Abstract
Background
Islets are susceptible to damage by proinflammatory cytokines, via activation of transcription factor NF-κB. We hypothesized that inhibition of NF-κB activity will decrease cytokine-mediated β cell injury and improve islet transplant functional outcome.
Methods
We created a transgenic mouse expressing a degradation resistant N-terminally deleted IκBα (ΔNIκBα) under control of the TetOn system under the rat insulin promoter. Isolated islets from transgenic and control mouse strains were exposed to cytokines in vitro and assayed or transplanted.
Results
ΔNIκBα was significantly increased with doxycycline treatment according to Western blot. Cytokine-induced NF-κB activation was significantly decreased in transgenic (0.065±0.013 abs. value/µg protein) versus control islets (0.128±0.006; p<0.05). Suppression of cytokine-mediated NF-κB activity reduced expression of iNOS, MCP-1 and IP-10 RNA transcripts and significantly reduced nitric oxide production in transgenic islets (0.084±0.043 µM/µg protein) versus controls (0.594±0.174; p<0.01). The insulin stimulation index in islets exposed to cytokines was higher in transgenic versus controls (1.500 ± 0.106 vs. 0.800 ± 0.098; p<0.01). Syngeneic transplants of a marginal mass of intraportally infused transgenic islets resulted in a reversion to euglycemia in 69.2% of diabetic recipients at a mean of 7.8±1.1 days, versus 35.7% of control islet recipients reverting at a mean of 15.8±2.9 days (p<0.05).
Conclusions
Conditional and specific suppression of NF-κB activity in β cells protected islets from cytokine-induced dysfunction, in vitro and in vivo. These results provide a proof of principle that inhibition of NF-κB activity in donor islets enhances function and improves the outcome of islet transplantation.
Introduction
Islet transplantation is a promising treatment for select patients with type 1 diabetes.1,2 Methods to mitigate islet injury, and specifically β cell injury, in the early engraftment period are hypothesized to improve functional results. Immune-mediated injury of transplanted islets involves both classic allo-specific T-cell responses as well as early host non-specific inflammatory responses involving infiltration of the islet graft by macrophages and release of proinflammatory cytokines such as IL-1β, TNF-α and IFN-γ.3–6 Proinflammatory cytokines act directly on the β cells, upregulating inducible nitric oxide synthase (iNOS), that is detrimental to β cell viability and function through generation of nitric oxide and subsequent peroxynitrite formation.7–10 In addition, induction of macrophage chemokine protein-1 (MCP-1) and interferon-γ inducible protein-10 (IP-10), which draw immune cells into the islet graft7,11–14, and interleukin-15 (IL-15), a powerful growth factor and activator of T cells14,15, exacerbate an injurious host response. Prolonged exposure to these cytokines leads to cell death by apoptosis in both murine and human islets.16–18
The transcription factor NF-κB is activated in β cells by proinflammatory cytokines and plays a critical role in regulating expression of iNOS, MCP-1, IP-10 and IL-15 in mouse and human islets.7,14 In resting β cells, NF-κB is bound by its inhibitory protein IκBα and resides in the cytoplasm. Upon stimulation, IκBα is phosphorylated at two serine residues in the N-terminal region. This results in IκBα dissociation from NF-κB allowing NF-κB to translocate to the nucleus and upregulate a wide variety of genes. We have previously shown that stably transfected MIN6 cells expressing a mutant IκBα protein lacking the two serine phosphorylation sites (IκBαM) inhibited cytokine-induced NF-κB activity and protected the cells from dysfunction and cell death.19 Additionally, using the same MIN6 cell line, we have demonstrated that cytokine-induced chemokine expression was reduced when NF-κB activity was inhibited.20 Protection of β cells from proinflammatory cytokines is not limited to mouse β cells, as expression of an IκBα super repressor in human islets, delivered using an adenoviral vector, protected those cells from IL-1β-mediated dysfunction and cell death.21
While inhibition of NF-κB protects β cells from the deleterious effects of IL-1β, TNF-α and IFN-γ in vitro, nothing is known about the effects of inhibiting NF-κB in an in vivo transplant setting. Eldor, et. al. demonstrated that inhibition of NF-κB activity specifically in the β cells, using the TetOn system under the control of the rat insulin promoter to express an N-terminally deleted IκBα protein, protected the islets from multiple low dose streptozotocin injections.22 Here we present data using a similar mouse model to conditionally inhibit NF-κB activity in the β cells (RIP-rtTA-luciferase(Renilla); luciferase(Firefly)-TetO-ΔNIκBα). Inhibition of NF-κB in islets isolated from the transgenic animals resulted in enhanced islet survival and function both in vitro and in an in vivo marginal mass isogeneic islet transplant model.
Methods and Materials
Creation of transgenic mouse model
A double transgenic mouse strain Tg(PRIP-rtTA-M2-hRL/Ptet-ΔNIκBα-Luc) was generated. The rtTA-M2 gene is a revised/mutated version of the original rtTA generated by the laboratory of Dr. Hermann Bujard at the University of Erlangen, Germany. rtTA-M2 functions at 10-fold lower doxycycline concentrations than rtTA, has enhanced stability in eukaryotic cells, and causes less background expression in the absence of doxycycline. A plasmid containing the rtTA-M2 was obtained from Dr. Bujard (PhCMV-rtTA-M2). We then generated a construct PRIP-rtTA-M2-hRL by replacing the promoter sequence with the 683bp rat insulin II promoter, ligating the PRIP-rtTA-M2 sequence with a synthetic Renilla Luciferase reporter sequence (hRluc, Promega), linked via the internal ribosome entry site (IRES, Clontech). The purified DNA fragment was microinjected into the pronuclei of C57BL/6 × BALB/c zygotes. This line was crossbred with another transgenic mouse line, Tg(Ptet-ΔNIκBα-Luc), a generous gift from Dr. Yinon Ben-Neriah from The Hebrew University-Hadassah Medical School, Israel. This strain of mouse expresses a ΔNIκBα and a Firefly luciferase reporter gene under the control of a tetracycline-responsive bi-directional vector.23 Several double transgenic mouse lines were generated and the line with the highest expression of rtTA coupled with the strongest induction of ΔNIκBα was used for all experiments reported here.
