Abstract
The reconstitution of biosynthetic pathways from heterologous hosts can help define the minimal genetic requirements for pathway function and facilitate detailed mechanistic studies. Each of the three pathways for the assembly of cytochrome c in nature (called systems I, II, and III) has been shown to function recombinantly in Escherichia coli, covalently attaching heme to the cysteine residues of a CXXCH motif of a c-type cytochrome. However, recombinant systems I (CcmABCDEFGH) and II (CcsBA) function in the E. coli periplasm, while recombinant system III (CCHL) attaches heme to its cognate receptor in the cytoplasm of E. coli, which makes direct comparisons between the three systems difficult. Here we show that the human CCHL (with a secretion signal) attaches heme to the human cytochrome c (with a signal sequence) in the E.coli periplasm, which is bioenergetically (p-side) analogous to the mitochondrial intermembrane space. The human CCHL is specific for the human cytochrome c, whereas recombinant system II can attach heme to multiple non-cognate c-type cytochromes (possessing the CXXCH motif.) We also show that the recombinant periplasmic systems II and III use components of the natural E.coli periplasmic DsbC/DsbD thiol-reduction pathway.
Keywords: cytochrome c assembly, CCHL, heme attachment, periplasmic, thioreduction
1. Introduction
All c-type cytochromes function in the prokaryotic periplasm, the thylakoid lumen, or the mitochondrial intermembrane space. (See [1–4] for recent reviews on functions and assembly of c-type cytochromes). Typical c-type cytochromes have two thioether bonds that are formed between two cysteines and the two vinyl groups of heme (at a CXXCH motif, where the histidine acts as an axial ligand to the iron). This covalent linkage prevents heme loss and indirect evidence shows that most c-type cytochromes are degraded without heme attachment [5, 6]. The assembly of a c-type cytochrome requires that the heme and the apocytochrome are already transported to the periplasm or mitochondrial intermembrane space prior to attachment. Additionally, in vitro the heme iron and the two cysteines must be reduced for attachment, this attachment occurring spontaneously at the alpha carbons of the vinyl groups [7, 8].
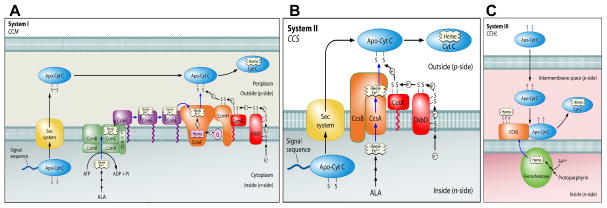
Three systems have been described for cytochrome c assembly (Fig 1). Many Gram negative bacteria (including Escherichia coli), archaea, and plant mitochondria use the system I pathway encoded by ccm (cytochrome c maturation) genes (Fig 1A). System I is the most studied pathway and it involves apocytochrome c thiol-reduction by a specific periplasmic thioredoxin protein called CcmG (HelX) [9–11], which is itself reduced by the transmembrane DsbD (or the related CcdA) [12–14]. Other proteins are involved in heme export (CcmABCD), heme chaperoning (CcmE), apocytochrome trafficking and thiol redox (CcmH), and heme reduction (CcmF) prior to heme attachment to apocytochrome c by CcmF/H complex (recently reviewed in [1, 2]). Although the molecular mechanisms underlying apocytochrome c recognition and heme attachment by CcmF/H are poorly understood, many studies are in agreement that system I can assemble a wide variety of c-type cytochromes, with only the CXXCH as the recognition determinant for attachment (e.g. [15–21]).
Figure 1.
Schematic models of the three c-type cytochrome assembly pathways (Systems I, II, and III). The cartoons are reproduced in modified form from Kranz, et al. 2009 [1].
System II is present in some Gram negative bacteria (e.g. Helicobacter, Wolinella), Gram positives (e.g. Mycobacterium, Bacillus), cyanobacteria, and the chloroplast (Fig 1B). Genetic analyses of bacteria with system II have shown that a specific periplasmic thioredoxin (CcsX) [22], and again the generic DsbD (or CcdA) protein [22–26] are required. System II also involves export of heme, recognition of the apocytochrome c, and heme attachment, but these functions are carried out by the CcsBA integral membrane protein [27]. This was established by using a recombinant E. coli deleted for all its ccm genes, which was able to synthesize periplasmic cytochrome c4 when only the Helicobacter fused ccsBA gene was expressed [27–29]. Although most organisms with system II have separate ccsB and ccsA genes, a few organisms have naturally fused ccsBA genes. Further studies on this recombinant CcsBA have indeed shown that the protein acts as a heme exporter and cytochrome c synthetase [27]. However, questions remain for the recombinant CcsBA as to what comprises the substrate recognition determinants and how the apocytochrome c is reduced (e.g. whether Dsb proteins are involved) [28, 30]. Here we address these questions.
System III appears to be the simplest, comprised of an enzyme first discovered by yeast genetic analyses [31–33], referred to as cytochrome c heme lyase (CCHL) (Fig 1C). This enzyme has also been referred to as holocytochrome c synthase (HCCS), cytochrome c synthase, or cytochrome c synthetase e.g. [34–36]. Here we use CCHL to refer to the system III enzyme. Despite evidence that it is a soluble protein in the mitochondrial intermembrane space, and its involvement in some rare human genetic defects [34–36], it is a poorly understood enzyme [37]. The evidence that it represents the sole cytochrome c synthetase comes in part from reconstitution of its activity in the E.coli cytoplasm, using its cognate cytochrome c (e.g. [15, 38–40]). Interestingly, although yeast has two separate CCHLs, one for cytochrome c [31] and a second related enzyme for cytochrome c1 (CC1HL) [33], humans have a single CCHL [3, 34]. The human CCHL appears to recognize both cytochromes c and c1. Moreover, the yeast CCHL can be over-expressed or easily selected to recognize both cytochromes [3, 41]. Recently, the recombinant cytoplasmic CCHL from yeast was used to begin to investigate apocytochrome c recognition determinants (ie. residues or regions in cytochrome c required for heme attachment by CCHL) [39, 40, 42]. One of the caveats to studying cytoplasmic synthesis of c-type cytochromes is that no direct comparison to the periplasmic recombinants (systems I or II) can be made with respect to apocytochrome c folding and/or suitability for heme attachment. Here, we test whether a periplasmic recombinant human CCHL with a periplasmic human cytochrome c is functional (to allow such comparisons). We also address what endogenous periplasmic thiol reduction proteins are necessary.
2. Materials and Methods
2.1 Bacterial growth conditions
E. coli were grown aerobically in Luria-Bertani (LB) media (Difco) at 37°C with shaking at 300 rpm. Antibiotic concentrations are as follows: 50 μg mL−1 carbenicillin (carb), 20 μg mL−1 chloramphenicol (cm) and 100 μg mL−1 kanamycin (km). Expression of pRGK333 (CcmABCDEFGH; system I) [28], pRGK368 (CcsBA; system II) [29], pRGK399 (pMALp2x:CCHL; system III), and pRGK400 (pET226xHis:CCHL; system III) was induced by 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) and expression of pRGK332 (Cyt c4) [28], pRGK389 (Cyt c2; pBADc2:6xHis), pRGK390 (human CycS; pBADCycS:6xHis), and pRGK401 (human CycS:R. capsulatus Cyt c2 chimera; pBADCycS-c2:6xHis) was induced by 0.2% arabinose.
