Abstract
The developing brain is particularly sensitive to environmental teratogens, such as methylmercury (MeHg), which may induce cell death. Although several mechanisms of MeHg-induced apoptosis have been defined in culture models, pathways mediating caspase-3 activation in vivo remain unclear, especially in the developing hippocampus. To explore apoptotic mechanisms, Sprague-Dawley rats were exposed to 5 μg/g MeHg or PBS vehicle on postnatal day 7 (P7) and the hippocampus was assessed at various times for levels of apoptotic proteins. MeHg induced a 38% increase in Bax protein and an increase in cytosolic cytochrome c at 4 h, followed by later increases in caspase-9 (40% at 12 h; 33% at 24 h) and caspase-8 (33% at 24 h), compared to controls. MeHg also induced an increase in executioner caspase-3, a protease activated by both mitochondrial-dependent caspase-9 and mitochondrial-independent caspase-8. To further define pathways, we used a forebrain culture model and found that the MeHg-induced increases in caspase-3 and caspase-8 were completely blocked by a caspase-9-specific inhibitor, while caspase-9 induction was unperturbed by the caspase-8 inhibitor. These observations suggest that MeHg acts primarily through the mitochondrial-dependent cascade to activate caspase-3 in forebrain precursors, a pathway that may contribute to previously documented neurotoxicity in developing hippocampus. In turn, using the endpoint protein, caspase-3, as a sensitive marker for neural injury, we were able to detect hippocampal cell death in vivo at ten-fold lower levels of MeHg exposure (0.6 μg/g) than previously reported. Thus mitochondrial-dependent cell death in the hippocampus may serve as a sensitive index for teratogenic insults to the developing brain.
Keywords: Methylmercury, hippocampus, apoptosis, caspase-3, mitochondria
1. Introduction
As a persistent environmental toxicant, MeHg continues to raise concerns about negative effects on human brain development. Studies indicate that MeHg exposure from a fish diet may correlate inversely with some developmental milestones in learning (Grandjean et al., 1997, Grandjean et al., 1998, Murata et al., 1999, Rice, 2000, Grandjean et al., 2004). However, results from these studies are confounded by the beneficial effects of a fish diet (Marsh et al., 1995, Myers et al., 1995a, Myers et al., 1995b, Shamlaye et al., 1995, Myers et al., 1997, Davidson et al., 1998, Davidson et al., 1999, Davidson et al., 2008). In turn, to control for other factors present in a fish diet, studies have been performed in animal models and have shown deficiencies in learning tasks following both acute and chronic exposure to MeHg (Rice and Gilbert, 1982, Reed et al., 2006, Falluel-Morel et al., 2007, Onishchenko et al., 2007). Among other developmental abnormalities, deficits in spatial learning and memory may suggest that MeHg is affecting regions of the hippocampus, a brain structure critical to these functions and not usually associated with mercury toxicity.
The hippocampus is one of the few areas of the brain that undergoes continuous neurogenesis (Kaplan and Hinds, 1977, Kaplan and Bell, 1984, Eriksson et al., 1998). During development, the neural progenitor cells divide continuously, with waves of programmed cell death to cull damaged cells (Yuan and Yankner, 2000). These mitotic neural progenitors can be sensitive targets of many environmental toxicants, like MeHg (Tamm et al., 2006). Increased expression levels of apoptotic markers can be indicators of cellular damage elicited by exogenous factors.
Many biological responses occurring during apoptosis are caused by cysteine proteases that are part of a large family of proteins known as caspases. These proteins are homologous and their enzymatic activity is divided into two classes: initiator and effector caspases (Riedl and Shi, 2004). When an initiator caspase, such as caspase-8 or −9, is activated, the apoptotic cascade begins but can also be interrupted. In contrast, when an effector caspase, such as caspase-3, is cleaved, the apoptotic cascade is further along and is virtually irreversible. As components of the apoptotic response in mammalian cells, caspases can be activated through two pathways: extrinsic and intrinsic, depending on the origin of the death stimulus. The extrinsic pathway is initiated by extracellular ligands that bind to cell-surface death receptors that can activate caspase-8 (Siegel et al., 1998, Riedl and Shi, 2004). The intrinsic or mitochondrial pathway may be triggered by recruitment of pro-apoptotic members of the B-cell lymphoma 2 (Bcl-2) family, such as Bcl 2-associated X protein (Bax), to the outer mitochondrial membrane. Oligomerization of pro-apoptotic proteins, like Bax, facilitates the translocation of cytochrome c from the mitochondria to the cytosol (Oltvai et al., 1993, De Giorgi et al., 2002). Cytochrome c then joins the holoenzyme, known as the apoptosome, to promote the self-cleavage of caspase-9 (Bossy-Wetzel et al., 1998, Martinou and Green, 2001, Green and Kroemer, 2004). Both caspase-8 and caspase-9 are initiator caspases that cleave and activate the effector caspase, caspase-3, a central component of the apoptotic response. Caspase-3 has been found to play a normal role in apoptosis in the developing brain (Kuida et al., 1996). Although an endogenous enzyme, increases in caspase-3 expression may indicate a toxic insult and cleave cellular targets to produce functional deficits and cell death.
In our previous studies, summarized in Table 1, we found that MeHg can act during development to damage the hippocampus, targeting the neurogenic precursors and impairing later learning behaviors (Burke et al., 2006, Falluel-Morel et al., 2007). In the P7 rat, proliferating neural progenitors are present in the dentate gyrus, while post-mitotic neurons reside in Ammon’s horn (CA1-3) (Altman and Bayer, 1990). In response to P7 MeHg exposure, a number of proliferation markers were decreased including thymidine incorporation, BrdU labeling, cyclin D1, cyclin D3 and cyclin E, suggesting the metal had an effect on the progenitor population. In parallel, in hippocampal regions where cell division was occurring at the time of mercury exposure, there were later decreases at P21 in the total volume as well as neuronal cell number (dentate gyrus: hilus and granule cell layer). In contrast, in non-mitotic regions (CA1-3: pyramidal cell layer, stratum radiatum, and stratum oriens) MeHg exposure induced no such changes. In addition, acute MeHg exposure increased the levels of activated caspase-3 in the P7 hippocampus (Burke et al., 2006, Falluel-Morel et al., 2007). Together these data suggest that MeHg may be targeting mitotic cells in the hippocampal dentate gyrus through caspase-3 activation. In support of this, work in culture from other labs suggests MeHg acts via the mitochondrial-dependent cascade, increasing Bax, cytosolic cytochrome c, and caspase-9, to elicit cell death in primary neuronal precursors and cell lines (Tamm et al., 2006). While we previously found that a single MeHg exposure induced activation of caspase-3 in developing hippocampus, the roles of mitochondrial-dependent and –independent pathways in vivo remains undefined. Here we sought to define upstream mechanisms involved in MeHg-induced caspase-3 activation in the developing rat hippocampus.
Table 1. Summary of previous findings.
