post-MI, no difference could be observed among groups. The data reveal that syngeneic and allogeneic CDCs are equivalent in their paracrine benefits, both in magnitude and time course, and that sustained increased levels of VEGF, IGF-1 and HGF, at least during the first week post cell transplantation, underlie the functional benefit. Our experimental design (transplantation of rCDCs into rat hearts and the use of antibodies that detect both human and rat cytokine isoforms) cannot elucidate whether the increased myocardial levels of these factors are attributable to direct secretion by transplanted cells, to upregulation of host tissue humoral responses20, or both. Nevertheless, prior work shows that release of paracrine factors directly from CDCs is substantial in the early post-transplantation period5.
Figure 8.
Detection of beneficial paracrine factors by Western blotting. (A) Representative blots demonstrating increased secretion of VEGF, IGF1 and HGF during the first week post syngeneic and allogeneic CDC transplantation. Quantitative analysis of myocardial levels of VEGF (B), IGF-1 (C) and HGF (D) post MI (n=4–6/group at each timepoint). (* p<0.05 vs syngeneic, allogeneic groups; s: syngeneic; a: allogeneic; c: control)
Discussion
We report a the detailed spatiotemporal evaluation of the local and systemic immune responses following allogeneic CDC transplantation for myocardial repair. Allogeneic CDC transplantation without immunosuppression is safe, and produces structural and functional benefits post-MI by stimulating endogenous cardiac regeneration. This indirect mechanism of action, shared by syngeneic cells, explains why benefits persist despite the temporary engraftment of transplanted cells.
CDCs represent an attractive cell type for heart repair and regeneration. CDCs are clonogenic and exhibit multineage potential, thus fulfilling key criteria for heart-derived stem cells15. Over the past 6 years, we have demonstrated that CDCs can improve cardiac function post-MI in mice5,7,21, rats9,22,23 and pigs24,25. Importantly, several independent labs worldwide have reproduced the published methodology and verified CDCs’ identity and utility26–32. On the other hand, critiques of the cardiosphere methodology have appeared33,34, but, as we have pointed out in detailed rebuttals8,15, these studies did not follow published protocols for CDC isolation and expansion, and the methodological variations likely explain the negative results. With regard to clinical translation, highly positive results from a proof-of-concept clinical study utilizing autologous CDCs—the CADUCEUS (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction; NCT008933603) trial— have recently been reported35.
The avoidance of immunologic rejection renders autologous therapy attractive, but serious disadvantages dampen enthusiasm. Patient-specific tissue harvesting and cell processing result in a delay to therapy and introduce possible variations in cell potency related to patient age and disease4. Here, we tested the specific hypothesis that allogeneic CDCs are hypoimmunogenic in vivo and can survive in the infarcted myocardium for a critical period of time in order to stimulate endogenous reparative and regenerative pathways, resulting in sustained benefit. We find that allogeneic CDC transplantation without immunosuppression induces only a transient mild local immune reaction in a rat MI model. In the clinical setting, development of an immune response after allogeneic CDC delivery to the heart could theoretically lead to: a) immune-related myocardial damage (which based on our findings would be unlikely, since no foci of myocardial damage were detected even after xenogeneic CDC transplantation); and b) allosensitization of the cell recipient, which in turn could: i) complicate repeat dosing with the same batch of cells (a problem that could be easily overcome by administering cells from different donors); and ii) complicate future organ transplantation (if the CDC donor and organ donor share similar HLA haplotypes). We did not detect any circulating anti-donor antibodies after allogeneic CDC transplantation, implying that no significant increase in panel reactive antibodies would occur; the absence of such sensitization at baseline increases the likelihood of allograft survival36. In addition, the small inoculum associated with CDC therapy (compared for example with the volumes used in blood transfusions) make sensitization of the recipient improbable. Nevertheless, the possibility of recipient allosensitization should be investigated in large animals as a prelude to studies in human subjects.