Isolation of pancreatic islets from donor mice
Unless specified, all donor mouse islets used in the study were isolated from control (−rtTA/+ΔNIκBα) and transgenic (+rtTA/+ΔNIκBα) mice that were treated with doxycycline (Sigma Aldrich) in the drinking water (2mg/mL) for 3 weeks prior to the isolation to induce expression of the transgene. Islets were isolated as described previously.24,25 The islets were then cultured in RPMI 1640 containing 10% FBS, 100 U/mL penicillin G, 100 µg/mL streptomycin sulfate and 2µg/mL doxycycline at 37°C, 5% CO2.
Western blot analysis
The isolated islets as stated above were lysed using the RIPA lysis buffer. Protein from each was run on 4–20% Tris-HCl gels and transferred to PVDF membranes. The primary antibody against IκBα (C-21 rabbit polyclonal antibody, Santa Cruz Biotechnologies) was added at a dilution of 1:100 in TBST, 5% blocker for 1 hour at room temperature. Secondary antibody was added at a dilution of 1:2000 in TBST for 1 hour at room temperature. The blot was developed using the Amersham ECL detection system (GE Healthcare).
Luciferase assay
Islets were isolated from control (−rtTA/+ΔNIκBα) and transgenic (+rtTA/+ΔNIκBα) mice treated with or without doxycycline (2 mg/mL) for 3 weeks. Islets were lysed using the Passive Lysis Buffer from Promega and luciferase activity of both Renilla and Firefly luciferase measured using Promega’s Dual Luciferase Reporter Assay system.
Bioluminescent imaging of transgenic mice
Mice were anesthetized using isoflurane and injected with either the Renilla luciferase substrate coelenterazine (Promega) or the firefly luciferase substrate luciferin (BioGold) to detect the presence of the RIP-rtTA-luciferase(Renilla) and luciferase(Firefly)-TetO-ΔNIκBα transgene, respectively. For the Firefly luciferase detection, mice were previously given doxycycline in the drinking water (2mg/mL) for 3 weeks prior to imaging. Imaging was done using the Xenogene IVIS 100 imaging instrument approximately 6 minutes after substrate injection.
NF-κB Activation Assay
Islets were isolated from control (−rtTA/+ΔNIκBα) and transgenic (+rtTA/+ΔNIκBα) mice that had received doxycycline in the drinking water for 3 weeks prior to isolation. The islets were cultured overnight in RPMI 1640 with doxycycline (2mg/mL). The islets were then stimulated with 50 U/mL IL-1β, 1000 U/mL TNF-α and 750 U/mL IFN-γ for 1 hour. Nuclear lysates were prepared using the Nuclear Extract kit from Active Motif (Carlsbad, CA). Activated NF-κB was measured using Active Motif’s NF-κB Activation ELISA Assay. Protein concentrations were determined using the Prostain Protein Quantification kit (Active Motif). Data were presented as absorbance units per µg of protein and are a combination of 3 independent experiments.
Analysis of gene expression changes in response to cytokine treatment using semiquantitative RT-PCR
Control (−rtTA/+ΔNIκBα) and transgenic (+rtTA/+ΔNIκBα) islets were isolated from donors treated with doxycycline (2 mg/mL) in the drinking water for 3 weeks. Islets were cultured with or without the cytokine mix in RPMI 1640 with doxycycline (2 µg/mL) for 24 hours. RNA was extracted using Trizol (Molecular Research Center, Cincinnati, OH) and BCP (Molecular Research Center). The amount of RNA in each sample was quantified by measuring the absorbance at 260 nm and 1 µg of RNA used for reverse transcription using Promega’s Reverse Transcription System. PCR was then preformed on the subsequent cDNA products for GAPDH, iNOS, MCP-1, IP-10 and IL-15 using the following reaction cycle: 1 cycle of 95°C for 2 minutes; 35 cycles of 95°C for 1 minute, 55°C for 1 minute, 72°C for 1 minute; 1 cycle of 72°C for 10 minutes. GoTaq DNA Polymerase and dNTPs were obtained from Promega. PCR primers were ordered from Integrated DNA Technologies, Inc. (Coralville, IA). Gel electrophoresis was run on the PCR products. GAPDH was used as a control to ensure that the same amount of cDNA was added to each PCR reaction tube.
Measurement of Nitric Oxide Production
Control (−rtTA/+ΔNIκBα) and transgenic (+rtTA/+ΔNIκBα) islets were isolated from mice treated with doxycycline (2mg/mL) in the drinking water for 3 weeks. Islets were cultured with or without cytokine mixture (CM: 50 U/mL IL-1β, 1000 U/mL TNF-α, 750 U/mL IFN-γ; R&D Systems) in RPMI 1640 with doxycycline (2mg/mL) for 24 hours. Media was collected from all conditions and the nitrite concentration measured using the Griess Reaction (Promega). The islets were collected, centrifuged at 2000 ×g for 4 minutes at 4°C and lysed. The amount of protein in each islet sample was assayed using the DC Protein Assay (BioRad) and the amount of nitrite produced standardized to the protein concentrations. Data are presented as nitrite concentration (µM) per µg protein and are a combination of 4 independent experiments.
Glucose stimulated insulin secretion assay
Control (−rtTA/+ΔNIκBα) and transgenic (+rtTA/+ΔNIκBα) islets were isolated from donors previously treated with doxycycline for 3 weeks. Islets were treated with or without CM in RPMI 1640 media supplemented with doxycycline (2 µg/mL) for 24 hours. Islets (approximately 15 per replicate) were allowed to equilibrate in Krebs solution (25 mM HEPES, 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 0.1% BSA [pH7.4]) containing 2.8 mM glucose. Next, the islets were incubated for 1 hour in Krebs solution containing 2.8 mM glucose and subsequently for another hour in 28 mM glucose-containing Krebs solution. The media from each 1-hour incubation was collected for insulin measurement using an insulin EIA kit (Alpco, Salem, NH). Data are presented as a ratio of the amount of insulin secreted by the islets exposed to high (28 mM) versus low glucose (2.8 mM). Data represent a combination of 3 independent experiments.