These data demonstrates the involvement of proliferating cells in acute MeHg toxicity in the P7 rat hippocampus possibly leading to later cell deficits and behavioral abnormalities
| Changes observed in hippocampus after P7 MeHg exposure Data are from Burke et al. 20061 and Falluel-Morel et al. 20072 |
|---|
| Markers of proliferation (P8) |
 G1 phase proteins: Cyclin E1,2, Cyclin D12, Cyclin D32 G1 phase proteins: Cyclin E1,2, Cyclin D12, Cyclin D32
|
 S phase markers: BrdU labeling2, Thymidine incorporation1,2 S phase markers: BrdU labeling2, Thymidine incorporation1,2
|
| Markers of cell death (P8) |
 Caspase-3 protein levels1,2 Caspase-3 protein levels1,2
|
 Co-labeling of caspase-3 and BrdU2 Co-labeling of caspase-3 and BrdU2
|
| Region-specific effects in proliferating populations (P8, P21) |
 Total hippocampal cell number (DNA content)2 Total hippocampal cell number (DNA content)2
|
 Total neuron number2 Total neuron number2dentate gyrus granule neurons (not CA1-3 pyramidal neurons) |
 Total volume2 Total volume2dentate gyrus hilus and granule layer (not CA1-3 pyramidal cell layer) |
| Hippocampal-dependent behaviors (P35) |
 Spatial learning and memory2 Spatial learning and memory2
|
2. Materials and methods
2.1 Animals
Sprague–Dawley rats were purchased from Hilltop Lab Animals (Philadelphia, PA). Rats were housed in a temperature-and light-controlled animal care facility and given food and water ad libitum. E14.5 female Sprague-Dawley rats were sacrificed and embryos were used for cortical cultures. Females with twelve, 4-day-old, cross-fostered, male pups (P4) were received on Mondays. P7 rats were injected subcutaneously with vehicle or MeHg (0.1 – 10 μg/g in a 100 μL bolus) at 11 am and hippocampal tissues were collected at various times from 2 to 24 hours post injection. All animal procedures were approved by the Robert Wood Johnson Medical School institutional animal care and utilization committee and conformed to NIH Guidelines for animal use.
2.2 Materials
Methylmercury chloride (CH3HgCl) was purchased from Sigma (St Louis, MO). A 1.5 mg/mL stock solution in 0.1 M phosphate-buffered saline (PBS) was prepared by agitation immediately before use.
2.3 Western blot
Samples were obtained from P7 rats 2, 4, 6, 12 or 24 hours after injection of vehicle or MeHg (5 μg/g). Total cellular proteins were extracted by using lysis buffer containing 1% Triton X-100, 50 mM Tris-HCl and 10 mM EDTA as previously reported (Falluel-Morel et al., 2007). The homogenate was centrifuged (20,000 g, 4°C, 15 min) and the proteins contained in the supernatant were precipitated by addition of ice-cold 100% trichloroacetic acid (TCA). The extract was centrifuged (15,000 g, 4°C, 15 min) and washed three times with a mix of alcohol/ether (30:70 v/v). The pellet was denatured in Laemmli buffer (50 mM Tris-HCl (pH 7.5) containing 20% glycerol, 0.7 M 2-mercaptoethanol, 0.004% (w/v) bromophenol blue and 3% (w/v) SDS at 100°C for 5 min, and electrophoresed on a 12% SDS/PAGE. After separation, proteins were electrically transferred onto a PVDF membrane (Millipore, Bedford, MA). The membrane was incubated with the blocking solution (1% BSA in Tris-buffered saline containing 0.05% Tween 20) at room temperature for 1 h and revealed with antibodies against cleaved caspase-3, caspase-8, and caspase-9 (Cell Signaling; 9664L, 9429, 9507s), Bax and cytochrome c (Santa Cruz, Santa Cruz, CA; sc-571 and sc-6254), using a chemiluminescence detection kit (ECL System, Amersham, Sweden). Autoradiographic films (Amersham) were quantified using an image analysis system (Biorad, Hercules, CA).
2.4 Mitochondrial and cytosolic fractionation
Hippocampal homogenates were suspended in isotonic buffer supplemented with a protease inhibitor mixture (aprotinin, leupeptin, PMSF; Sigma). Nuclei and unbroken cells were separated at 900 × g for 10 min at 4°C. This supernatant was centrifuged at 9500 × g for 15 min at 4C. Pellet (mitochondrial fraction) and supernatant (cytosol) were collected. Supernatant was precipitated and washed as regular Western blot samples. The pellet was resuspended in isotonic buffer, freeze-fractured with liquid nitrogen, and dissolved in Laemmli denaturing buffer.
2.5 Caspase-3 immunohistochemistry
Animals were perfused transcardially with 0.9% NaCl followed by 1% paraformaldehyde in 0.1 M PBS. Brains were dissected and post-fixed in 1% paraformaldehyde in PBS for 12 h at 4°C, cryoprotected with PBS containing 30% sucrose, embedded in Tissue-Tek (Sakura, Tokyo, Japan), frozen and cut into 12μm thick coronal sections on a microcryotome (Leica, Heidelberg, Germany). Coronal sections were cut from whole brains in a 1 in 10 series for the entire rostro-caudal extent of the hippocampus. For the P8 brain, the 1:10 series yielded approximately 20 sections through the entire hippocampus for each animal, which were mounted on 4 slides in total. Preliminary studies indicated that the greatest levels of caspase staining and response to MeHg occurred on the second and third slides. For these analyses the second slide was used, resulting in analogous regional analysis between brains; approximate rostro-caudal coordinates are 5.86mm – 5.40mm from Intraural or 3.14mm – 3.60mm from Bregma (Kaplan and Bell, 1984). Quantification of positive cells was performed on 4 sections/animal, 9 animals/group derived from three independent experiments. For antigen unmasking, sections were incubated in 0.01 M boiling citrate buffer. Endogenous peroxidases were inactivated using hydrogen peroxide (0.3% in methanol, 10 min). Sections were then incubated in 5% normal goat serum for 1 h, followed by rabbit antibody to cleaved-caspase-3 (1 : 200 ; Cell Signaling, Beverly, MA) overnight at 4°C in 0.5% Triton X-100 and 1% normal goat serum in PBS. Biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) was applied as second antibody and avidin-biotin peroxidase complex (ABC kit; Vector Laboratories) served as amplification reagent. Diaminobenzidine served as chromogen to localize the peroxidase, followed by counterstain with Toluidine blue (Sigma).
2.6 Pyknotic nuclei
Pyknosis was identified on the basis of characteristic morphological shrinkage and distortion of cell nuclei, using 4, 6-diamidino-2-phenylindoe (DAPI) staining, a protocol adapted from published studies (Shirai et al., 2006). Coronal sections (12μm) showing bilateral hippocampi were assessed. Four sections per animal from the second slide in a 1:10 series were analyzed. The total number of pyknotic cells in the hilus is presented as the mean ± SEM.
2.7 Cortical cell culture
Our culture model was selected in order to examine effects of MeHg on proliferating forebrain precursors, a model that effectively reflected developmental neurotoxicity in vivo, as previously reported (Burke et al., 2006; Falluel-Morel et al., 2007). To obtain a homogeneous forebrain precursor population, dorsolateral cerebral cortices from E14.5 rat embryos were separated from overlying meninges and basal ganglia. Cells were mechanically dissociated, plated on 0.1 mg/mL poly-d-lysine coated culture dishes, and incubated at 37°C with 5% CO2 in defined medium (Lu and DiCicco-Bloom, 1997, Falluel-Morel et al., 2007) composed of Dulbecco’s Modified Eagle’s Medium and Ham’s F12 medium (50 : 50 v/v; Invitrogen, Grand Island, NY) and containing penicillin (50 U/mL), streptomycin (50 μg/mL), transferrin (100 μg/mL) (Calbiochem, La Jolla, CA), putrescine (100 μmol/L), progesterone (20 nmol/L), selenium (30 nmol/L), glutamine (2 mmol/L), glucose (6 mg/mL), and bovine serum albumin (10 mg/mL). Unless otherwise noted, components were obtained from Sigma. Cells (3 × 105) were seeded in 24-well plates for caspase inhibition studies. Protein analysis required 4 × 106 cells plated in 60 mm dishes. Cells were plated 0.5 h prior to addition of caspase inhibitors or vehicle, and MeHg was added to the media 1 h after cell plating so that initial adhesion was not disturbed by the toxicant (Burke et al., 2006).