We have also shown that transient and rather paltry short-term cell survival suffices to produce dramatic lasting benefits. Despite lower cell engraftment, allogeneic CDC transplantation generates structural and functional benefits which are indistinguishable from syngeneic transplantation, and persist 6 months post-MI. The equivalence of allogeneic and syngeneic transplantation is not surprising once we recognize and accept the central paradox: few, briefly-present transplanted cells are sufficient to produce large, durable benefits by amplifying endogenous pathways of repair and regeneration, rather than by directly generating new transplanted tissue. This indirect “amplifier effect”, impressive as it may be, is not yet fully understood2. Even though we show that allogeneic CDCs stimulate host cardiomyocyte cycling, endogenous stem cell recruitment and angiogenesis in the post-MI setting, it is unclear whether these phenomena can account for the totality of the observed benefit; other mechanisms could involve cytoprotection of the host tissue, or modulation of inflammatory processes resulting in better infarct healing. In addition, it is unclear how much of the benefit is attributable to the identified paracrine factors; IGF1 and HGF have been shown to mobilize resident cardiac stem cells37, while VEGF is well-known to stimulate angiogenesis38. Alternatively, other factors39 may also play important roles. Identification of the appropriate “cocktail” of beneficial growth factors and incorporation into a formulation enabling sustained and controlled local release after cardiac delivery is a conceptually attractive approach. However, cell-mediated contact-dependent mechanisms may also contribute to the observed effects.
Regardless of the mechanism, in practice, the current work opens up a new treatment paradigm: CDCs could be grown in large numbers from allogeneic heart tissue in a central facility under strict quality control and banked for future use, enabling safe and effective myocardial repair in a timely, cost-efficient manner. Potential sources of allogeneic heart tissue include hearts explanted from organ donors but not used for transplantation, cadaveric hearts from the recently deceased, and surgical discards. Hearts obtained from organ donors (but not used for transplantation) have the inherent advantage that donors are, by definition, healthy and have been previously HLA-typed and screened for infectious diseases, with the tissue maintained viable and sterile until processed. Hearts from organ donors after cardiac death are particularly attractive, since they are rarely used for transplant, although kidneys, liver, and pancreas are commonly used40. In 2008 there were 832 organ donors after cardiac death in the US, and no hearts were used for cardiac transplantation41; these hearts represent one pool from which source tissue can be obtained for allogeneic CDC culture. Cadaveric hearts from healthy, non-infectious donors could be also be used, however, the tissue is not optimally stored and samples would have to be obtained with low post-mortem intervals. Surgical discards are yet another source option; while these specimens are more abundant, donors are apt to have an existing cardiac disorder or other comorbidities (which may or may not hamper cell quality).
In conclusion, we demonstrate that allogeneic CDC transplantation without immunosuppression is safe, promotes cardiac regeneration and improves heart function in a rat MI model, mainly through stimulation of endogenous repair mechanisms. This indirect mechanism of action rationalizes the lasting benefit brought about by ephemeral transplanted cells, in that the new tissue originates from the recipient rather than the donor. This work motivates the testing of allogeneic human CDCs as a potential clinical product for cellular cardiomyoplasty.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by NIH (R01 HL083109) and the Cedars-Sinai Board of Governors Heart Stem Cell Center. EM holds the Mark S. Siegel Family Chair of the Cedars-Sinai Medical Center.
Footnotes
Conflict of Interest Disclosures: EM and LM are founders and equity holders in Capricor, Inc. LM receives salary from Capricor, Inc. KM and JT receive consulting fees from Capricor, Inc. The remaining authors report no conflicts.