2.2 Plasmid construction
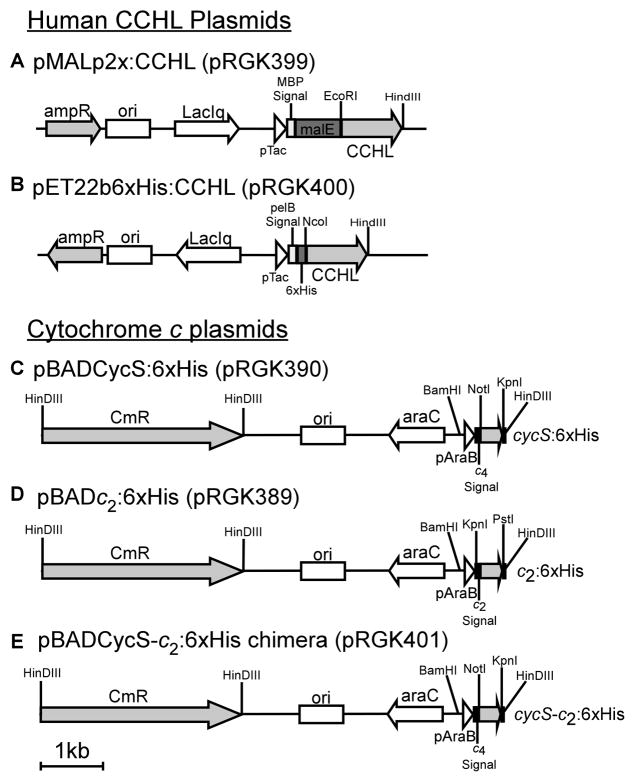
The human CCHL was amplified from a cDNA clone (Origene) and cloned in frame into the EcoRI and HindIII sites of pMalp2x (New England Biolabs) to create pRGK399, a periplasmically targeted maltose binding protein (MBP) fusion (see Table 1 for strains and plasmids, Table S1 for oligonucleotide sequences). An N-terminal hexahistidine-tagged periplasmically targeted (pelB signal) version of the human CCHL (pRGK400) was created by amplification from the above cDNA clone and in-frame insertion into the NcoI and HindIII sites of pET22b (EMD Biosciences). The cytochrome c2 gene cycA, with its natural signal sequence, was amplified from R. capsulatus DNA and cloned into the KpnI and PstI sites of pRGK330 [28] to create pRGK389. The human cytochrome c gene cycS was amplified from cDNA clone MGC12367 (ATCC) and cloned into NotI and KpnI sites of pRGK331 [28] that was amplified to use the cytochrome c4 signal sequence to create pRGK390. The human cytochrome c gene, containing a deletion of the DNA corresponding to amino acids 4–22, along with the surrounding plasmid was amplified from pRGK390 with phosphorylated oligonucleotides, containing the R. capsulatus Cyt c2 DNA corresponding to amino acids 2–20, and blunt end ligated to create pRGK401.
Table 1.
Description of strains and plasmids used in this study
| Strain or Plasmid | Description & Purpose | Reference |
|---|---|---|
| RK103 | Δccm E.coli strain deleted for all ccm genes | [28] |
| RK106 | ΔccmΔdsbA E. coli strain Generated by P1 phage transduction using strains JW3832 and RK103 |
This study |
| RK107 | ΔccmΔdsbB E. coli strain Generated by P1 phage transduction using strains JW5182 and RK103 |
This study |
| RK108 | ΔccmΔdsbC E. coli strain Generated by P1 phage transduction using strains JW2861 and RK103 |
This study |
| RK109 | ΔccmΔdsbD E. coli strain Generated by P1 phage transduction using strains JW5734 and RK103 |
This study |
| RK110 | ΔccmΔdsbG E. coli strain Generated by P1 phage transduction using strains JW0597 and RK103 |
This study |
| RK112 | Δccm BL21(DE3) E. coli stain Generated by P1 transduction with RK103 |
This study |
| TC127034 | cDNA clone from Origene-plasmid template for construction of pRGK390 and pRGK391 | Origene |
| MGC12367 | E. coli strain from ATCC with plasmid PCR template for construction of pRGK392 | ATCC |
| pRGK333 | Expression of GST-tagged CcmABCDEFGH (system I) from E. coli | [28] |
| pRGK368 | Expression of GST-tagged CcsBA (fused system II) from H. hepaticus | [29] |
| pRGK330 | pBAD24 based, chloramphenicol resistant plasmid for arabinose inducible expression | [28] |
| pRGK399 | Periplasmic expression MBP-tagged human cytochrome c heme lyase (CCHL; system III) | This study |
| pRGK400 | Periplasmic (pelB signal) of N-terminal 6x: His-tagged human CCHL | This study |
| pRGK331 | Expression of cytochrome c4 from B. pertussis fused to alkaline phosphatase. PCR template for construction of pRGK392 | [28] |
| pRGK332 | Expression of cytochrome c4 reporter from B. pertussis | [28] |
| pRGK389 | Expression of cytochrome c2 reporter from R. capsulatus | This study |
| pRGK390 | Periplasmic (cyt c4 signal) expression of human cytochrome c CycS reporter | This study |
| pRGK401 | Periplasmic (cyt c4 signal) expression of chimeric human CycS and R. capsulatus cytochrome c2 reporter | This study |
2.3 Knockout strain construction
E. coli strains with knockout mutations in dsb genes were obtained from the Keio strain collection [43, 44]. The knockout mutations were transduced into RK103 (Δccm) [28] by P1 phage transduction [45]. Briefly, P1 phage were grown first in LB media, 5 mM CaCl2, 0.2% glucose, with a dsb mutant strain as the donor strain. Cells were killed by addition of chloroform to the growth media and the phage were isolated. Dilutions of isolated phage (100 μL) were then incubated with 100 μL RK103 cell culture (O.D.600=0.8–1.0) in 5 mM CaCl2 and 100 mM MgSO4 for 30 minutes at 37°C. Physical interaction between the phage and cells was disrupted by addition of 66.7 mM sodium citrate pH 5.5 then the culture was added to 1 mL LB broth and incubated for one hour at 37°C with shaking at 300 rpm. Transductants were selected by growth on LB-Kan plates and verified by PCR.
2.4 Protein purification
Hexahistidine-tagged proteins were purified with Talon affinity resin (Clonetech) from whole cell lysates. E. coli cultures of RK103 (Δccm) containing pRGK399 (pMalp2x:CCHL) or pRGK333 (system I) and pRGK390 (pBADCycS:6xHis) or RK112 (BL21(DE3) Δccm) containing pRGK400 (pET226xHis:CCHL) were inoculated (to 1%) with fresh overnight culture in LB medium supplemented with the appropriate antibiotics. One liter cultures were grown to an OD600 of 0.8–1.0 and protein over-expression was induced with IPTG and arabinose or IPTG alone for four hrs (system I matured) or 12–14 hrs (system III matured). Following induction the cells were harvested at 6,000g for 10 min and the cell pellet was resuspended in 3.5 ml, per gram of cell pellet, of 1 x Talon buffer (50 mM sodium phosphate buffer, 300 mM NaCl, pH 7.0) that was supplemented with 0.1 mg ml−1 egg white lysozyme (Sigma-Aldrich) and 1 mM phenylmethanesulfonyl fluoride (PMSF; Sigma-Aldrich). Following gentle agitation on ice (20 min), the cell suspensions were sonicated (on ice) for 2 × 3 min using a Branson 250 sonicator (50% duty, 70% output), and cellular debris was removed by centrifugation (10,000g, 15 min, 4°C). The clarified lysate (Load) was passed by gravity flow over Talon resin, washed with 10 column volumes of 1 x Talon buffer (Wash 1), five column volumes of 1 x Talon buffer containing 5 mM imidazole (Wash 2), and eluted with five column volumes of 1 x Talon buffer containing 150 mM imidazole (Elution). To ensure that all hexahistidine-tagged protein was eluted from the resin, the cobalt ions were stripped with five column volumes of 1 x Talon buffer containing 0.2 M EDTA (Strip). The elution fraction was concentrated approximately 10-fold with an Amicon 10 K spin filter (ThermoFisher Scientific). The human CycS:6xHis was further purified (>95%) by passage over DEAE Sepharose Fast Flow (GE Healthcare). The 1 x Talon buffer (containing 150 mM imidazole) was exchanged for a Tris based buffer (20 mM Tris, 25 mM NaCl, pH 8.0; loading buffer) and the final volume was brought to four ml. The buffer-exchanged Talon purified CycS:6xHis was loaded onto a 2.5 ml packed bed volume DEAE column, the flow through was collected along with another 2 x column volumes of loading buffer (total nine ml), and the column was washed with successive 2 x column volumes of loading buffer containing 0.1 M, 0.3 M, 0.5 M, and 1 M NaCl, respectively. Using these conditions (ie. loading buffer at pH 8.0) the human CycS:6xHis did not bind with the DEAE resin and thus, was present in the flow through. For mass spectrometry analysis the DEAE purified CycS:6xHis (matured by both system III and system I) was dialyzed against sterile distilled de-ionized H20 and concentrated as above.