2.8 Fluorochrome Inhibition of Caspases
E14.5 cortical precursor cells were treated with selective caspase-8 inhibitor (60 μM, z-IETD-FMK, Sigma), caspase-9 inhibitor (80 μM, z-LEHD-FMK, Sigma), or vehicle 30 minutes prior to the 4h-exposure to MeHg (1.5 μM) or vehicle. Fluorescence-producing substrates for detection of activated caspases-3, −8 and −9 (CaspaTag Caspase In Situ Assay Kit, Chemicon (Milipore), Billerica, MA) were added 1 hour before the end of MeHg exposure. Cells were visualized under a fluorescence microscope with the excitation filter set at 490 nm and converted substrate emissions detected at 520 nm. Positive cells in each field at 20X were counted and recorded. Data were obtained from five fields/well and experiments were performed three times.
2.9 Statistical Evaluation
A one-way ANOVA followed by a Tukey-Kramer multiple comparison test was used for analysis of experiments involving three groups or more. An unpaired t-test with Welch’s correction was used for analysis of experiments involving two groups. Data were analyzed using Statview.
3. Results
3.1 MeHg-induced apoptosis is mediated through the mitochondrial-dependent pathway in developing hippocampus
Our previous studies reported that activation of caspase-3 by MeHg in vivo contributed to hippocampal cell loss in postnatal rats (P7) and subsequent juvenile learning deficits. MeHg exposure of P7 rats elicited a 2-fold increase in protein levels of activated caspase-3 in the hippocampus 24 h after injection. Further, cell death was directly associated with the proliferating hippocampal precursors themselves as revealed by caspase-3/BrdU double-labeling (Table 1) (Falluel-Morel et al., 2007). However, it is not known whether caspase-3 is activated by a mitochondrial-dependent or alternatively a mitochondrial-independent pathway. Mitochondrial involvement in apoptosis occurs through the release of cytochrome c from the inner mitochondrial membrane into the cytosol, where it complexes with caspase-9 and other proteins. The release of cytochrome c may be facilitated by the formation of pores on the outer mitochondrial membrane through Bax protein oligomerization (Hengartner, 2000, De Giorgi et al., 2002). To examine the roles of Bax protein and cytochrome c release in caspase activation following MeHg exposure, P7 rats were injected with 5 μg/g MeHg or vehicle and hippocampi were removed at 2, 4, 6, 12, and 24 h and processed for western blot analysis.
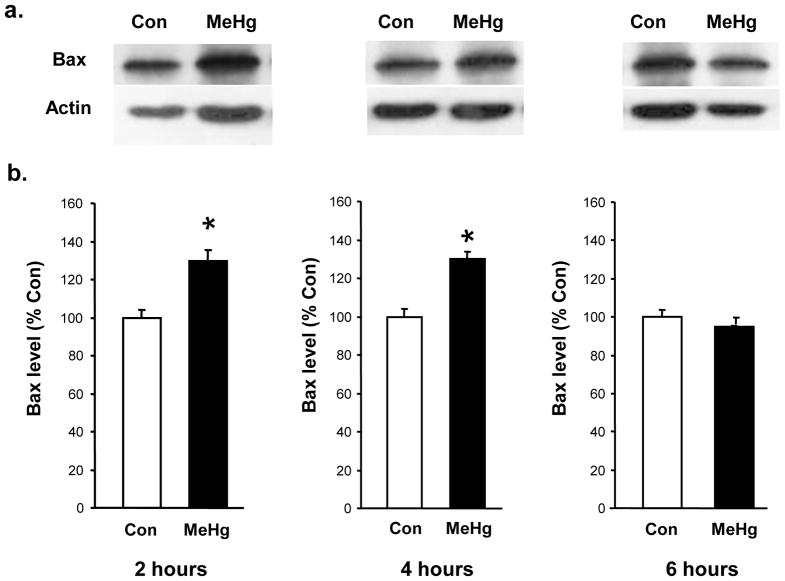
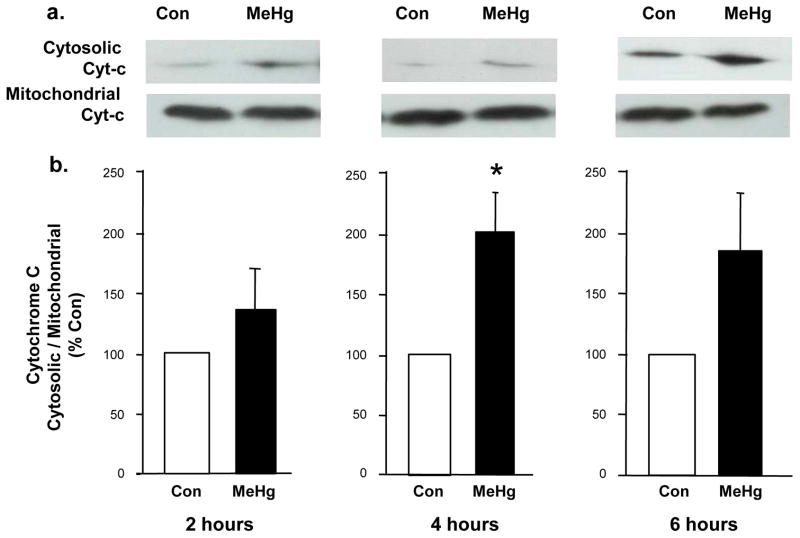
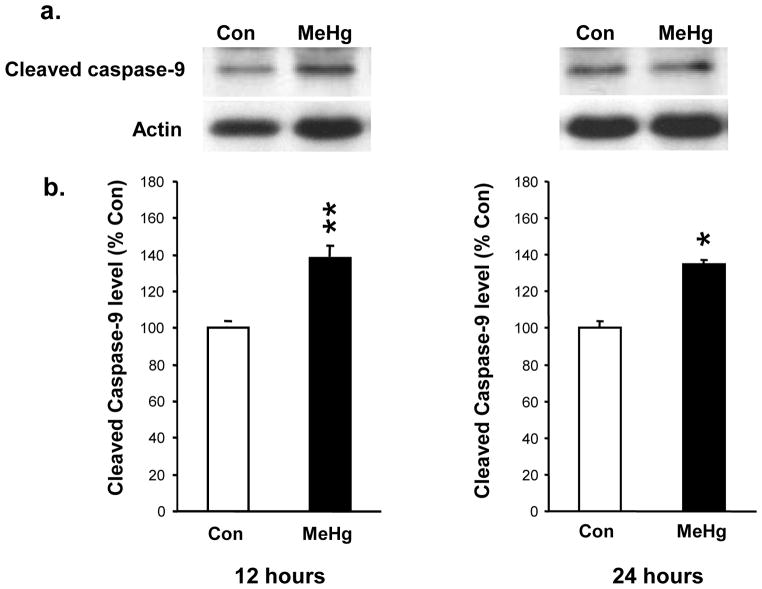
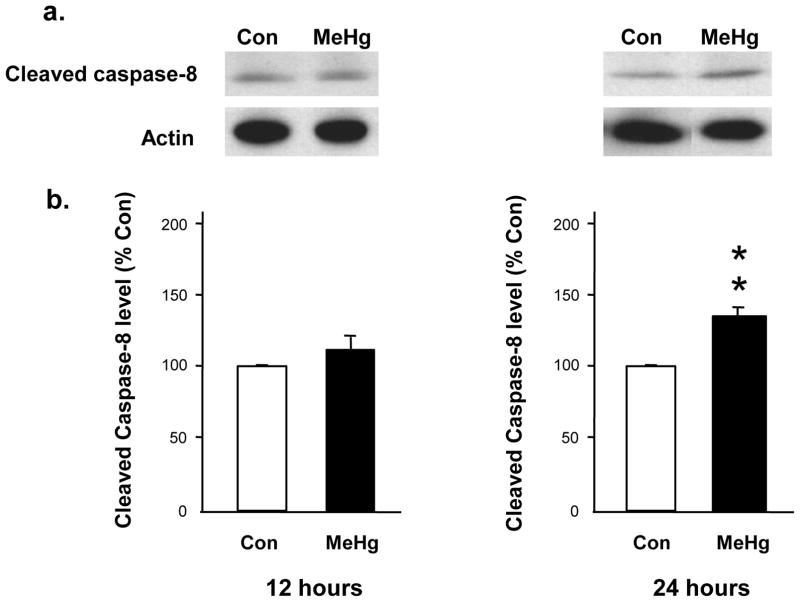
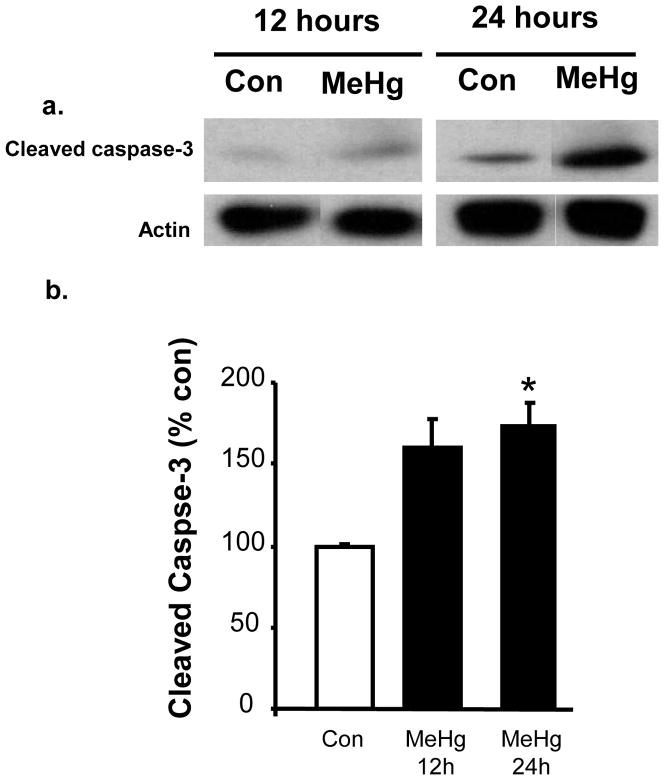
MeHg induced a 34% increase in the level of Bax protein at 2h and 27% increase at 4h, with a return to normal by 6 h (Fig. 1). Since increases in Bax protein levels may suggest initiation of the mitochondrial-dependent cascade, we sought evidence of cytochrome c release by defining cytosolic protein levels at 2, 4, and 6 h. At 4 h after MeHg exposure, there was an increase in cytosolic cytochrome c protein levels, reflected by a 100% increase in the cytosolic/mitochondrial fraction ratio (Fig. 2), which persisted until 6 h. Since cytochrome c release into the cytosol may facilitate apoptosome formation with cleavage and activation of caspase-9, we determined protein levels 12 or 24 h after MeHg exposure. MeHg increased levels of active caspase-9 by 40% at 12 h and 33% at 24 h (Fig. 3). In contrast, caspase-8, a caspase not associated with mitochondria, exhibited no significant change at 12 h when caspase-9 was already induced, but it did exhibit a 33% increase by 24h (Fig. 4). Since both caspase-9 and −8 are initiator caspases that can cleave caspase-3 into its active form, we assessed levels of activated caspase-3. MeHg induced an almost two-fold increase in protein levels of activated caspase-3 at 24 h (n=5, p<0.05; Fig. 5) reproducing previous results (Burke et al., 2006, Falluel-Morel et al., 2007), suggesting that MeHg exposure produced a mitochondrial-dependent process of apoptosis in the postnatal hippocampus.