References
- 1.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 2.Malliaras K, Marbán E. Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br Med Bull. 2011;98:161–185. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CADUCEUS. CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction ( NCT00893360) doi: 10.1016/j.jacc.2013.08.724. Available at: www.clinicaltrials.gov. [DOI] [PMC free article] [PubMed]
- 4.Dimmeler S, Leri A. Aging and Disease as Modifiers of Efficacy of Cell Therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 8.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R, Marbán E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marbán E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggeri L, Capanni M, Martelli MF, Velardi A. Cellular therapy: exploiting NK cell alloreactivity in transplantation. Curr Opin Hematol. 2001;8:355–359. doi: 10.1097/00062752-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Atoui R, Shum-Tim D, Chiu RC. Myocardial regenerative therapy: immunologic basis for the potential “universal donor cells”. Ann Thorac Surg. 2008;86:327–334. doi: 10.1016/j.athoracsur.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schär M, Gerstenblith G, Weiss RG, Marbán E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 13.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 14.Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 15.Davis DR, Ruckdeschel Smith R, Marbán E. Human cardiospheres are a source of stem cells with cardiomyogenic potential. Stem Cells. 2010;28:903–904. doi: 10.1002/stem.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 17.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 20.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marbán E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis DR, Kizana E, Terrovitis, Barth AS, Zhang Y, Smith RR, Miake J, Marbán E. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heartbiopsies. J Mol Cell Cardiol. 2010;49:312–321. doi: 10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrovitis J, Lautamäki R, Bonios, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marbán E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee ST, White AJ, Matsushita, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R, Marbán E. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 25.Johnston PV, Sasano T, Mills, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marbán E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghila Rani KG, Kartha CC. Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart. Growth Factors. 2010;28:157–165. doi: 10.3109/08977190903512628. [DOI] [PubMed] [Google Scholar]
- 27.Gaetani R, Ledda M, Barile L, Chimenti I, De Carlo F, Forte E, Ionta V, Giuliani L, D’Emilia E, Frati G, Miraldi F, Pozzi D, Messina E, Grimaldi S, Giacomello A, Lisi A. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovascular Research. 2009;82:411–420. doi: 10.1093/cvr/cvp067. [DOI] [PubMed] [Google Scholar]
- 28.Mishra R, Vijayan K, Colletti E, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC, Jr, Wold LE, Kaushal S. Characterization and Functionality of Cardiac Progenitor Cells in Congenital Heart Patients. Circulation. 2011;123:364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takehara N, Tsutsumi Y, Tateishi, Ogata T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M, Komeda M, Yamagishi M, Yaku H, Tabata Y, Matsubara H, Oh H. Controlled Delivery of Basic Fibroblast Growth Factor Promotes Human Cardiosphere-Derived Cell Engraftment to Enhance Cardiac Repair for Chronic Myocardial Infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 30.Tang YL, Zhu W, Cheng, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic Preconditioning Enhances the Benefit of Cardiac Progenitor Cell Therapy for Treatment of Myocardial Infarction by Inducing CXCR4 Expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, Gaballa MA. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovascular Research. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koninckx R, Daniëls A, Windmolders S, Carlotti F, Mees U, Steels P, Rummens JL, Hendrikx M, Hensen K. Mesenchymal stem cells or cardiac progenitors for cardiac repair? A comparative study. Cell Mol Life Sci. 2011;68:2141–2156. doi: 10.1007/s00018-010-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen DC, Andersen P, Schneider M, Jensen HB, Sheikh SP. Murine “Cardiospheres” Are Not a Source of Stem Cells with Cardiomyogenic Potential. Stem Cells. 2009;27:1571–1581. doi: 10.1002/stem.72. [DOI] [PubMed] [Google Scholar]
- 34.Shenje LT, Field LJ, Pritchard C, Guerin CJ, Rubart M, Soonpaa MH, Ang KL, Galiñanes M. Lineage Tracing of Cardiac Explant Derived Cells. PLoS One. 2008;3:e1929. doi: 10.1371/journal.pone.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makkar R, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman DS, Czer L, Marbán L, Mendizabal A, Johnston PV, Russell S, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. The CADUCEUS (CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction) Trial. Circulation. 2011 (Abstract) (in press) [Google Scholar]
- 36.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 37.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stastna M, Chimenti I, Marbán E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–253. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinbrook R. Organ donation after cardiac death. N Engl J Med. 2007;357:209–213. doi: 10.1056/NEJMp078066. [DOI] [PubMed] [Google Scholar]
- 41.The 2009 Annual Report of the OPTN and SRTR: Transplant Data 1999–2008. Available at www.ustransplant.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.