2.5 E. coli cellular protein fractionation
Isolation of periplasmic proteins was by a modification of the method of Feissner, et al. [28]. E. coli cultures (100 ml) of RK103 (Δccm) or RK112 (BL21(DE3) Δccm) containing a system plasmid, pRGK333 (pGEXCcmABCDEFGH), pRGK399 (pMALp2x:CCHL) and a reporter plasmid, pRGK400 (pET226xHis:CCHL) and pRGK390 (pBADCycS:6xHis), pRGK389 (pBADc2:6xHis) or pRGK401 (pBADCycS-c2:6xHis chimera) were grown to an OD600 of 0.6 to 0.8, IPTG (1mM) and arabinose (0.2%, when required) were added for protein over-expression, and induction proceeded for 12–14 hrs. Cells were harvested at 6,000g for 10 min at 4°C and washed once at room temperature with 10 mM Tris pH8.0. The washed cell pellet was resuspended in 10 ml of 100 mM Tris containing 20% (w/v) sucrose, warmed to 37°C, 0.1 mg ml−1 lysozyme was added, and the suspension was gently rocked at 37°C for 15 min. Periplasmic proteins were released from the spheroplasts by treatment with EDTA (10 mM final concentration) at 37°C and separated by centrifugation at 12,000g for 10 min. The supernatant was saved as the periplasmic protein fraction. The spheroplasted cell pellet was resuspended in phosphate buffered saline (10 mM phosphate, 137 mM NaCl, 3 mM KCl, pH7.4), sonicated on ice for 3 × 20 sec (50% duty, 15% output on a Branson Model 250 sonicator), and the lysate was cleared via centrifugation at 12,000g for 15 min. The membranes were separated from the cytoplasm by ultracentrifugation at 100,000g for one hour and solbulized in PBS containing 1% n-Dodecyl-β-D-maltoside. Crude protein extraction for cytochrome c assembly activity assays was accomplished with B-PER (Bacterial Protein Extraction Reagent; Pierce-Thermo Scientific). Briefly, five-ml LB cultures, supplemented with the appropriate antibiotics, of a given strain of E. coli Δccm (or Δccm with single dsbA, dsbB, dsbC, dsbD, or dsbG deletions) containing the indicated system plasmid (pRGK333, pRGK368, pRGK399, or pRGK400) and/or the indicated cytochrome c over-expression plasmid (pRGK332, pRGK389, pRGK390, or pRGK401) were inoculated with 200 μl of fresh overnight culture and grown for 2.5 hrs shaking at 37°C. Protein over-expression was induced with IPTG and arabinose for 4 hrs (system I matured cytochrome c) or 12–14 hrs (system II and III matured cytochrome c). For experiments containing DTT, a final concentration of 3 mM DTT was added to the five ml cultures at the time of IPTG induction, incubation proceeded for 30 min, and then the cytochrome c reporters were induced with arabinose for the times given above. The cells were pelleted at 4,000g for 10 min and resuspended in 200 μl B-PER.
2.6 Heme stains and Western blots
Total protein concentration was determined by BCA assay (Pierce Thermo Scientific) or A280 with a Nanodrop Spectrophotomer (Thermo Scientific). Protein fractions were subjected to SDS-PAGE, transferred to a Hybond C nitrocellulose membrane (GE Healthcare) and heme stains were conducted as described [46] using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce-Thermo Scientific). Western blots were performed with anti-His antibody (1:5000; Santa Cruz Biotechnology) with protein A peroxidase as the secondary label and chemiluminescent signal was developed with SuperSignal West Femto. Both heme stains and Western blots were visualized with an LAS-1000plus Luminescent Image Analyzer CCD camera system (Fujifilm/GE Healthcare).
2.6 Other methods
Reduced (sodium dithionite; Sigma-Aldrich) and as purified (air oxidized) cytochrome c UV-Vis absorption spectra were recorded on a UV2101PC scanning spectrophotometer (Shimadzu). ESI-MS was performed by the Proteomics and Mass Spectrometry Facility at the Donald Danforth Plant Science Center on DEAE purified human CycS:6xHis.
3. Results and Discussion
3.1 Expression of a functional CCHL (ie. cytochrome c synthase) in E.coli
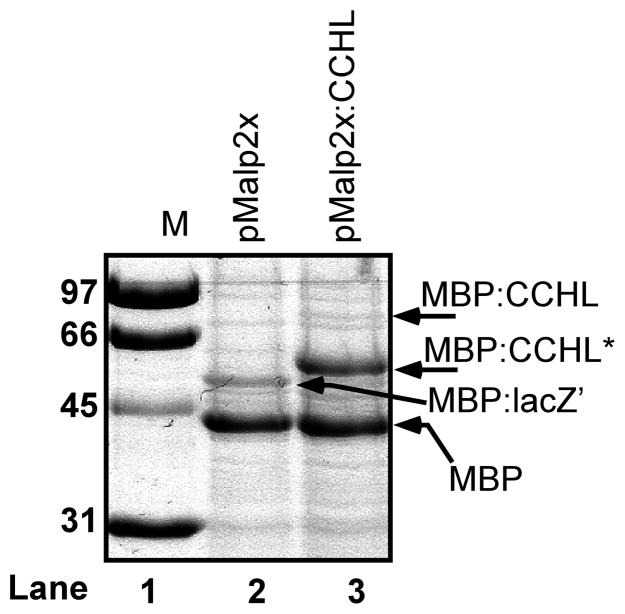
The human gene encoding CCHL was cloned in-frame to the E.coli gene (malE) encoding the periplasmic maltose binding protein (MBP) (Fig 2A). Note that MBP contains its natural signal sequence for export to the periplasm, and such fusion proteins can be purified in one step on amylose-agarose columns. E.coli RK103 (Δccm) with this plasmid as well as RK103 with pMal-p2x (malE-only plasmid without CCHL) were induced with IPTG, periplasmic fractions were separated by SDS-PAGE, and stained with Coomassie Blue (Fig 3). Both preparations show a major polypeptide the size of MBP (42KD). It is often the case that fusion proteins are proteolyzed at the fusion junction resulting in MBP. With pMalp2X, a larger polypeptide (~53 KD) that represents the fusion of lacZ′ from the blue/white screening vector is also present (labeled as MBP:lacZ′ in Fig 3). This polypeptide is absent from preparations of the MBP:CCHL, as expected, and two new polypeptides are observed. One of these is likely the full length MBP:CCHL, migrating at approximately 83 KD, the other is a truncated product of 59 KD (labeled MBP:CCHL*).
Figure 2.
Recombinant plasmids with the human system III (A and B) and indicated c-type cytochromes (C, D, and E).
Figure 3.
Human cytochrome c heme lyase expressed as a MBP:CCHL fusion protein in the E. coli periplasm. Coomassie Blue stained SDS-PAGE profile of periplasmic shock proteins from IPTG-induced RK103 (Δccm) expressing pMalp2x (lane 2) and pMalp2X:CCHL (lane 3). Protein molecular weight standards are shown (labeled M) in lane 1 with indicated molecular masses (left).