Transplantation of a marginal islet mass intrahepatically to streptozotocin-induced isogeneic recipients
Control (C57BL/6) and transgenic (+rtTA/+ΔNIκBα) were isolated from donors treated with doxycycline (2 mg/mL) in the drinking water for 3 weeks. Islets were cultured in RPMI 1640 containing 2 µg/mL doxycycline overnight prior to transplantation. Recipient mice (C57BL/6) were made diabetic via a single injection of streptozotocin (220 mg/kg body weight). Mice were considered diabetic after demonstrating 2 consecutive blood glucose readings above 300 mg/dL as measured using the One Touch Basic blood glucose meter (Lifescan, Milpitas, CA). 50 islets (control or transgenic) were hand counted and transplanted to the liver of recipient mice via the portal vein as described previously.25,26 Recipient mice were treated with doxycycline (2 mg/mL) in the drinking water post transplantation. Blood glucose levels were measured daily for the first 3 weeks, then 3 times per week after that. Mice were considered normoglycemic when 2 consecutive blood glucose readings were below 200 mg/dL. Data are presented as the percentage of mice achieving normoglycemia in each group versus days post transplantation. N=14 for the control islet recipient group and N=13 for the transgenic islet recipient group.
Statistical analyses
The student’s t-test and ANOVA tests were utilized for statistical analysis, where appropriate. P values of less than 0.05 were considered statistically significantly different.
Results
Generation of transgenic mouse strain
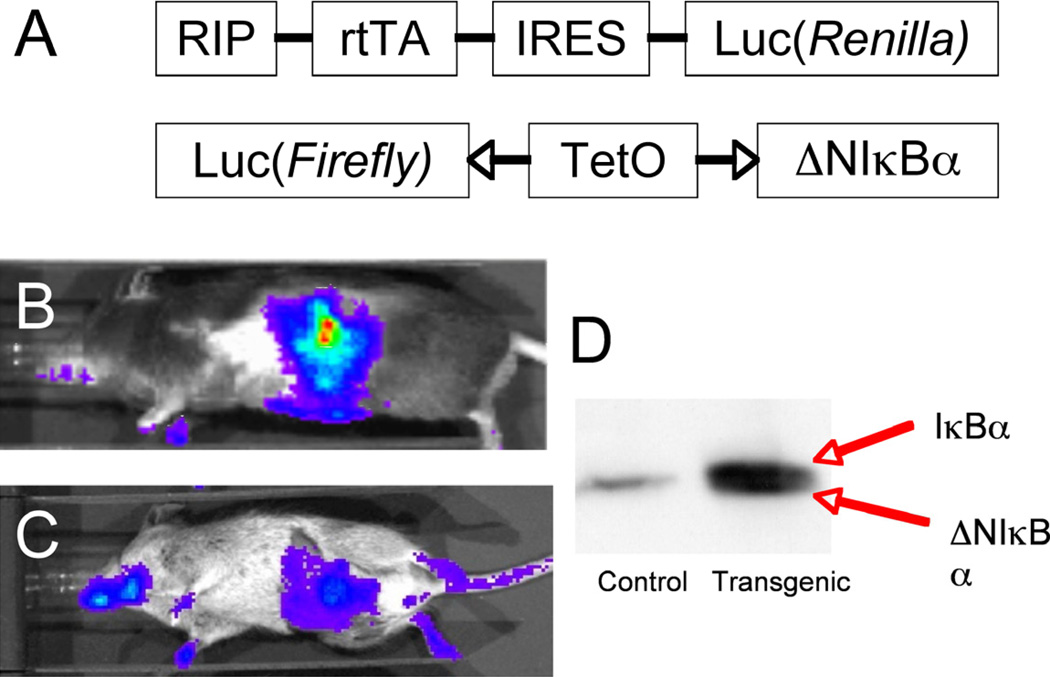
To generate a transgenic mouse strain in which we are able to inhibit NF-κB activity specifically and conditionally in the adult β cell, we utilized the TetOn system and bred a C57BL/6 RIP-rtTA-luc(Renilla) mouse strain with a Balb/c luc(Firefly)-TetO-ΔNIκBα strain (Figure 1A). Figure 1B and 1C shows the RIP-rtTA-luc(Renilla) and double transgenic strain, imaged using the Xenogen IVIS 100 system, demonstrating the presence of the rtTA transgene in 1B and the combination of transgenes (rtTA and ΔNIκBα) in 1C. The resultant double transgenic mice were then bred back on a C57BL/6 background for at least 12 generations to obtain pure C57BL/6 double transgenic mice. Figure 1D shows a western blot for IκBα of control (−rtTA/+ΔNIκBα) and double transgenic (+rtTA/+ΔNIκBα; referred to as transgenic from here on out) lysates, with the two bands in the double transgenic lane corresponding to the endogenous and N-terminally deleted IκBα proteins.
Figure 1. Characterization of double transgenic mouse line.
Double transgenic mice were generated by crossing the two strains shown in (A) and breeding them back on a C57BL/6 strain for at least 12 generations to generate a pure C57BL/6 double transgenic mouse. B. RIP-rtTA-luc(Renilla) mouse injected with coelenterazine and imaged using the Xenogen IVIS 100 system. C. Double transgenic mouse treated with doxycycline in the drinking water (2 mg/mL) for 3 weeks, then injected with luciferin and imaged using the Xenogen IVIS 100 system. D. Western blot for IκBα.
Characterization of Transgenic Mouse Model
To characterize the relative expression levels of the rtTA and ΔNIκBα transgenes, we utilized the fact that the Renilla and Firefly luciferase expression directly correlate with the expression of the rtTA and ΔNIκBα transgenes. The transgenic mouse line demonstrated a much greater Renilla luciferase expression (2,900,000.00 RLU/islet versus 2391.00 under no doxycycline treatment) and induction of the ΔNIκBα transgene in the transgenic mouse line by doxycycline treatment resulted in a 967.47 fold increase as compared to no doxycycline treatment (268,956.00 RLU/islet versus 278.00 RLU/islet) (Table 1). These results indicate that at baseline, the transgenic mice express the rtTA transgene and that treatment with doxycycline induces significant expression of the ΔNIκBα transgene.
Table 1. In Vitro Luciferase assay to measure relative expression level of the rtTA transgene and induction of ΔNIκBα.