Figure 1. Effect of 5 μg/g MeHg on Bax levels in postnatal rat hippocampus.
P7 rats were exposed to a single sc injection of 5 μg/g MeHg or vehicle and then hippocampi were harvested at various times (2–6 hours). Hippocampal homogenates were analyzed for Bax protein levels by western blot. Blot quantifications were normalized to actin. Representative immunoblots are shown above (a) while densitometric intensity values (expressed as % Control) are shown below (b). Data represent the mean ± SEM from three independent experiments performed in triplicate. *p<0.01 vs control. There was a significant increase in Bax protein levels in the hippocampus 2 and 4h-post-injection.
Figure 2. Effect of 5 μg/g MeHg exposure on cytochrome c release in postnatal rat hippocampus.
P7 rats were exposed to a single sc injection of 5 μg/g MeHg or vehicle and then hippocampi were harvested at various times (2–6 hours). Cytochrome c release from mitochondria to the cytosol was measured by performing western blot on separated cytosolic and mitochondrial fractions of hippocampal homogenates. Representative immunoblots are shown above (a) while densitometric intensity values (expressed as % of control) are shown below (b) as a ratio of cytosolic fraction/mitochondrial fraction. Data represent the mean ± SEM from three independent experiments performed in triplicate. *p<0.05, **p<0.01. At 4h-post-injection, there was a significant shift of cytochrome c from the mitochondria to the cytosol in MeHg exposed animals.
Figure 3. Effect of 5 μg/g MeHg exposure on cleaved caspase-9 protein levels in postnatal rat hippocampus.
P7 rats were exposed to a single sc injection of 5 μg/g MeHg or vehicle and then hippocampi were harvested at 12 or 24 h. Hippocampal homogenates were analyzed for cleaved caspase-9 protein levels by western blot. Representative immunoblots are shown above (a) while densitometric intensity values are shown below (b). Data represent the mean ± SEM from three independent experiments performed in triplicate. *p<0.05, **p<0.005. MeHg increased the levels of caspase-9 protein in the hippocampus at 12 and 24 h.
Figure 4. Effect of 5 μg/g MeHg exposure on cleaved caspase-8 protein levels in postnatal rat hippocampus.
P7 rats were exposed to a single sc injection of 5 μg/g MeHg or vehicle and then hippocampi were harvested at 12 or 24 h. Hippocampal homogenates were analyzed for cleaved caspase-8 protein levels by western blot. Representative immunoblots are shown above (a) while densitometric intensity values are shown below (b). Data represent the mean ± SEM from three independent experiments performed in triplicate. **p<0.005. MeHg increased the levels of caspase-8 protein in the hippocampus at 24 h.
Figure 5. Effect of 5 μg/g MeHg exposure on cleaved caspase-3 protein levels in postnatal rat hippocampus.
P7 rats were exposed to a single sc injection of 5 μg/g MeHg or vehicle and then hippocampi were harvested at 24 h. Hippocampal homogenates were analyzed for cleaved caspase-3 protein levels by western blot. Representative immunoblots are shown above (a) while densitometric intensity values are shown below (b). Data represent the mean ± SEM from three independent experiments performed in triplicate. *p<0.05. MeHg increased the levels of caspase-3 protein in the hippocampus at 24 h.