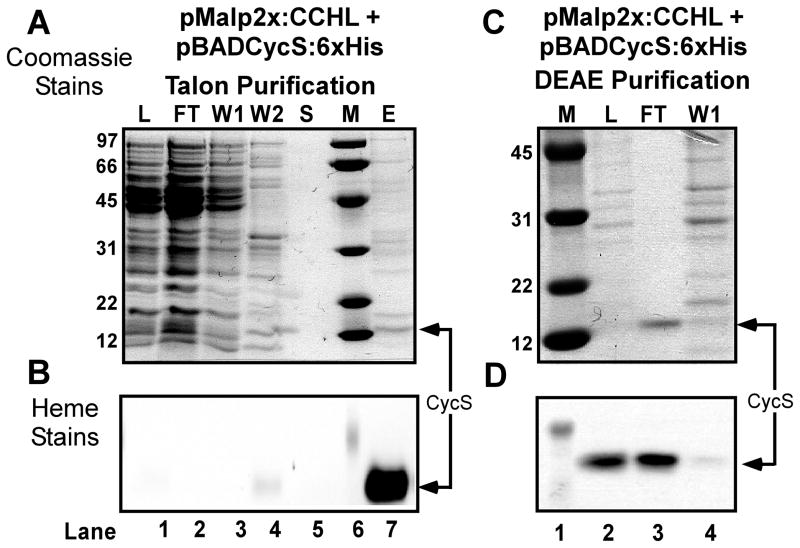
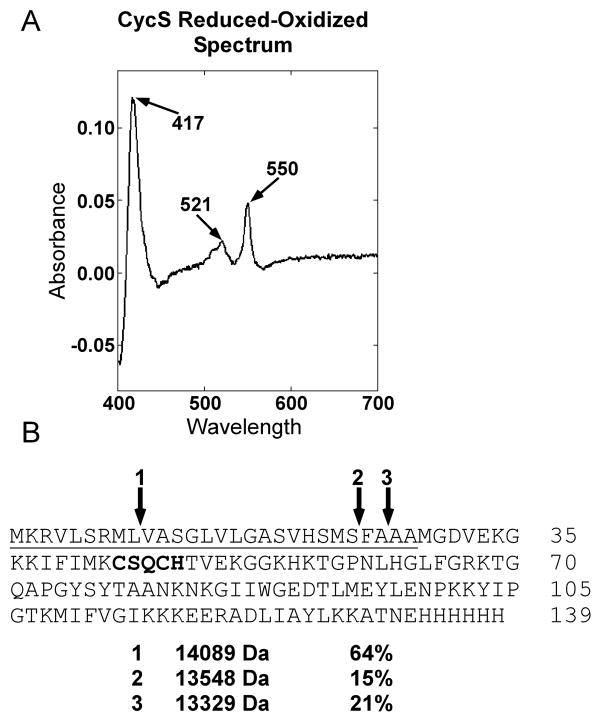
To determine if the human CCHL that contains the MalE periplasmic signal sequence is functional in E.coli, the human cytochrome c gene (cycS) was engineered into an arabinose-inducible pBAD plasmid (Fig 2C) which is compatible with pMalp2x. A periplasmic signal sequence from Bordetella pertussis cytochrome c4 was engineered in frame to the N-terminus of CycS, and cloned downstream of the arabinose-inducible promoter. We have previously shown that the cytochrome c4 signal is efficiently processed in the periplasm of E. coli Δccm expressing recombinant system I and II [28]. For affinity purification a hexahistidine tag was engineered onto the C-terminus of CycS. Expression and heme attachment to this periplasmically targeted CycS was initially tested using the E.coli system I pathway, all eight ccm genes expressed from pRGK333 in E. coli RK103 (Δccm). The human cytochrome c was expressed and assembled in the periplasm, as is evidenced by the heme stain of polypeptides separated by SDS PAGE (Supp Fig 1), which suggests that CycS:6xHis with the c4 signal is properly transported and folded in the periplasm. These results are in agreement with system I cytochrome c maturation requiring apocytochrome export to the periplasm for heme insertion and folding [47]. [Note that because the heme is covalently attached, even upon SDS PAGE the heme remains with the denatured polypeptide and is detected by staining for heme.] We next tested strain RK103 (E.coli Δccm) containing pRGK399 (pMalp2x:CCHL) and pRGK390 (pBADCycS:6xHis) for holoCycS:6xHis maturation. A culture containing the periplasmically targeted MBP:CCHL and CycS:6xHis was induced and the hexahistidine tagged CycS was purified over a cobalt column (Fig 4A). Although the imidazole-eluted fraction showed multiple polypeptides (Fig 4A, lane 7), one major polypeptide at 13 KD stained intensely for heme (Fig 4B, lane 7). Subsequent purification of the imidazole-eluted fraction over a DEAE column yielded (>95%) pure CycS (Fig 4C, lane 3). This polypeptide (13 KD) stained for heme (Fig 4D), and, upon UV-Vis spectroscopy, the pure protein showed the characteristic alpha absorption peak at 550 nm (Fig 5A). The purified preparation was analyzed by ESI-MS to establish whether the signal sequence was cleaved and to confirm covalent heme attachment (Fig 5B). All masses are given as +/− 1 Dalton. Three cleavage products were detected of the masses indicated in Fig 5B (see “1, 2, 3”) and are predicted with an attached heme of 616 Daltons. (Note that the relative percentage of each holoCycS, as determined from mass spectrometry peak profiles, are given to the right of the mass.) Even though each of the three predicted polypeptides (Fig 5B, see “1, 2, 3”) was found to be cleaved at a potential E.coli signal peptidase recognition site [48–50] we cannot rule out some nonspecific proteases are also responsible for this cleavage (in periplasm or cytoplasm). No polypeptides without heme were detected, which is consistent with the premise that the apocytochrome polypeptide is often degraded if heme is not attached (e.g. see [1, 4]). CycS matured by system I was also purified, as given above, and showed identical absorption maxima as system III matured CycS and a single polypeptide at 13329 Da was detected via ESI-MS (data not shown). These results, e.g. the presence of full-length MBP:CCHL in unpurified periplasmic shock fractions (Fig 3 lane 3) and holoCycS that has the same mass and spectral characteristics as system I matured, suggest that at least some of the holoCycS is matured in the E. coli periplasm.
Figure 4.
Purification of hexahistidine tagged human cytochrome c (CycS) produced in IPTG- and arabinose-induced RK103 (Δccm) by pMALp2X:CCHL (MBP:CCHL; pRGK 399). SDS-PAGE of protein purification fractions stained with (A and C) Coomassie Blue and (B and D) heme stain. Hexahistidine-tagged Talon purification fractions (panel A) are indicated above each lane, where lane 1 (L; crude extract load), lane 2 (FT; Flow Through), lane 3 (Wash 1), lane 4 (Wash 2), lane 5 (EDTA strip), and lane 7 (imidazole elution). Note: The imidazole elution was performed before the EDTA strip. DEAE purification fractions are indicated above panel C where lane 2 (Talon elution as load), lane 3 (flow through), and lane 4 (0.3 M NaCl wash). Protein molecular weight standards are shown (labeled M) in lane 6 (A and B) and lane 1 (C and D) with the masses (kDa) shown on the left of A and C. The position of CycS is shown in the flow through with an arrow on the right.
Figure 5.
Reduced minus oxidized UV-Vis absorption spectra and ESI mass spectrometry analysis of purified human cytochrome c matured by MBP:CCHL. (A) UV-Vis absorption spectra of human CycS expressed from RK103 (Δccm) containing pRGK399 (pMalp2x:CCHL) and pRGK390 (pBADCycS:6xHis). The absorption maxima are denoted with arrows. (B) Amino acid sequence of the cytochrome c4 signal:CycS. The signal sequence is underlined, the CXXCH heme binding motif is bolded and the cleavage sites, as determined by ESI-MS, are denoted with arrows. The molecular masses (Da) and relative percentages of each cleaved product, as deduced from the mass spectrometry peak profile, are shown below the amino acid sequence.