Control islets demonstrated only background levels of both Renilla and Firefly luciferase activity. The transgenic mouse line demonstrated high levels of Renilla luciferase activity and a 967.47 fold induction of Firefly luciferase activity when treated with doxycycline.
| Mice | Dox | Firefly luciferase (RLU/islet) |
Fold (+DOX/−DOX) |
Fold (+DOX/control) |
Renilla luciferase (RLU/islet) |
|---|---|---|---|---|---|
| − | 71.00 | 2,391.00 | |||
| (−) Control | + | 74.00 | 1.00 | 1.00 | 2,362.00 |
| − | 278.00 | 2,900,000.00 | |||
| Transgenic | + | 268,956.00 | 967.47 | 3,788.11 | 2,900,000.00 |
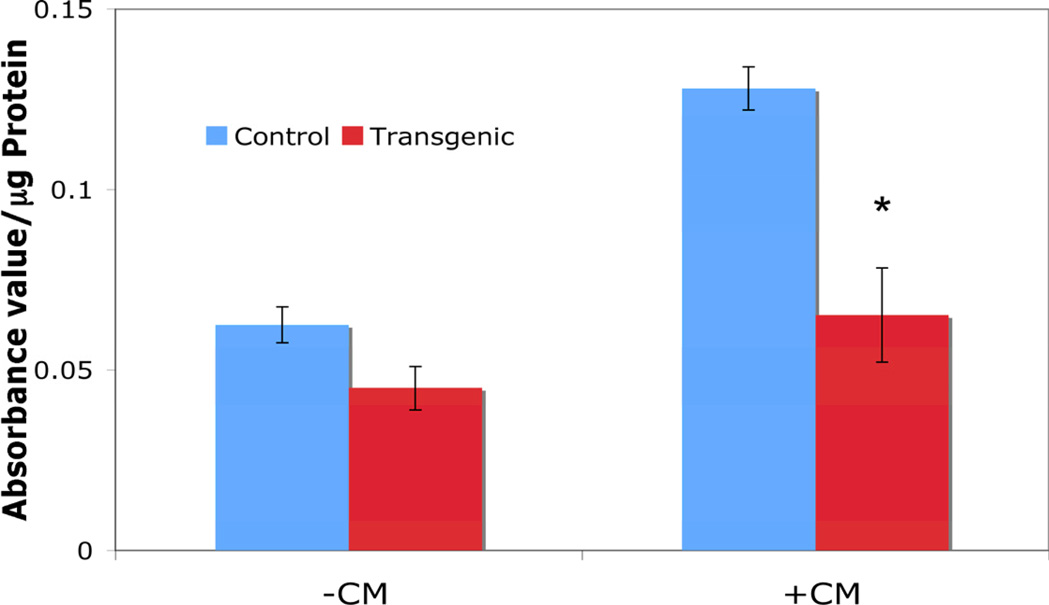
To determine whether the expression of the ΔNIκBα transgene was sufficient to inhibit NF-κB activity in the β cells, islets were isolated from mice treated with doxycycline and cultured overnight before treatment with proinflammatory cytokines (50 U/mL IL-1β, 1000 U/mL TNF-α, 750 U/mL IFN-γ) for 1 hour. NF-κB activation was reduced by 49% in transgenic islets compared to control (0.065 ± 0.013 abs. value/µg protein versus 0.128 ± 0.006; p<0.05; Figure 2). These results demonstrate that doxycycline treatment for 3 weeks induces sufficient ΔNIκBα transgene expression to inhibit NF-κB activation in response to cytokine exposure.
Figure 2. Activation of NF-κB in Control and Transgenic Islets by Proinflammatory Cytokines.
Treatment with cytokine mix (CM: 50 U/mL IL-1β, 1000 U/mL TNF-α and 750 U/mL IFN-γ induced less activation of NF-κB in transgenic islets as compared to control islets. * indicates p<0.05
Inhibition of NF-κB Alters Gene Expression in Islets Treated with Proinflammatory Cytokines
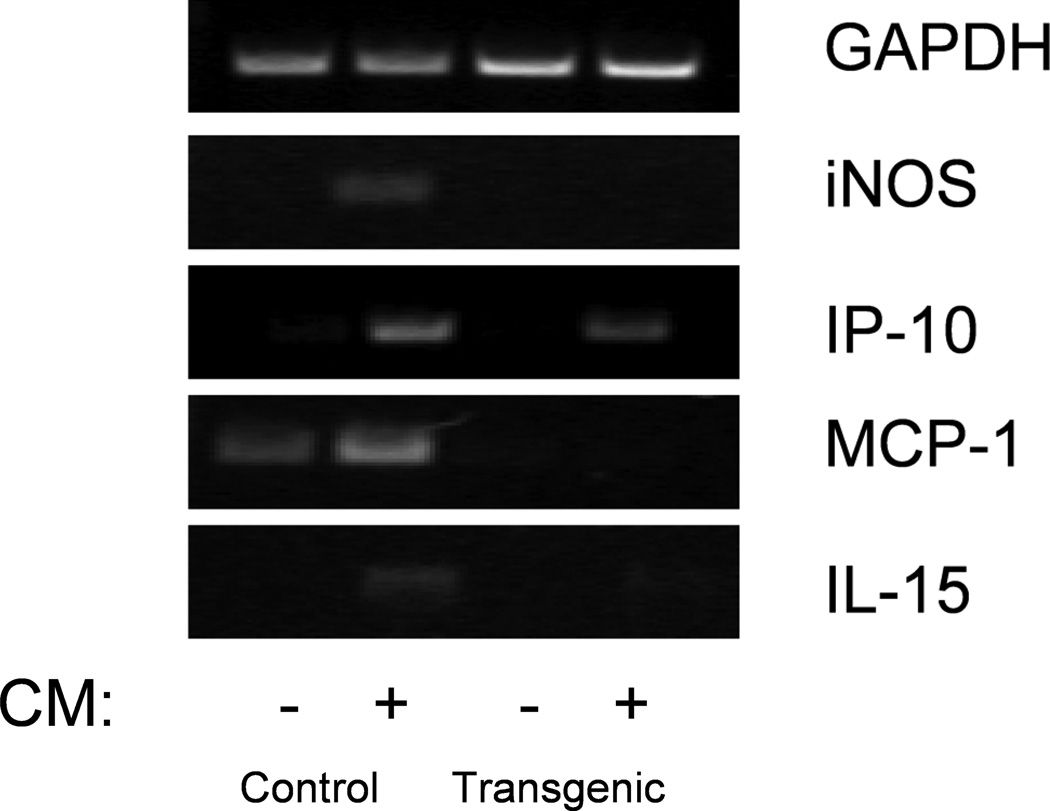
Gene expression changes in control and transgenic islets were analyzed using semiquantitative RT-PCR and gel electrophoresis on RNA samples obtained from islets treated with and without proinflammatory cytokines for 24 hours (Figure 3). All samples were standardized to GAPDH expression levels. In control islets, cytokine treatment led to increased expression of iNOS, MCP-1, IP-10 and IL-15, all of which were reduced or eliminated when NF-κB activity was inhibited in the transgenic islets. These data indicate that inhibition of NF-κB in the islets decreases expression of cytokine-induced chemokines and deleterious genes.