3.2 Caspase-9 is required for activation of caspase-3 and caspase-8 in vitro
The foregoing in vivo experiments indicate that MeHg exposure of P7 rats elicits an early increase in caspase-9 and later increases in caspace-8 and caspase-3 in hippocampus. Caspases exhibit catalytic and substrate recognition motifs that are highly conserved (Cryns and Yuan, 1998). The substrate preferences or specificities of individual caspases have been exploited for the development of peptides that successfully compete and irreversibly bind to a particular caspase (Talanian et al., 1997, Cryns and Yuan, 1998, Garcia-Calvo et al., 1998). While the roles of caspase-9 and −8 in subsequent activation of caspase-3 may be most informatively addressed by using caspase-specific inhibitors in vivo, the small size and ongoing nursing of P7 rat pups precluded use of ICV pumps for drug delivery. Thus to determine the relative contributions of the initiator caspases in caspase-3 activation, we used select caspase inhibitors in a previously developed cell culture model of E14.5 cerebral cortical precursors (Burke et al., 2006, Falluel-Morel et al., 2007). This model uses a relatively homogeneous and abundant population of proliferating neuronal (not glial) precursors that maintain survival in culture. It is important to have neural precursors because, based on previously published data on developing hippocampus, the mitotic precursors are especially sensitive to MeHg exposure in vivo (Table 1). In contrast to this precursor model, the postnatal hippocampus is comprised of primarily postmitotic, differentiated cells of diverse lineages including neurons, astrocytes, oligodendrocytes and microglia, as well as neural stem cells, making consistent target cell composition and identification difficult. In addition, cultures from the postnatal hippocampus would not provide the robust number of neuronal precursors generated from E14.5 cortical dissections.
To study activation of caspases, primary cortical precursors from E14.5 rats were plated for a total of 5 h, a time when MeHg exposure produces no changes in cell survival. We employed 1.5 μM MeHg because this exposure elicited decreases in DNA synthesis and activation of caspase-3 similar to those observed in the hippocampus in vivo (Burke et al., 2006, Falluel-Morel et al., 2007). Specific caspase inhibitors (z-IETD-FMK or z-LEHD-FMK) were added 30 min after plating and MeHg was added 1 h after plating. Non-fluorescent substrates of caspase-3, −8, and −9 that yield fluorescent signal upon cleavage were added 3 h after MeHg, and cells were collected 1 h later for analysis.
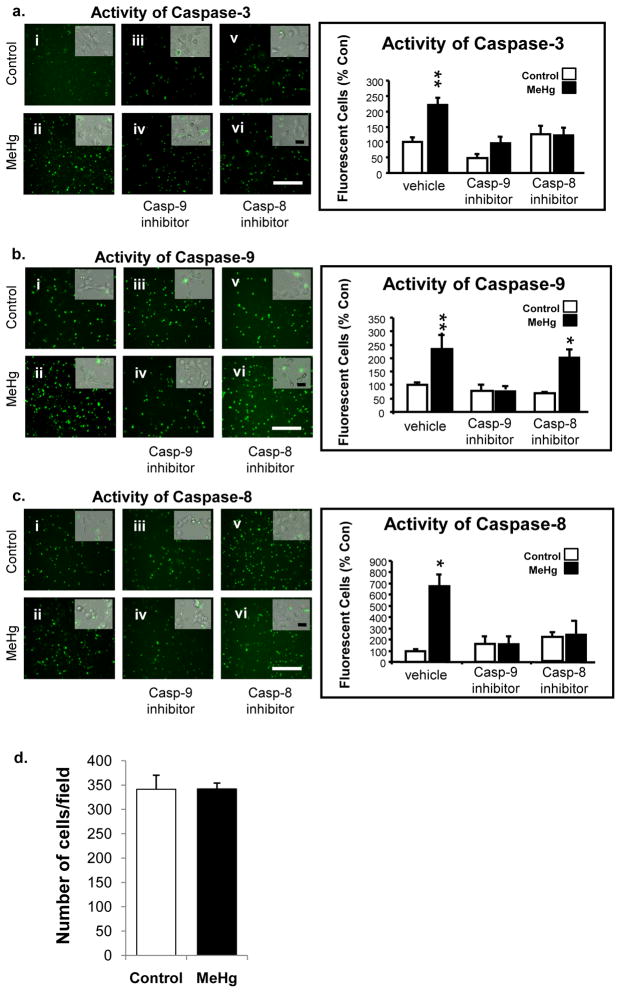
For these studies cells were exposed to 1.5 μM MeHg, a concentration of MeHg that yields no differences in cell numbers at 6 h (Fig. 6d), as previously published (Burke et al., 2006). Four hours after MeHg addition, there were increases in cells exhibiting activation of caspase-3, −8, and −9 (Fig. 6: a-i, a-ii, b-i, b-ii, c-i, c-ii). Clear increases in the proportion of cells expressing activated cell death caspases indicate an induction of apoptosis. Additionally, increases in caspase-8 and caspase-9 activity in vivo and in vitro, raise the possibility that MeHg may activate both mitochondrial-dependent and -independent cascades. To further define mediating pathways and interrelationships, we used caspase-8 and caspase-9 specific inhibitors in the same model.
Figure 6. Caspase activity in the presence of 1.5 μM MeHg and effects of caspase inhibitors in primary cultures.
E14.5 cortical neurons were treated with caspase inhibitors (caspase-8 or −9) at −0.5 h and 1.5 μM MeHg at 0 h. Specific non-fluorescent caspase substrates (caspase-3, −8, or −9) were added at 3 h and fluorescence indicating enzyme activation was analyzed at 4 h. Five fields were analyzed for each condition and the experiment was performed three times (N=3). Representative fields for caspase-3, caspase-9 or caspase-8 activities are shown on the left panel (a, b, c, respectively) and quantification of the number of fluorescent cells is presented as a percent of control on the right. Insets are merged phase and fluorescence images of the living cultures assessed at 5 h. Scale bar = 100 μm (10 μm for inlay); *p<0.05, **p<0.005. In the presence of MeHg, the caspase-9 inhibitor blocked the increase of caspase-9 activity as well as the activity of both caspase-3 and caspase-8. In the presence of the caspase-8 inhibitor and MeHg, there was no effect on the activity of caspase-9. (d) Histogram representing no change in the number of total cells/field between control and MeHg groups.
When caspase-9 was inhibited, activities of all three MeHg induced caspases were reduced to control levels (Fig. 6, panel a-iii, a-iv, b-iii, b-iv, c-iii, c-iv), suggesting that caspase-9 likely leads to activation of both caspase-3 and caspase-8. Specifically, 4 h after exposure to MeHg, caspase-3 activity increased more than 2-fold. However, in the presence of the caspase-9 inhibitor, caspase-3 activity levels were no different than control, suggesting a decrease in MeHg-induced caspase-3 activity with caspase-9 inhibition. In the presence of MeHg, caspase-8 activity was markedly increased, but in the presence of caspase-9 inhibitor, the increase was almost entirely blocked, suggesting caspase-9 activity is required for caspase-8 activation.
In contrast, when the caspase-8 inhibitor was present, the MeHg-induced activity of caspase-9 failed to decline significantly (Fig. 6: b-v, b-vi), suggesting that caspase-9 is upstream of caspase-8. Moreover, caspase-3 activation was blocked by both inhibitors (Fig. 6: a-iii, a-iv, a-v, a-vi), suggesting it may respond to either upstream initiator caspase.