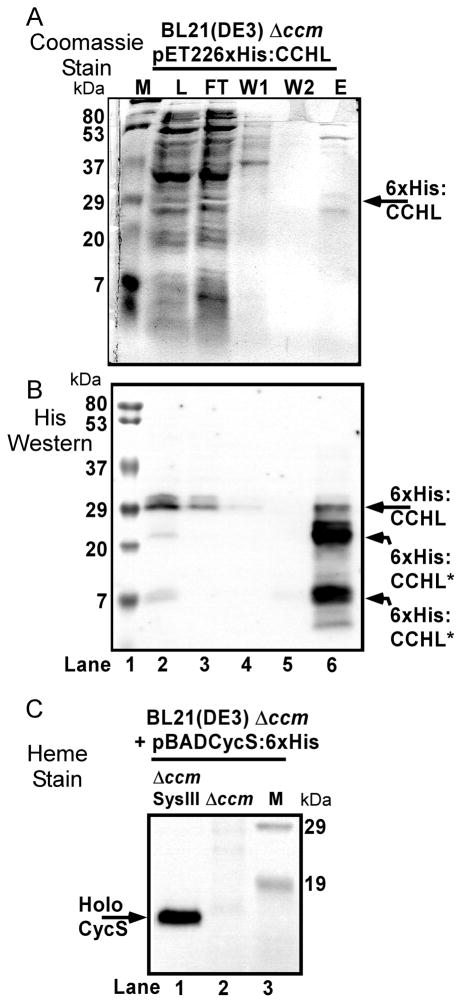
This is the first report that CCHL may be functional in the E.coli periplasm, thus we wanted to confirm the potential periplasmic synthesis using a different secretion signal for CCHL (from pelB), and a different affinity tag for purification, an N-terminal hexahistidine tag rather than a MBP fusion (see Fig 2B). We chose the an N-terminal hexahistidine tag because previous work with cytoplasmically expressed yeast CCHL suggested the N-terminal 6xHis tag was more soluble and was over-expressed to higher levels than the non-hexahistidine tagged version [15, 38]. The 6x:His-tagged CCHL, over-expressed from pRGK400 (pET226xHis:CCHL), could be partially purified over a cobalt column (Fig 6A), and various polypeptides reacted with hexahistidine antisera upon Western blotting (Fig 6B). From the Western blot, full-length CCHL of approximately 30 KD was present in the load fraction (Fig 6B, lane 2) and purified fraction (lane 6), which suggests that at least some of the CCHL is properly folded and stable. Two other immunoreactive polypeptides were observed in the pure fraction (Fig 6B, lane 6), yet it is clear that in the load (lane 2) full-length CCHL is the major polypeptide. Thus, some of the CCHL is proteolyzed during purification. When this plasmid construct is transformed into E.coli BL21(DE3) Δccm (RK112) that also possesses the cycS gene (with signal; pBADCycS:6xHis), human cytochrome c is synthesized, as detected by heme staining of periplasmic shock proteins (Fig 6C, lane 1) and no holocytochrome is detected (lane 2) in the absence of CCHL (pET226xHis:CCHL). We conclude that CCHL can fold and properly function with its cognate cytochrome c in the bacterial periplasm. An important implication of these results is that some reduced heme is available in the periplasm for cytochrome c assembly by CCHL. Possibly this is a ramification of the peripheral association of CCHL [51] with the outer leaflet of the cytoplasmic membrane, where the amphipathic and still reduced heme may bind directly to CCHL for subsequent attachment. We have previously speculated that non-specific “flippases” may flip heme from inner leaflet to outer leaflet in a manner similar to phospholipid “flipping”, as a mechanism to “export” heme for CCHL [1] (see Fig 1C). Nevertheless, the levels of human cytochrome c matured by periplasmic CCHL are very low when compared to system I (see below). Since a previous study indicated that cytoplasmically matured yeast cytochrome c (by cytoplasmic CCHL) was up to two-fold more than a recombinant system I [15], we estimate that in our study at least ten- to one-hundred fold lower levels are periplasmically attached (compared to system I, thus to cytoplasmic CCHL). This may be a ramification of lower levels of available reduced heme, or to the secretory or folding requirements in the periplasm.
Figure 6.
Hexahistidine-tagged human CCHL (with a periplasmic signal) attaches heme to human cytochrome c. Cobalt (Talon) resin purification of IPTG-induced 6xHis:CCHL (pET226xHis:CCHL; pRGK400) expressed in RK112 (BL21(DE3) Δccm). Purification fractions run on SDS-PAGE and detected with (A) Coomassie Blue stain and (B) 6xHis antisera by Western blot. Protein fractions are given above each lane, where lane 2 (L; crude extract load), lane 3 (flow through), lane 4 (wash 1), lane 5 (wash 2), lane 6 (imidazole elution). (C) Heme stain of periplasmic shock proteins from IPTG- and arabinose-induced RK112 containing pRGK400 and pRGK390 (lane 1; labeled SysIII Δccm) or pRGK390 only (lane 2; labeled Δccm). Protein molecular weight standards are shown (labeled M) in lane 1 (A and B; masses on left) and lane 3 (C; masses on right). The position of 6xHis:CCHL and its proteolytic products (A and B) and of holoCycS:6xHis (C) are denoted with arrows.
3.2 Apocytochrome c recognition requirements of systems II and III
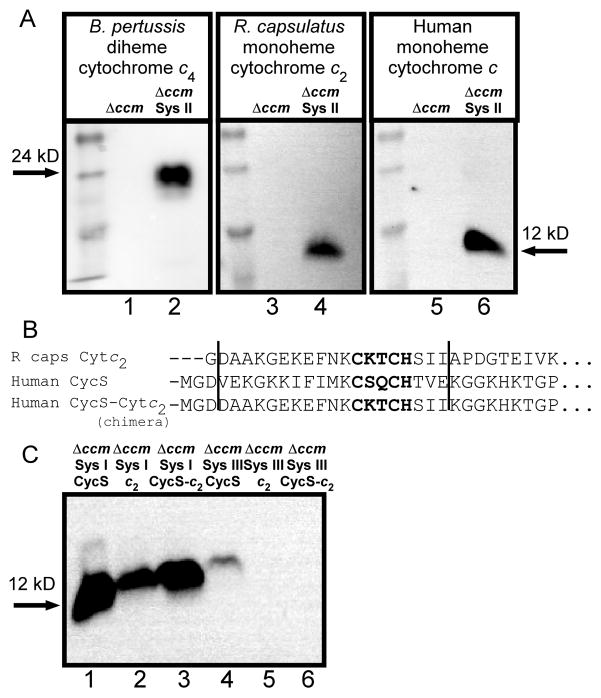
Many studies have been carried out on the substrate recognition of system I, with the conclusion that naturally only the CXXCH appears to be necessary [16–20, 52]. However the substrate recognition requirements for system II are not as well studied. A recent report using our Helicobacter pylori ccsBA recombinant approach indicated that multiple c-type cytochromes were matured [21], suggesting that system II requires only the CXXCH motif for recognition. To further examine recognition determinants of system II, three heterologous cytochromes c were expressed (Fig 2C, D, and pBADCytc4:6xHis [28]) in E. coli strain RK103 (Δccm) in the presence or absence of the H. hepaticus CcsBA system II fusion protein. Heme stains of B-PER-isolated fractions were conducted to detect assembled cytochromes c. In the absence of CcsBA, no cytochrome c is assembled (Figure 7A, lanes 1, 3 and 5). However, when CcsBA is present, all three periplasmic cytochromes c contain covalently attached heme (Figure 7A, lanes 2, 4 and 6), demonstrating that system II is capable of maturing diverse cytochromes c. These include the diheme cytochrome c4 from B. pertussis (system II), cytochrome c2 from R. capsulatus (system I), and the human (system III) cytochrome c. Our results are in agreement with the study by Goddard et al. who showed that CcsBA from H. pylori matures monoheme cytochromes from the system I organisms Paracoccus denitrificans and Hydrogenobacter thermophilus [21]. We concur that the feature of apocytochromes c recognized by CcsBA is the CXXCH motif. Note that rare “alternative” CcsBA synthetases have been shown to recognize motifs other than CXXCH, such as CXXCK and CX15CH [53, 54], but that here we have studied the more common system II CcsBA.