Figure 3. Gene expression analysis of control and transgenic islets exposed to cytokines by semiquantitative RT-PCR.
Control islets exposed to CM for 24 hours upregulated iNOS, MCP-1, IP-10 and IL-15. Transgenic islets receiving the same treatment demonstrated reduced expression of iNOS, MCP-1, IP-10 and IL-15 as compared with control islets treated with CM.
Nitric Oxide Production by Islets in Response to Cytokine Exposure
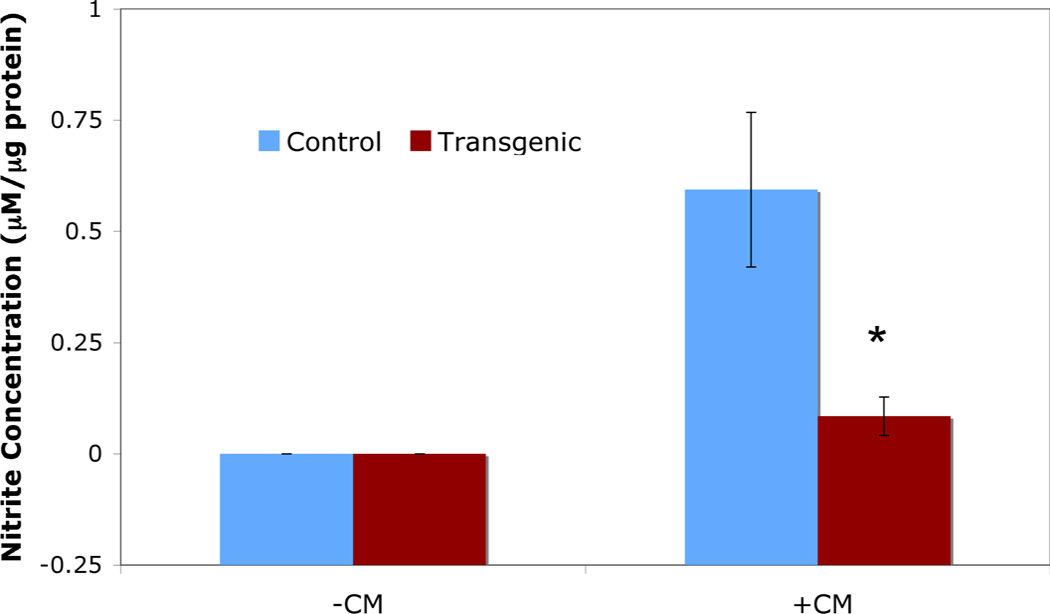
When islets are exposed to proinflammatory cytokines, one of the major genes upregulated is iNOS. While direct measurement of NO is difficult, measurement of nitrite, a byproduct of iNOS activity, is much easier. Transgenic islets exposed to proinflammatory cytokines (50 U/mL IL-1β, 1000 U/mL TNF-α, 750 U/mL IFN-γ) for 24 hours demonstrate a 7-fold reduction in nitrite levels (0.084 ± 0.043 µM nitrite/µg protein) versus control islets (0.594 ± 0.174 µM nitrite/µg protein; p<0.01; Figure 4). These results demonstrate that inhibition of NF-κB activity by the ΔNIκBα transgene results in decreased activity of iNOS in islets exposed to proinflammatory cytokines.
Figure 4. Nitric Oxide production in control and transgenic islets exposed to proinflammatory cytokines.
Exposure of islets to CM for 24 hours resulted in increased NO production in control islets as compared to transgenic islets. *indicates p<0.01
Inhibition of NF-κB Activity Enhances Glucose Stimulated Insulin Secretion When Islets Are Exposed to Proinflammatory Cytokines
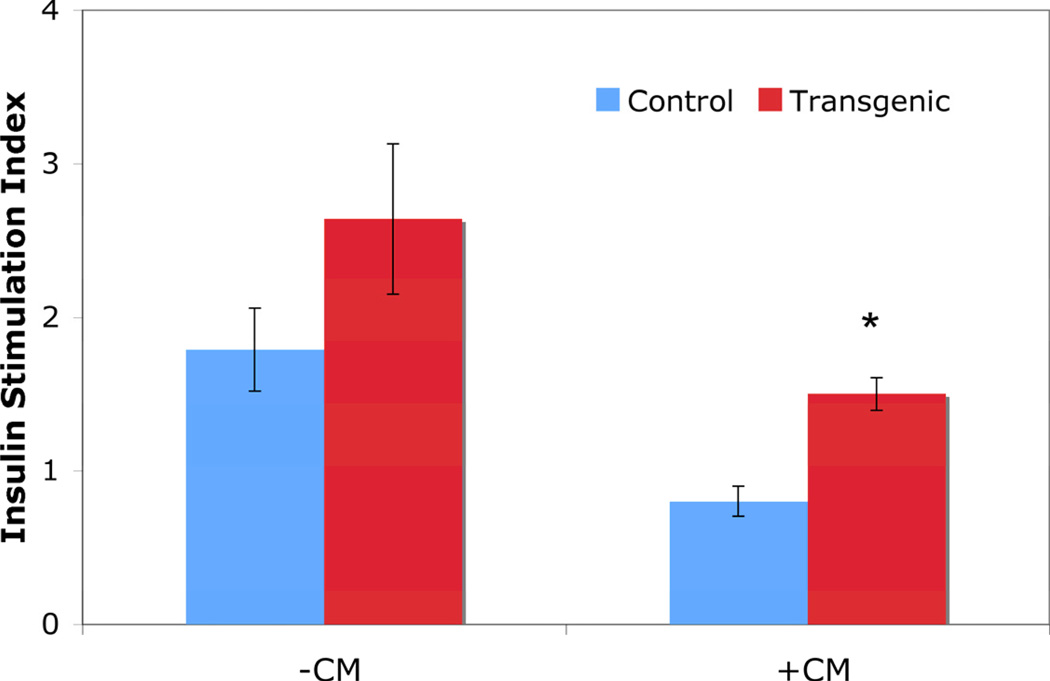
Exposure of islets to proinflammatory cytokines decreases the islets’ ability to secrete insulin in response to glucose stimulation. When transgenic islets were exposed to proinflammatory cytokines for 24 hours, they demonstrated an increased insulin stimulation index (the ratio of the amount of insulin secreted at 28 mM glucose divided by the amount of insulin secreted at 2.8 mM glucose) as compared to control islets (1.500 ± 0.106 versus 0.800 ± 0.098; p<0.01; Figure 5). These data demonstrate that inhibition of NF-κB activity protects the islets’ ability to secrete insulin when exposed to proinflammatory cytokines.