3.3 Low-exposures of MeHg induce apoptosis markers in the developing hippocampus
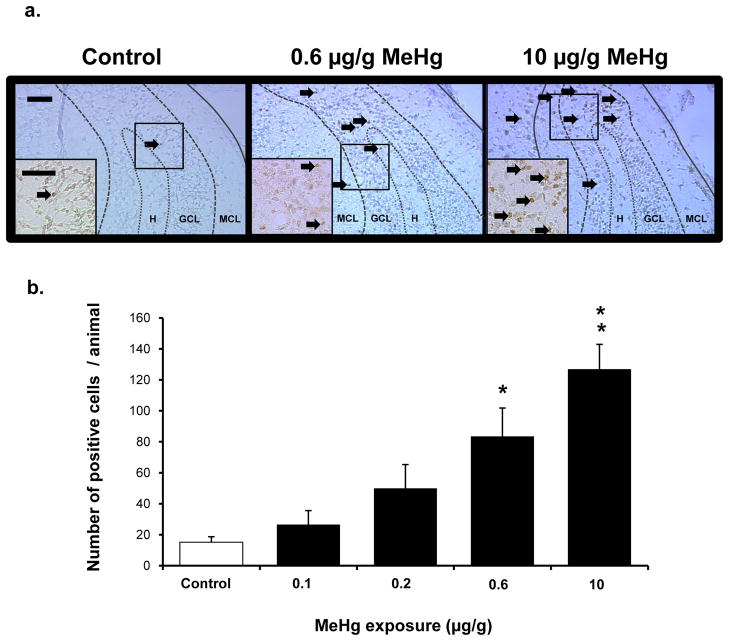
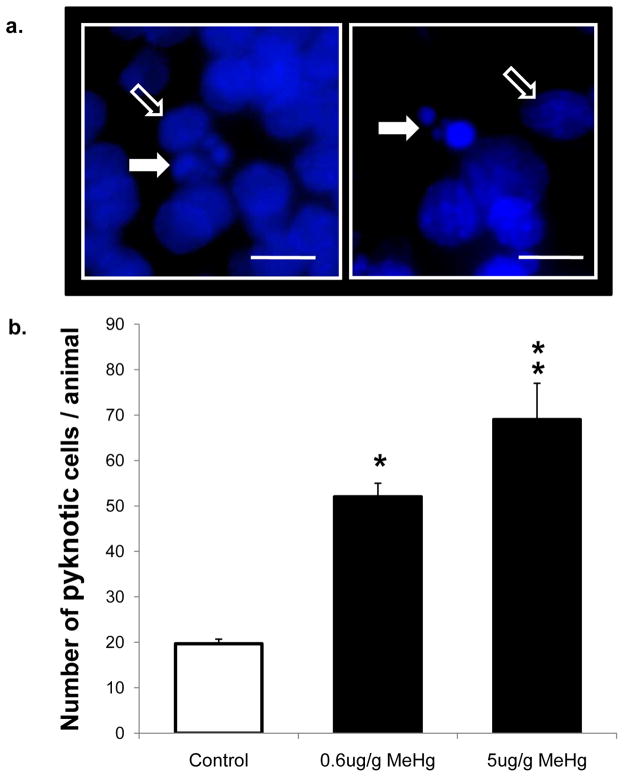
Given that we observed the greatest changes in protein levels of caspase-3 in vivo after MeHg exposure, we used this molecular marker to define the lowest exposures of the neurotoxicant that elicit hippocampal injury. Further, we employed immunohistochemical analysis because it allowed single cell assessment of the vulnerable stem cells that were proliferating within the dentate gyrus (Falluel-Morel et al., 2007). An exposure of 0.6 μg/g MeHg elicited a 5-fold increase in caspase-3 immunoreactive cells in the hippocampus 24 h after MeHg injection (Fig. 7), with a trend for exposure at 0.2 μg/g (p=0.07). This magnitude of effect is in the range of that previously reported for 5 μg/g MeHg on apoptotic markers (Falluel-Morel et al., 2007) and similar to the 8-fold increase observed at a far higher exposure of 10 μg/g used as positive control in this current study (Fig. 7). In order to further analyze the presence of apoptosis after MeHg exposure, pyknotic cells were counted. There was a 3–4 fold increase in pyknotic nuclei at both 0.6 μg/g MeHg and 5 μg/g MeHg 24h after exposure (Fig. 8). Parallel increases in caspase-3 immunoreactive cells and pyknotic nuclei in the hilus further support the hypothesis that increased caspase-3 activation reflects cell death induction by MeHg exposure in vivo.
Figure 7. Effects of low levels of MeHg on caspase-3 activation in postnatal hippocampus.
P7 rats received a single sc injection of various exposures of MeHg ranging from 0.1 to 10 μg/g, were euthanized at 24 h and processed for immunostaining (see Materials and methods). Cleaved caspase-3 positive cells were counted in hippocampi (including CA1-3 and dentate gyrus) from 5 animals per group, using four sections per animal. Data are expressed as the total number ± SEM of positive cells detected on 4 sections from each of 5 animals per group. *p<0.005, **p<0.0001. Representative fields of the dentate gyrus are shown above at 200x and 400x (a) and quantification of the number of caspase+ cells is presented as average number of cells per animal (b). In control animals, very few caspase-3-positive cells were detected in the hippocampus, while a dose-dependent increase in positive cells was observed in MeHg-exposed animals. Scale bar = 50 μm. H: hilus, GCL: granule cell layer, Mol: molecular cell layer.
Figure 8. Pyknotic nuclei present in the hippocampal hilus 24 h after exposure to 0.6 μg/g and 5 μg/g MeHg.
P7 rats received a single sc injection of MeHg (0, 0.6, or 5μg/g) and were euthanized at 24 h. Pyknotic cells were identified morphologically with DAPI and counted in the hilus of the hippocampi. Above images present representative pyknotic morphologies with nuclear DNA fragments characteristic of apoptosis (white arrows) and non-pyknotic nuclei (open arrows) at 630x (a). Quantification of the number of pyknotic cells is presented below (b). In control animals, few pyknotic cells were detected in the hilus, while a dose-dependent increase in pyknotic cells was observed in MeHg-exposed animals. Data are expressed as the total number ± SEM of positive cells detected on 4 sections from each of 3 animals per group. Animals were taken from three independent experimental litters. *p<0.005, **p<0.0001. Scale bar = 10 μm.
4. Discussion
Our observations suggest that MeHg exposure of the developing hippocampus in vivo induces mitochondrial-dependent cell death via sequential activation of caspase-9 and caspase-3. Further, by using single cell analysis, we find that an unexpectedly low MeHg exposure induces cell death in the hippocampal dentate gyrus, a region where previous studies found mitotic precursors as vulnerable targets.
The initial studies used an acute-exposure animal model in which P7 rat pups were injected subcutaneously with 5 μg/g MeHg. This level of MeHg exposure is half that used previously to define the kinetics of mercury entry into the brain, which measured hippocampal tissue burdens of 714 ppb total Hg at 7 hours (Burke et al., 2006). This concentration of Hg seems to approach clinical relevance when compared to levels found in Seychellois infant brain tissues (~200 – 300 ppb) (Lapham et al., 1995). Levels of MeHg in our culture models were based on reproducing the biological effects of 5 μg/g MeHg exposure on hippocampal mitosis and apoptosis, and were not extrapolated from the putative concentration in the dentate gyrus. Most human exposures to MeHg occur from chronic low levels; however, our acute model provides a means to analyze the temporal sequence of events that occur in an apoptotic cascade at relevant tissue levels, a model used previously by Rodier and colleagues (Rodier et al., 1984). Serendipitously, by utilizing single cell analysis, a ten-fold lower level of MeHg (0.6 μg/g) was found to produce 3–5-fold increases in caspase-3 activation and pyknotic nuclei. MeHg is effecting a minor subpopulation of postnatal hippocampal cells, thus using whole hippocampal homogenates, composed primarily of unaffected cells, may limit sensitivity of detecting the lowest exposure that elicits toxic effects.