Figure 7.
Maturation of various c-type cytochromes by recombinant CcsBA (system II) and CCHL (system III). (A) Representative heme stains (three trials) of arabinose-induced, soluble B-PER extracts (40 μg) from RK103 (Δccm; lanes 1, 3, and 5) or RK103 containing pRGK368 (Sys II; CcsBA) and pRGK332 (lane 2; pBADCytc4:6xHis), pRGK389 (lane 4; pBADCytc2:6xHis), or pRGK390 (lane 6; pBADCycS:6xHis). (B) Amino acid sequence alignment of the N-termini of R. capsulatus cytochrome c2, the human Cyc S, and the chimeric human CycS:c2. The vertical lines denote the 19 amino acid region that was “swapped” in the chimera. (C) Heme stain of IPTG- and arabinose-induced periplasmic shock proteins (approx. 100 μg) from RK103 containing pRGK333 (SysI; system I) and pRGK390 (lane 1; pBADCycS:6xHis), pRGK389 (lane 2; pBADCytc2:6xHis), pRGK401 (lane 3; CycS-c2; pBADCycS-c2:6xHis chimera) or pRGK399 (SysI; pMALp2xCCHL) containing pRGK390 (lane 4), pRGK389 (lane 5), pRGK401 (lane 6). The sizes of the respective holocytochromes c are denoted with arrows.
It has been largely accepted that the mitochondrial CCHLs may have more stringent recognition requirements than the bacterial systems (e.g. more than CXXCH) [31, 33]. Recombinant studies of CCHLs in the E.coli cytoplasm [15, 38–40] and in yeast mitochondria [3, 41, 42] point to expanded recognition requirements (ie. more than simply CXXCH or XXCH) [55]. Recently, studies using the cytoplasmic recombinant CCHL have started to define exact residues in apocytochrome c that are important for the cognate CCHL recognition [39, 40]. To address possible folding defects (rather than recognition defects), the same recombinant c-type cytochromes were assembled by system I in E.coli [39]. However these controls (system I) require the engineering of a signal sequence, thus not allowing exact comparison of substrates (or folding environments, periplasm versus cytoplasm). One of the uses of the recombinant system III (CCHL) described here is to facilitate direct comparisons of identical substrates in the same cellular compartment (periplasm). We test this approach by focusing on the human cytochrome c and the structurally related soluble monoheme cytochrome c2 [56–58] from R. capsulatus. Three substrates were compared: the human cytochrome c, the R.capsulatus cytochrome c2, and a chimeric molecule in which the human cytochrome c had a 19 residue region (encompassing the CXXCH) substituted with the analogous region of the R.capsulatus cytochrome c2 (Fig 7B). System I recognized and assembled all three substrates (Fig 7C, lanes 1–3), which further confirms that system I can mature a wide variety of c-type cytochromes. However, the human CCHL recognized only its cognate human cytochrome c (Fig 7C, lane 4) and not the cytochrome c2 (lane 5) or chimera (lane 6). Because the expression level of system III was lower than that of system I, as noted previously with recombinant system II [28, 29], we concentrated by six-fold the recombinant periplasmic shock proteins and still no holocytochrome c2 or chimera are detected (data not shown). We conclude that CXXCH is not sufficient for CCHL recognition, and that other residues within this region (ie. of the 19 residues of the human cytochrome c defined in Fig 7B) are important for recognition by human CCHL. This conclusion is consistent with previous cytoplasmic studies in E.coli that suggested the N-terminus (up to and including the CXXCH) of the apocytochrome c are important and sufficient for yeast CCHL activity (e.g. [40, 47]). Further analysis will be necessary to elucidate the exact residues necessary for recognition.
3.3 The periplasmic thiol redox requirements of recombinant system II and III
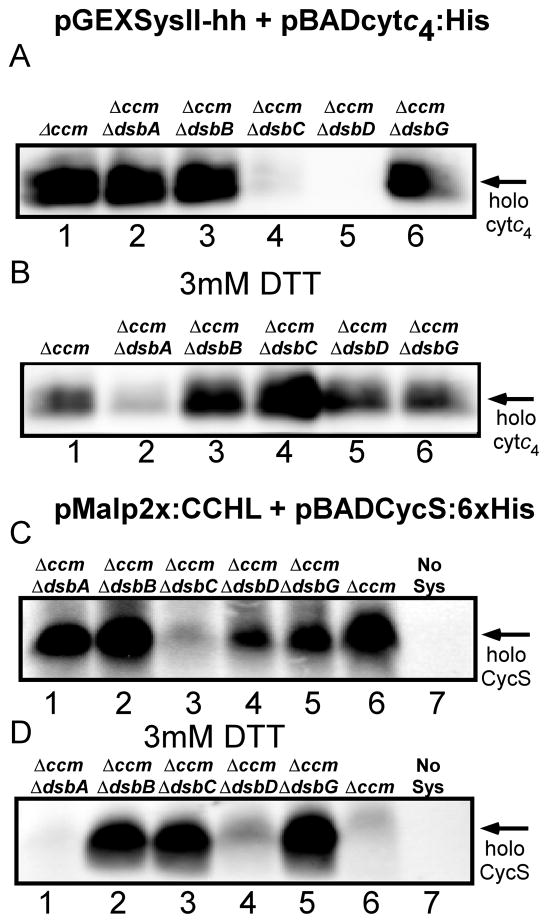
When results on the first functional recombinant system II were published [28], a commentary on the achievement [30] suggested that one significant question was what periplasmic thiol oxidation and reduction pathway(s) from E.coli were used. For secreted (and membrane) proteins the E. coli Dsb system is one of the best understood pathways for disulfide bond formation [59–61]. DsbA and DsbB represent the major pathway for oxidation of disulfides in the periplasm. For thiol reduction, the integral membrane protein DsbD transfers reducing equivalents from the cytoplasm to periplasmic thioredoxin-type proteins such as DsbC, or to CcmG (HelX) in the case of the natural system I in E.coli (see Fig 1A). (Note that deletion of the ccm operon, necessary for studies on the recombinant system II and III, removes ccmG also). In order to determine which Dsb proteins, if any, are required for recombinant systems, deletions of selected dsb genes were constructed in a Δccm background. Using the recombinant system plasmids and selected cytochrome c expression plasmids, the attachment of heme was investigated in each strain (Fig 8). For system II, the dsbD and dsbC mutants produced little to no cytochrome c4 (Fig 8A), and these defects were largely corrected by adding exogenous DTT (Fig 8B). Neither the dsbA nor dsbB mutant showed defects in assembly, suggesting that formation of a disulfide is unnecessary under these growth conditions. Various studies have suggested that sometimes DsbA and/or DsbB are necessary for the natural system I mediated synthesis, and sometimes they are dispensable [13, 14, 62]. Such variation could be due to aerobic versus anaerobic growth conditions, but we have not studied this further. The requirement for DsbC and DsbD suggests that some of the apocytochrome c4 (CXXC) may undergo oxidation (e.g. oxygen-mediated), and DsbD-/DsbC-mediated thiol reduction is necessary. DsbC encodes a periplasmic thioredoxin-like protein, thus we propose that it functions in the reduction of apocytochrome c directly, and is re-reduced by DsbD. In the case of recombinant system III (CCHL) and its cognate human cytochrome c (Fig 8C, D), DsbC is partially required (holoCycS is reduced by 80%), while the dsbD mutant showed only 30% reduction (when averaged over three trials) in cytochrome c assembly. It is possible that as the human cytochrome c is exported it is more rapidly used by CCHL, thus its two cysteines are reduced and ready for heme attachment to CXXCH. (Alternatively, some of the holoCycS may be matured by unsecreted, cytoplasmically located CCHL.) In both systems II and III, DTT inhibits cyctochrome c production in the absence of DsbA (and in Δccm). This result could be due to the osmofragility of dsbA− strains, thus a pleiotrophic effect [63]. We conclude that under the aerobic conditions used, both recombinant systems II and III require some thiol reduction and this periplasmic reduction is mediated by the natural E.coli DsbC and/or DsbD proteins. For system III, the lack of a strong DsbD requirement suggests that DsbC may be acting in a DsbD-independent manner, thus DsbC can obtain reductant from other periplasmic proteins directly or from reduced DsbA, as shown previously [63, 64]. In addition to the thiol reduction requirement, DsbC might protect the apocytochrome c cysteines from sulfenylation, which was recently reported as a function of DsbC [65]. The need for DsbC in system III underscores the conclusion that heme attachment can occur in the periplasm by this recombinant CCHL system, albeit at much reduced levels when compared to system I (or to cytoplasmic CCHL). The absolute dependence of recombinant system II on DsbC and DsbD (and correction by exogenous DTT), addresses how recombinant, periplasmic c-type cytochromes in E. coli can be reduced using various recombinant assembly systems (e.g. see [28, 30, 64]
Figure 8.