Figure 5. Glucose stimulated insulin secretion assay.
Treatment with CM for 24 hours resulted in a lower insulin stimulation index in the control islets compared with transgenic islets. * indicates p<0.01
Transgenic Islets Perform Better than Control Islets in a Syngeneic Marginal Islet Mass Transplant Model
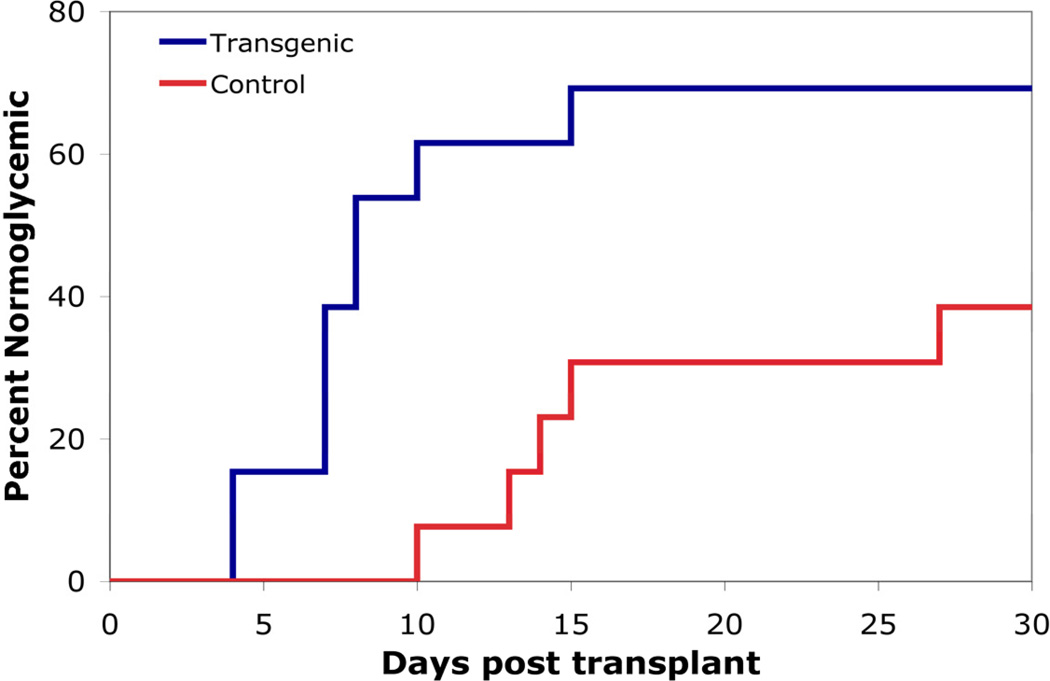
To investigate the benefit of inhibiting NF-κB activity in the islet transplant setting, 50 islets, control or transgenic, were transplanted to the liver of C57BL/6 streptozotocin-induced diabetic mice. Fifty islets was chosen as the marginal islet mass to be transplanted as previous studies demonstrated that this number of islets was sufficient to cause reversion to euglycemia in approximately 8 days.26 Normoglycemia is defined in this instance as 2 consecutive blood glucose readings below 200 mg/dL. Transplantation of transgenic islets caused reversion to normoglycemia in 69.2% of recipients, with an average of 7.8 ± 1.1 days to cure (N=13). Comparatively, transplantation of control islets caused reversion to normoglycemia in 35.7% of recipients, with an average of 15.8 ± 2.9 days to cure (N=14, p<0.05; Figure 6). These results demonstrate that inhibition of NF-κB in islets protects them in an in vivo transplant setting, allowing for increased engraftment and faster times to normoglycemia.
Figure 6. Syngeneic marginal islet mass transplantation to streptozotocin-induced diabetc mice.
Fifty control or transgenic islets were transplanted intrahepatically to diabetic recipients. Transgenic islet transplants reverted mice to euglycemia faster and had a higher success rate as compared to control islet transplants.
Discussion
Transplantation of pancreatic islets has been shown to be a very effective short-term treatment for type 1 diabetes by ameliorating the need for exogenous insulin to maintain a euglycemic state. Unfortunately, long-term durability of insulin independence has been challenging. It is estimated that as much as 50% of the intraportally infused islet graft is injured in the immediate post-transplant period. Given the stresses that islets are subjected to immediately post transplantation, specifically exposure to the proinflammatory cytokines IL-1β, TNF-α and IFN-γ released by macrophages in response to local inflammation, inhibition of NF-κB, the transcription factor activated by those cytokines, is a compelling strategy to improve islet survival and function.
To inhibit NF-κB in the β cells, we generated a transgenic mouse in which NF-κB activity could be inhibited conditionally and specifically. Knockout of either an NF-κB subunit, such as p65, or one of the components of the IKK complex, such as IKKβ, results in liver degradation and eventually embryonic lethality in mice.27,28 Additionally, inhibition of NF-κB during embryonic development in pancreatic progenitor cells by expression of a nondegradable IκBα protein resulted in adult mice with hyperglycemia and had altered glucose stimulated insulin secretion.29
Here we demonstrate that conditional and specific inhibition of NF-κB in adult β cells, through expression of an N-terminally deleted IκBα using the TetOn system under the control of the rat insulin promoter. Use of this transgenic mouse strain allows for inhibition of NF-κB in the β cells prior to transplantation but not during development, ensuring that there is no lethality or disruption to normal islet formation. Treatment of the transgenic mice with doxycycline induced significant transgene expression and a concomitant decrease of cytokine-induced NF-κB activity by ~50% compared with control islets.