In the developing hippocampus, the sequential increases in Bax, release of cytochrome c, cleavage of caspase-9 and activation of caspase-3 demonstrate the involvement of the mitochondrial-dependent cascade after MeHg exposure in vivo. Further work using specific caspase inhibitors substantiated the primary role of caspase-9 in caspase-3 activation. Previously, it was known that MeHg-induced caspase-3 activation in the brain and in cells engaged in mitotic S-phase (Falluel-Morel et al., 2007); however the upstream mechanisms were unknown. Involvement of the mitochondrial-dependent cascade has been shown in culture models after MeHg exposure (Tamm et al., 2006); however this has not been demonstrated in the developing hippocampus. In addition, the involvement and necessity of caspase-9 activity in caspase-3 cleavage was defined in vitro. Similar changes in the levels of mitochondrial proteins in vivo suggest that caspase-9 may play the same role in both model systems. By virtue of a cascade, we might expect that proteins downstream would have the most robust changes. We took practical advantage of these mechanistic insights by translating the most robust signal as an early indicator of neuronal injury that may lead to later behavioral deficits. Caspase-3 is an effector caspase known to play a role in apoptosis and cell differentiation (Chaly et al., 1984, Fernando et al., 2005, Ohsawa et al., 2008, Ohsawa et al., 2010). By identifying significant increases in caspase-3 activation and nuclear pyknosis on single cell analysis, we were able to detect hippocampal cell death in vivo at ten-fold lower levels of MeHg exposure (0.6 μg/g) than previously reported. Thus mitochondrial-dependent cell death in the hippocampus may serve as a sensitive index for teratogenic insults to the developing brain.
In our initial studies of the developing hippocampus, MeHg-induced changes in proteins constituting the mitochondrial-dependent cascade were identified in vivo. Increases in pro-apoptotic Bax occurred 2 h before increases in cytosolic cytochrome c at 4 h. Subsequently there was a significant increase in cleaved caspase-9 at 12 and 24 h and an increase in caspase-8 activation at 24 h. Although these changes may appear small, the sequence and timing is consistent with what is known in more robust models of toxicity. We believe that the small changes we detect are the consequences of measuring the response of only a vulnerable subpopulation of proliferating forebrain cells contributing to the protein measures.
Our culture model was selected in order to focus analysis on MeHg effects on proliferating forebrain precursors that may model the effects we observed in vivo. Previous work in vivo demonstrated the vulnerability of mitotic cells to MeHg exposure (Table 1). We selected a culture system consisting of proliferating forebrain neural precursors to further define the mechanism by which MeHg induces activation of caspase-3 in a mitotic population. Increased activity of caspase-3, −8, and −9 was detected in this culture model following MeHg exposure, paralleling effects we observed in vivo. While caspase-8 was induced both in vivo and in culture, inconsistencies in the magnitude of activation in the two models may be due to differences in the proportion of vulnerable mitotic cells, in the length of metal exposure or the origins/developmental ages of the model cells. Inhibition studies revealed that although there is considerable contribution of caspase-8 activity in caspase-3 cleavage, this may take place downstream of caspase-9. The upstream role of caspase-9 was demonstrated when caspase-8 activity was completely blocked by caspase-9 inhibition, whereas inhibition of caspase-8 had no effect on caspase-9 activity. A downstream role for caspase-8 in vivo may be inferred from its late induction occurring at 24 h, whereas caspase-9 was elevated already by 12 h. Although caspase-8 and −9 may be differentially regulated in neural precursor cells (Tamm et al., 2004, Knight et al., 2010, Ohsawa et al., 2010), their role in caspase-3 activation was demonstrated when inhibition of caspase-8 or −9 led to a decrease in caspase-3 activity. Although some reports have suggested non-specific activity with high concentrations of the caspase-8 inhibitor, z-IETD-FMK (Siegel, 2006), our study indicates that caspase-9 activity is unperturbed, and therefore consistent with the selectivity of the caspase-8 inhibitor. Selectivity of caspase inhibitors may have been achieved through the short incubation times we used (<5 h), limiting the non-specific interactions temporally. Considered in concert with the in vivo increases in Bax and cytosolic cytochrome c at early times, these results suggest that while other initiator caspases may cleave caspase-3, MeHg works primarily through the mitochondrial-dependent activation of caspase-9. The contribution of caspase-8 to caspase-3 activation is significant; however, it may be downstream of caspase-9 activation. These observations in the brain are important because previous work found MeHg acts through mitochondrial pathways in other organs and cell culture models, including neuronal cell lines (Tamm et al., 2006, Ceccatelli et al., 2007, Cuello et al., 2010). Our work extends these studies by directly relating upstream components of the mitochondrial cascade to the effects of MeHg in vivo on the developing hippocampus.
Our model is focused on determining the effects of MeHg on the developing hippocampus, a region critical for learning and memory. Multiple human and animal studies have found negative effects on learning and memory following MeHg exposure (Zenick, 1974, Olson and Bousch, 1975, Grandjean et al., 1997, Grandjean et al., 1998, Rice, 2000, Murata et al., 2004, Falluel-Morel et al., 2007, Onishchenko et al., 2007). Direct behavioral studies in humans certainly emphasize the concern for society. Yet, the mechanism through which MeHg affects cell survival in the hippocampus may provide insight on how the toxicant affects human learning and memory. In this paper we have begun to use these mechanistic insights to define an early indicator of cell loss, which may lead to behavioral deficits in the rat that begin to model deficits observed in human studies. Single cell analysis of tissue sections looking at the most responsive marker of change, caspase-3, enabled us to detect significant changes following a level of MeHg (0.6 μg/g) that begins to approximate human exposures (Lapham et al., 1995, Burke et al., 2006). In addition, this level of MeHg exposure that elicits significant increases in caspase-3 and pyknosis is equivalent to a single daily exposure in some chronic exposure paradigms that model human exposures (Newland and Reile, 1999). Our previous studies have demonstrated that MeHg-induced apoptosis occurred in proliferative precursors in the hilus with caspase-3 co-labeling cells in S-phase (Falluel-Morel et al., 2007). Although cells undergoing neurogenesis were known to be targets of MeHg, our studies demonstrate how remarkably sensitive proliferative neural progenitor cells can be to toxic exposures in vivo. These studies also lay the foundation for future investigations of the structural and behavioral effects of these low MeHg exposures. The literature points to a number of mechanisms upstream of the mitochondrial pathway in vivo including calcium signaling, microtubule disruption, protein synthesis disruption, and induction of oxidative stress (Sager et al., 1983, Vogel et al., 1985, Sager and Matheson, 1988, Atchison and Hare, 1994, Barone et al., 1998, Dey et al., 1999, Ceccatelli et al., 2007). Using the same models presented here, we may begin to define these upstream mechanisms.
Acknowledgments
This work was supported by grants from the National Institute of Health NS062591, ES07148, ES05022, ES11256; United States Environmental Protection Agency R829391; and a fellowship from La Fondation pour la Recherche Médicale SPE2006. We would also like to thank Dr. K. Reuhl and Dr Lisa Opanashuk for critical reading and valuable comments.