Assembly of c-type cytochromes by CcsBA and CCHL in E. coli strains deleted of the indicated genes. Representative heme stains (based on three trials) of soluble B-PER extracts (40 μg) of IPTG- and arabinose-induced ccm or ccm dsb knockout(s) (indicated above each lane), expressing pRGK368 (CcsBA) and pRGK332 (pBADCytc4:6xHis; A and B) or pRGK399 (pMalp2x:CCHL) and pRGK390 (pBADCycS:6xHis; C and D). Panels C and D lane 7 (No Sys) represent IPTG- and arabinose-induced E. coli ccm containing only pRGK390. B-PER extracts of induced E.coli strains where exogenous 3 mM DTT was included during growth and induction are shown in B and D. The positions of holoCytc4 and holoCycS are denoted with arrows.
Supplementary Material
Highlights.
Human CCHL matures human cytochrome c in the E. coli periplasm but at significantly lower levels than cytoplasmic CCHL
E. coli DsbC and DsbD mediate thiol reduction for recombinant systems II and III
The major feature of apocytochromes recognized by CcsBA is CXXCH
Residues in addition to CXXCH are important for apocytochrome recognition by CCHL
Acknowledgments
This work was supported by NIH GM47909 to RGK. We thank Dr. Leslie Hicks for the ESI-MS analyses and discussions.
ABBREVIATIONS
- CCHL
cytochrome c heme lyase
- 6x:His
hexahistidine
- B-PER
bacterial protein extraction reagent
- DEAE
diethylaminoethyl
- cyt
cytochrome
- MBP
maltose binding protein
- DTT
dithiothreitol
- GST
glutathione transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kranz RG, Richard-Fogal C, Taylor JS, Frawley ER. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol Mol Biol Rev. 2009;73:510–528. doi: 10.1128/MMBR.00001-09. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders C, Turkarslan S, Lee DW, Daldal F. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 2010;18:266–274. doi: 10.1016/j.tim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamel P, Corvest V, Giege P, Bonnard G. Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim Biophys Acta. 2009;1793:125–138. doi: 10.1016/j.bbamcr.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson SJ, Stevens JM, Allen JW, Robertson IB. Cytochrome c assembly: a tale of ever increasing variation and mystery? Biochim Biophys Acta. 2008;1777:980–984. doi: 10.1016/j.bbabio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Dumont MD, Mathews AJ, Nall BT, Baim SB, Eustice DC, Sherman F. Differential stability of two apo-isocytochromes c in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990;265:2733–2739. [PubMed] [Google Scholar]
- 6.Goldman BS, Beckman DL, Bali A, Monika EM, Gabbert KK, Kranz RG. Molecular and immunological analysis of an ABC transporter complex required for cytochrome c biogenesis. J Mol Biol. 1997;268:724–738. doi: 10.1006/jmbi.1997.0992. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson DW, Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc Natl Acad Sci U S A. 1989;86:4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker PD, Nerou EP, Freund SM, Fearnley IM. Conversion of cytochrome b562 to c-type cytochromes. Biochemistry. 1995;34:15191–15203. doi: 10.1021/bi00046a027. [DOI] [PubMed] [Google Scholar]
- 9.Beckman DL, Kranz RG. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc Natl Acad Sci U S A. 1993;90:2179–2183. doi: 10.1073/pnas.90.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MD, Ferguson SJ. Paracoccus denitrificans CcmG is a periplasmic protein-disulphide oxidoreductase required for c- and aa3-type cytochrome biogenesis; evidence for a reductase role in vivo. Mol Microbiol. 1997;24:977–990. doi: 10.1046/j.1365-2958.1997.4061775.x. [DOI] [PubMed] [Google Scholar]
- 11.Monika EM, Goldman BS, Beckman DL, Kranz RG. A thioreduction pathway tethered to the membrane for periplasmic cytochromes c biogenesis; in vitro and in vivo studies. J Mol Biol. 1997;271:679–692. doi: 10.1006/jmbi.1997.1227. [DOI] [PubMed] [Google Scholar]
- 12.Missiakas D, Schwager F, Raina S. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 1995;14:3415–3424. doi: 10.1002/j.1460-2075.1995.tb07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metheringham R, Tyson KL, Crooke H, Missiakas D, Raina S, Cole JA. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol Gen Genet. 1996;253:95–102. doi: 10.1007/pl00013815. [DOI] [PubMed] [Google Scholar]
- 14.Turkarslan S, Sanders C, Ekici S, Daldal F. Compensatory thio-redox interactions between DsbA, CcdA and CcmG unveil the apocytochrome c holdase role of CcmG during cytochrome c maturation. Mol Microbiol. 2008;70:652–666. doi: 10.1111/j.1365-2958.2008.06441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders C, Lill H. Expression of prokaryotic and eukaryotic cytochromes c in Escherichia coli. Biochim Biophys Acta. 2000;1459:131–138. doi: 10.1016/s0005-2728(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 16.Allen JW, Tomlinson EJ, Hong L, Ferguson SJ. The Escherichia coli cytochrome c maturation (Ccm) system does not detectably attach heme to single cysteine variants of an apocytochrome c. J Biol Chem. 2002;277:33559–33563. doi: 10.1074/jbc.M204963200. [DOI] [PubMed] [Google Scholar]
- 17.Braun M, Thony-Meyer L. Biosynthesis of artificial microperoxidases by exploiting the secretion and cytochrome c maturation apparatuses of Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:12830–12835. doi: 10.1073/pnas.0402435101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen JW, Leach N, Ferguson SJ. The histidine of the c-type cytochrome CXXCH haem-binding motif is essential for haem attachment by the Escherichia coli cytochrome c maturation (Ccm) apparatus. Biochem J. 2005;389:587–592. doi: 10.1042/BJ20041894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JW, Ferguson SJ. What is the substrate specificity of the System I cytochrome c biogenesis apparatus? Biochem Soc Trans. 2006;34:150–151. doi: 10.1042/BST0340150. [DOI] [PubMed] [Google Scholar]
- 20.Allen JW, Sawyer EB, Ginger ML, Barker PD, Ferguson SJ. Variant c-type cytochromes as probes of the substrate specificity of the E. coli cytochrome c maturation (Ccm) apparatus. Biochem J. 2009;419:177–184. doi: 10.1042/BJ20081999. [DOI] [PubMed] [Google Scholar]
- 21.Goddard AD, Stevens JM, Rondelet A, Nomerotskaia E, Allen JW, Ferguson SJ. Comparing the substrate specificities of cytochrome c biogenesis Systems I and II: bioenergetics. FEBS J. 2010;277:726–737. doi: 10.1111/j.1742-4658.2009.07517.x. [DOI] [PubMed] [Google Scholar]