Inhibition of NF-κB activity lead to decreased expression of iNOS, MCP-1, IP-10 and IL-15 as measured by semiquantitative RT-PCR. These results correlated to previous studies using MIN6 cells in which inhibition of NF-κB resulted in a decrease in chemokine and iNOS expression after 24-hour cytokine treatment.19,20 It is significant to note the large decrease in MCP-1 expression reported since MCP-1 is considered an extremely potent chemokine. Previous work has demonstrated that blocking MCP-1, or its receptor, prolongs islet graft survival.12 It is impossible to know how much islet cells contribute to the total amount of MCP-1 produced post islet transplantation, however, any decrease in MCP-1 expression should enhance islet survival post transplantation.
Corresponding to the decreased expression of iNOS, a decrease in nitrite production, a byproduct of iNOS activity, was seen when NF-κB activity was inhibited. Nitric oxide is detrimental to β cells as it weakens free radical defenses, stresses the endoplasmic reticulum, and can result in cell death.8,10 Previous work by our lab, and others, have demonstrated that inhibition of nitric oxide production in β cells decreased cytokine-induced cell death.
We observed that the ability of islets to secrete insulin in response to glucose stimulation in vitro was preserved in cytokine-treated transgenic islets compared to controls. These results correlate to that reported using a similar transgenic mouse model in which NF-κB inhibition resulted in protection from multiple low dose streptozotocin injections, a treatment thought to damage β cells through nitric oxide production and DNA alkylation.22 Others reported the use of viral vectors to deliver the IκBα superrepressor to islet cells.21,30 All these results are consistent with previous work reported by our lab that examined the effect of proinflammatory cytokine exposure to MIN6 cells expressing a dominant-negative IκBα mutant.
The above observations suggest that inhibition of NF-κB activity in β cells should prove beneficial to islet function in a transplant setting. Syngeneic transplantation of a marginal islet mass to streptozotocin-induced diabetic C57BL/6 recipients were used to determine whether inhibition of NF-κB activity in islets would have a beneficial effect on transplant outcome. In syngeneic islet transplants, the islets are still subjected to the nonspecific immune response, including exposure to proinflammatory cytokines, but not the specific T-cell mediated immune response seen in allogeneic rejection. Inhibition of NF-κB in the donor islets resulted in approximately double the number of normoglycemic mice, with the time to normoglycemia occurring 8 days earlier as compared to control islet recipients. These results demonstrated a clear benefit of inhibiting NF-κB activity in donor mouse islets. It is possible that further inhibition of NF-κB, above the 50% inhibition achieved in these transgenic mice, may lead to enhanced in vitro islet function and further improve the survival of the transplanted islet mass. However, a low level of NF-κB activity is necessary for proper glucose stimulated insulin secretion31 and inhibiting NF-κB further could result a decrease in islet function, which would possibly negate the improved islet transplant results shown here.
The results were similar to those obtained when a marginal islet mass is transplanted to mice receiving 15-deoxyspergualin (DSG).32 In those experiments, DSG treatment decreased the number of days post islet transplant of hyperglycemia and increased the percentage of allogeneic recipients that reverted to euglycemia after receiving a marginal islet mass transplant. It was concluded that DSG acted by blunting the nonspecific injury to β cells mediated by macrophage-derived proinflammatory cytokine release. DSG has been shown to inhibit translocation of NF-κB to the nucleus in LPS-stimulated 70Z/3.12 murine pre-B-cell line33, and blockade of NF-κB activity in macrophages can lead to decreased cytokine production as well as nitric oxide production.34–36 Here we demonstrate a similar beneficial effect through inhibiting NF-κB in β cells. It should be noted that lessening the effects of proinflammatory cytokine exposure on islets, either through blockade of cytokine release by macrophages or through inhibition of one of the major cytokine-induced signaling pathways in β cells, provides enhanced function and survival of the islets post transplantation.
Syngeneic transplant experiments allowed a focus on the effect of early host non-specific immune responses. It will be very interesting to investigate the effect of inhibiting NF-κB activity in β cells in an allogeneic setting, as various chemokines such as MCP-1 and IP-10 are upregulated by β cells in the presence of cytokines. By decreasing their expression levels, rejection of allogeneic islet grafts may be delayed due to less infiltration of the islet graft by host immune cells. Future research using this transgenic mouse strain will be directed towards determining what role, if any, NF-κB activity in β cells plays in islet graft rejection in an allogeneic setting.
Human islets used for clinical intraportal transplants are susceptible to very similar stresses as designed in the murine functional islet transplant model of intraportal implantation described. The detrimental effect on islet engraftment and function caused by immediate host non-specific proinflammatory cytokine production by resident macrophages in the liver may be blunted by inhibition of NF-κB activation. In the clinical scenario, the period of pre-transplant islet culture may provide an ideal window in which to treat the islets to protect against in situ cytokine-mediated NF-κB activation. There are a number of NF-κB small molecule inhibitors that are currently being used in research, including 6-amino-4-(4-phenoxyphenylethylamino)quinazoline (QNZ)37–39, dehydroxymethylepoxyquinomicin (DHMEQ)40 and the NF-κB essential modulator (NEMO)-binding domain (NBD) peptide.41 Pretreatment of the islets with one or more inhibitors may provide similar protection against proinflammatory cytokines as seen with the transgenic mice presented here. Additionally, gold nanoparticles (AuNP) have been used to deliver antisense DNA to both human and mouse islets.42 These AuNPs were able to fully penetrate into the islet cells without altering their viability or functionality, both in vitro and in vivo. Attaching antisense DNA, or siRNA, sequences to AuNPs targeted against NF-κB itself or a member of the NF-κB signaling pathway, would allow for inhibition of NF-κB activity in intact human islets.
Acknowledgements
The authors would like to thank Dr. Yinon Ben-Neriah from The Hebrew University-Hadassah Medical School in Israel for his generous gift of the Tg(Ptet-ΔNIκBα-Luc) mice strain and Courtney Larson and Jason West from Northwestern University for their assistance with breeding the double transgenic mice strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by National Institutes of Health Grant 1 U42 RR023242-01 (D.B.K.).
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Award Number T32DK007169 (J.S.R.).