Footnotes
Conflict of interest statement
No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J Comp Neurol. 1990;301:343–364. doi: 10.1002/cne.903010303. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Barone S, Jr, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Brain Res Dev Brain Res. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke K, Cheng Y, Li B, Petrov A, Joshi P, Berman RF, Reuhl KR, DiCicco-Bloom E. Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E. Neurotoxicology. 2006;27:970–981. doi: 10.1016/j.neuro.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccatelli S, Tamm C, Zhang Q, Chen M. Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol Behav. 2007;92:87–92. doi: 10.1016/j.physbeh.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Chaly N, Bladon T, Setterfield G, Little JE, Kaplan JG, Brown DL. Changes in distribution of nuclear matrix antigens during the mitotic cell cycle. J Cell Biol. 1984;99:661–671. doi: 10.1083/jcb.99.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Cuello S, Goya L, Madrid Y, Campuzano S, Pedrero M, Bravo L, Camara C, Ramos S. Molecular mechanisms of methylmercury-induced cell death in human HepG2 cells. Food Chem Toxicol. 2010;48:1405–1411. doi: 10.1016/j.fct.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myer GJ, Shamlaye C, Cox C, Gao P, Axtell C, Morris D, Sloane-Reeves J, Cernichiari E, Choi A, Palumbo D, Clarkson TW. Association between prenatal exposure to methylmercury and developmental outcomes in Seychellois children: effect modification by social and environmental factors. Neurotoxicology. 1999;20:833–841. [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JM, Robson PJ, Duffy EM, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Clarkson TW. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29:767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ, Ichas F. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- Dey PM, Gochfeld M, Reuhl KR. Developmental methylmercury administration alters cerebellar PSA-NCAM expression and Golgi sialyltransferase activity. Brain Res. 1999;845:139–151. doi: 10.1016/s0006-8993(99)01887-9. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem. 2007;103:1968–1981. doi: 10.1111/j.1471-4159.2007.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P, Brunette S, Megeney LA. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J. 2005;19:1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Murata K, Budtz-Jorgensen E, Weihe P. Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J Pediatr. 2004;144:169–176. doi: 10.1016/j.jpeds.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ Res. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Knight JC, Scharf EL, Mao-Draayer Y. Fas activation increases neural progenitor cell survival. J Neurosci Res. 2010;88:746–757. doi: 10.1002/jnr.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Lapham LW, Cernichiari E, Cox C, Myers GJ, Baggs RB, Brewer R, Shamlaye CF, Davidson PW, Clarkson TW. An analysis of autopsy brain tissue from infants prenatally exposed to methymercury. Neurotoxicology. 1995;16:689–704. [PubMed] [Google Scholar]
- Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc Natl Acad Sci U S A. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DO, Clarkson TW, Myers GJ, Davidson PW, Cox C, Cernichiari E, Tanner MA, Lednar W, Shamlaye C, Choisy O, et al. The Seychelles study of fetal methylmercury exposure and child development: introduction. Neurotoxicology. 1995;16:583–596. [PubMed] [Google Scholar]
- Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- Murata K, Sakamoto M, Nakai K, Weihe P, Dakeishi M, Iwata T, Liu XJ, Ohno T, Kurosawa T, Kamiya K, Satoh H. Effects of methylmercury on neurodevelopment in Japanese children in relation to the Madeiran study. Int Arch Occup Environ Health. 2004;77:571–579. doi: 10.1007/s00420-004-0542-1. [DOI] [PubMed] [Google Scholar]
- Murata K, Weihe P, Araki S, Budtz-Jorgensen E, Grandjean P. Evoked potentials in Faroese children prenatally exposed to methylmercury. Neurotoxicol Teratol. 1999;21:471–472. doi: 10.1016/s0892-0362(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Tanner MA, Choisy O, Sloane-Reeves J, Marsh D, Cernichiari E, Choi A, et al. Neurodevelopmental outcomes of Seychellois children sixty-six months after in utero exposure to methylmercury from a maternal fish diet: pilot study. Neurotoxicology. 1995a;16:639–652. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Tanner MA, Marsh DO, Cernichiari E, Lapham LW, Berlin M, Clarkson TW. Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology. 1995b;16:711–716. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Shamlaye CF, Axtell CD, Cernichiari E, Choisy O, Choi A, Cox C, Clarkson TW. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology. 1997;18:819–829. [PubMed] [Google Scholar]
- Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol Sci. 1999;50:106–116. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Hamada S, Kuida K, Yoshida H, Igaki T, Miura M. Maturation of the olfactory sensory neurons by Apaf-1/caspase-9-mediated caspase activity. Proc Natl Acad Sci U S A. 2010;107:13366–13371. doi: 10.1073/pnas.0910488107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Hamada S, Yoshida H, Miura M. Caspase-mediated changes in histone H1 in early apoptosis: prolonged caspase activation in developing olfactory sensory neurons. Cell Death Differ. 2008;15:1429–1439. doi: 10.1038/cdd.2008.71. [DOI] [PubMed] [Google Scholar]
- Olson K, Bousch GM. Decreased learning capacity in rats exposed prenatally and postnatally to low doses of mercury. Bull Environ Contam Toxicol. 1975;13:73–79. doi: 10.1007/BF01684867. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Tamm C, Vahter M, Hokfelt T, Johnson JA, Johnson DA, Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27:721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC. Identification of functional domains affected by developmental exposure to methylmercury: Faroe islands and related studies. Neurotoxicology. 2000;21:1039–1044. [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Early chronic low-level methylmercury poisoning in monkeys impairs spatial vision. Science. 1982;216:759–761. doi: 10.1126/science.7079739. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Aschner M, Sager PR. Mitotic arrest in the developing CNS after prenatal exposure to methylmercury. Neurobehav Toxicol Teratol. 1984;6:379–385. [PubMed] [Google Scholar]
- Sager PR, Doherty RA, Olmsted JB. Interaction of methylmercury with microtubules in cultured cells and in vitro. Exp Cell Res. 1983;146:127–137. doi: 10.1016/0014-4827(83)90331-2. [DOI] [PubMed] [Google Scholar]
- Sager PR, Matheson DW. Mechanisms of neurotoxicity related to selective disruption of microtubules and intermediate filaments. Toxicology. 1988;49:479–492. doi: 10.1016/0300-483x(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Shamlaye CF, Marsh DO, Myers GJ, Cox C, Davidson PW, Choisy O, Cernichiari E, Choi A, Tanner MA, Clarkson TW. The Seychelles child development study on neurodevelopmental outcomes in children following in utero exposure to methylmercury from a maternal fish diet: background and demographics. Neurotoxicology. 1995;16:597–612. [PubMed] [Google Scholar]
- Shirai K, Mizui T, Suzuki Y, Kobayashi Y, Nakano T, Shirao T. Differential effects of x-irradiation on immature and mature hippocampal neurons in vitro. Neurosci Lett. 2006;399:57–60. doi: 10.1016/j.neulet.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Siegel RM. Caspases at the crossroads of immune-cell life and death. Nature. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Martin DA, Zheng L, Ng SY, Bertin J, Cohen J, Lenardo MJ. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–1253. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem. 2006;97:69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Tamm C, Robertson JD, Sleeper E, Enoksson M, Emgard M, Orrenius S, Ceccatelli S. Differential regulation of the mitochondrial and death receptor pathways in neural stem cells. Eur J Neurosci. 2004;19:2613–2621. doi: 10.1111/j.0953-816X.2004.03391.x. [DOI] [PubMed] [Google Scholar]
- Vogel DG, Margolis RL, Mottet NK. The effects of methyl mercury binding to microtubules. Toxicol Appl Pharmacol. 1985;80:473–486. doi: 10.1016/0041-008x(85)90392-8. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zenick H. Behavioral and biochemical consequences in methylmercury chloride toxicity. Pharmacol Biochem Behav. 1974;2:709–713. doi: 10.1016/0091-3057(74)90098-7. [DOI] [PubMed] [Google Scholar]