References
- 1.2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR) Cell Transplant. 2009;18:753–767. doi: 10.3727/096368909X470874. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Debray-Sachs M, Assan R, Bailey D, Hamburger J. Functional inhibition of isolated pancreatic cells, new technic for the detection of macrophage cytotoxicity. C R Acad Sci Hebd Seances Acad Sci D. 1978;287:1161–1164. [PubMed] [Google Scholar]
- 4.Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med. 1990;172:291–302. doi: 10.1084/jem.172.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwizer RW, Leiter EH, Evans R. Macrophage-mediated cytotoxicity against cultured pancreatic islet cells. Transplantation. 1984;37:539–544. doi: 10.1097/00007890-198406000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 7.Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhoffer M, et al. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J Biol Chem. 2001;276:48879–48886. doi: 10.1074/jbc.M108658200. [DOI] [PubMed] [Google Scholar]
- 8.McCabe C, Samali A, O'Brien T. Cytoprotection of beta cells: rational gene transfer strategies. Diabetes Metab Res Rev. 2006;22:241–252. doi: 10.1002/dmrr.615. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 10.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 11.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 12.Merani S, Truong WW, Hancock W, Anderson CC, Shapiro AM. Chemokines and their receptors in islet allograft rejection and as targets for tolerance induction. Cell Transplant. 2006;15:295–309. [PubMed] [Google Scholar]
- 13.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 14.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46:255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 15.Kirman I, Vainer B, Nielsen OH. Interleukin-15 and its role in chronic inflammatory diseases. Inflamm Res. 1998;47:285–289. doi: 10.1007/s000110050331. [DOI] [PubMed] [Google Scholar]
- 16.Campbell IL, Iscaro A, Harrison LC. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988;141:2325–2329. [PubMed] [Google Scholar]
- 17.Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138:2610–2614. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg JO, Eizirik DL, Sandler S, Tracey DE, Andersson A. Treatment with an interleukin-1 receptor antagonist protein prolongs mouse islet allograft survival. Diabetes. 1993;42:1845–1851. doi: 10.2337/diab.42.12.1845. [DOI] [PubMed] [Google Scholar]
- 19.Baker MS, Chen X, Cao XC, Kaufman DB. Expression of a dominant negative inhibitor of NF-kappaB protects MIN6 beta-cells from cytokine-induced apoptosis. J Surg Res. 2001;97:117–122. doi: 10.1006/jsre.2001.6121. [DOI] [PubMed] [Google Scholar]
- 20.Baker MS, Chen X, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappaB-dependent/NO-independent chemokine gene expression in MIN6 beta cells. J Surg Res. 2003;110:295–303. doi: 10.1016/s0022-4804(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 21.Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J Biol Chem. 2000;275:36509–36513. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- 22.Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, et al. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci U S A. 2006;103:5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavon I, Goldberg I, Amit S, Landsman L, Jung S, Tsuberi BZ, et al. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nat Med. 2000;6:573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 24.Baker MS, Chen X, Rotramel AR, Nelson JJ, Kaufman DB. Interferon regulatory factor-1 down-regulates cytokine-induced IP-10 expression in pancreatic islets. Surgery. 2003;134:134–141. doi: 10.1067/msy.2003.236. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81:1421–1427. doi: 10.1097/01.tp.0000206109.71181.bf. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Zhang X, Larson C, Chen F, Kissler H, Kaufman DB. The epididymal fat pad as a transplant site for minimal islet mass. Transplantation. 2007;84:122–125. doi: 10.1097/01.tp.0000266909.58117.e3. [DOI] [PubMed] [Google Scholar]
- 27.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, et al. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 29.Norlin S, Ahlgren U, Edlund H. Nuclear factor-{kappa}B activity in {beta}-cells is required for glucose-stimulated insulin secretion. Diabetes. 2005;54:125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- 30.Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, et al. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes. 2001;50:2219–2224. doi: 10.2337/diabetes.50.10.2219. [DOI] [PubMed] [Google Scholar]
- 31.Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, et al. Activation of NF-kappaB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J Biol Chem. 2005;280:30630–30637. doi: 10.1074/jbc.M502493200. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman DB, Gores PF, Field MJ, Farney AC, Gruber SA, Stephanian E, et al. Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes. 1994;43:778–783. doi: 10.2337/diab.43.6.778. [DOI] [PubMed] [Google Scholar]
- 33.Tepper MA, Nadler SG, Esselstyn JM, Sterbenz KG. Deoxyspergualin inhibits kappa light chain expression in 70Z/3 pre-B cells by blocking lipopolysaccharide-induced NF-kappa B activation. J Immunol. 1995;155:2427–2436. [PubMed] [Google Scholar]
- 34.Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, et al. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol. 1994;153:712–723. [PubMed] [Google Scholar]
- 35.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 37.Choi S, Kim JH, Roh EJ, Ko MJ, Jung JE, Kim HJ. Nuclear factor-kappaB activated by capacitative Ca2+ entry enhances muscarinic receptor-mediated soluble amyloid precursor protein (sAPPalpha) release in SH-SY5Y cells. J Biol Chem. 2006;281:12722–12728. doi: 10.1074/jbc.M601018200. [DOI] [PubMed] [Google Scholar]
- 38.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Fukazawa T, et al. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg Med Chem. 2003;11:383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]
- 39.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Hayashi H. A novel structural class of potent inhibitors of NF-kappa B activation: structure-activity relationships and biological effects of 6-aminoquinazoline derivatives. Bioorg Med Chem. 2003;11:3869–3878. doi: 10.1016/s0968-0896(03)00438-3. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi T, Matsumoto S, Matsushita M, Kamachi H, Tsuruga Y, Kasai H, et al. Donor pretreatment with DHMEQ improves islet transplantation. J Surg Res. 2010;163:e23–e34. doi: 10.1016/j.jss.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Huang HJ, Sugimoto S, Lai J, Okazaki M, Yamamoto S, Krupnick AS, et al. Maintenance of IKKbeta Activity Is Necessary to Protect Lung Grafts From Acute Injury. Transplantation. 2011 doi: 10.1097/TP.0b013e31820ba2a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rink JS, McMahon KM, Chen X, Mirkin CA, Thaxton CS, Kaufman DB. Transfection of pancreatic islets using polyvalent DNA-functionalized gold nanoparticles. Surgery. 2010;148:335–345. doi: 10.1016/j.surg.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]