- 22.Beckett CS, Loughman JA, Karberg KA, Donato GM, Goldman WE, Kranz RG. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol Microbiol. 2000;38:465–481. doi: 10.1046/j.1365-2958.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- 23.Schiott T, Throne-Holst M, Hederstedt L. Bacillus subtilis CcdA-defective mutants are blocked in a late step of cytochrome c biogenesis. J Bacteriol. 1997;179:4523–4529. doi: 10.1128/jb.179.14.4523-4529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Brun NE, Bengtsson J, Hederstedt L. Genes required for cytochrome c synthesis in Bacillus subtilis. Mol Microbiol. 2000;36:638–650. doi: 10.1046/j.1365-2958.2000.01883.x. [DOI] [PubMed] [Google Scholar]
- 25.Page ML, Hamel PP, Gabilly ST, Zegzouti H, Perea JV, Alonso JM, Ecker JR, Theg SM, Christensen SK, Merchant S. A homolog of prokaryotic thiol disulfide transporter CcdA is required for the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J Biol Chem. 2004;279:32474–32482. doi: 10.1074/jbc.M404285200. [DOI] [PubMed] [Google Scholar]
- 26.Feissner RE, Beckett CS, Loughman JA, Kranz RG. Mutations in cytochrome assembly and periplasmic redox pathways in Bordetella pertussis. J Bacteriol. 2005;187:3941–3949. doi: 10.1128/JB.187.12.3941-3949.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frawley ER, Kranz RG. CcsBA is a cytochrome c synthetase that also functions in heme transport. Proc Natl Acad Sci U S A. 2009;106:10201–10206. doi: 10.1073/pnas.0903132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feissner RE, Richard-Fogal CL, Frawley ER, Loughman JA, Earley KW, Kranz RG. Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol Microbiol. 2006;60:563–577. doi: 10.1111/j.1365-2958.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- 29.Richard-Fogal CL, Frawley ER, Feissner RE, Kranz RG. Heme concentration dependence and metalloporphyrin inhibition of the system I and II cytochrome c assembly pathways. J Bacteriol. 2007;189:455–463. doi: 10.1128/JB.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turkarslan S, Sanders C, Daldal F. Extracytoplasmic prosthetic group ligation to apoproteins: maturation of c-type cytochromes. Mol Microbiol. 2006;60:537–541. doi: 10.1111/j.1365-2958.2006.05148.x. [DOI] [PubMed] [Google Scholar]
- 31.Dumont ME, Ernst JF, Hampsey DM, Sherman F. Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J. 1987;6:235–241. doi: 10.1002/j.1460-2075.1987.tb04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson DW, Kohler H, Neupert W. Import of cytochrome c into mitochondria. Cytochrome c heme lyase. Eur J Biochem. 1987;164:147–157. doi: 10.1111/j.1432-1033.1987.tb11006.x. [DOI] [PubMed] [Google Scholar]
- 33.Zollner A, Rodel G, Haid A. Molecular cloning and characterization of the Saccharomyces cerevisiae CYT2 gene encoding cytochrome-c1-heme lyase. Eur J Biochem. 1992;207:1093–1100. doi: 10.1111/j.1432-1033.1992.tb17146.x. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer L, Ballabio A, Zoghbi HY. Cloning and characterization of a putative human holocytochrome c-type synthetase gene (HCCS) isolated from the critical region for microphthalmia with linear skin defects (MLS) Genomics. 1996;34:166–172. doi: 10.1006/geno.1996.0261. [DOI] [PubMed] [Google Scholar]
- 35.Prakash SK, Cormier TA, McCall AE, Garcia JJ, Sierra R, Haupt B, Zoghbi HY, Van Den Veyver IB. Loss of holocytochrome c-type synthetase causes the male lethality of X-linked dominant microphthalmia with linear skin defects (MLS) syndrome. Hum Mol Genet. 2002;11:3237–3248. doi: 10.1093/hmg/11.25.3237. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz QP, Cox TC. Complementation of a yeast CYC3 deficiency identifies an X-linked mammalian activator of apocytochrome c. Genomics. 2002;79:51–57. doi: 10.1006/geno.2001.6677. [DOI] [PubMed] [Google Scholar]
- 37.Steiner H, Kispal G, Zollner A, Haid A, Neupert W, Lill R. Heme binding to a conserved Cys-Pro-Val motif is crucial for the catalytic function of mitochondrial heme lyases. J Biol Chem. 1996;271:32605–32611. doi: 10.1074/jbc.271.51.32605. [DOI] [PubMed] [Google Scholar]
- 38.Pollock WB, Rosell FI, Twitchett MB, Dumont ME, Mauk AG. Bacterial expression of a mitochondrial cytochrome c. Trimethylation of lys72 in yeast iso-1-cytochrome c and the alkaline conformational transition. Biochemistry. 1998;37:6124–6131. doi: 10.1021/bi972188d. [DOI] [PubMed] [Google Scholar]
- 39.Kleingardner JG, Bren KL. Comparing substrate specificity between cytochrome c maturation and cytochrome c heme lyase systems for cytochrome c biogenesis. Metallomics. 2011;3:396–403. doi: 10.1039/c0mt00086h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens JM, Zhang Y, Muthuvel G, Sam KA, Allen JW, Ferguson SJ. The mitochondrial cytochrome c N-terminal region is critical for maturation by holocytochrome c synthase. FEBS Lett. 2011:1891–1896. doi: 10.1016/j.febslet.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 41.Bernard DG, Gabilly ST, Dujardin G, Merchant S, Hamel PP. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J Biol Chem. 2003;278:49732–49742. doi: 10.1074/jbc.M308881200. [DOI] [PubMed] [Google Scholar]
- 42.Corvest V, Murrey DA, Bernard DG, Knaff DB, Guiard B, Hamel PP. c-type cytochrome assembly in Saccharomyces cerevisiae: a key residue for apocytochrome c1/lyase interaction. Genetics. 2010;186:561–571. doi: 10.1534/genetics.110.120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thierauf A, Perez G, Maloy AS. Generalized transduction. Methods Mol Biol. 2009;501:267–286. doi: 10.1007/978-1-60327-164-6_23. [DOI] [PubMed] [Google Scholar]
- 46.Feissner R, Xiang Y, Kranz RG. Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes. Anal Biochem. 2003;315:90–94. doi: 10.1016/s0003-2697(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 47.Sanders C, Wethkamp N, Lill H. Transport of cytochrome c derivatives by the bacterial Tat protein translocation system. Mol Microbiol. 2001;41:241–246. doi: 10.1046/j.1365-2958.2001.02514.x. [DOI] [PubMed] [Google Scholar]
- 48.Carlos JL, Paetzel M, Brubaker G, Karla A, Ashwell CM, Lively MO, Cao G, Bullinger P, Dalbey RE. The role of the membrane-spanning domain of type I signal peptidases in substrate cleavage site selection. J Biol Chem. 2000;275:38813–38822. doi: 10.1074/jbc.M007093200. [DOI] [PubMed] [Google Scholar]
- 49.Paetzel M, Dalbey RE, Strynadka NC. The structure and mechanism of bacterial type I signal peptidases. A novel antibiotic target. Pharmacol Ther. 2000;87:27–49. doi: 10.1016/s0163-7258(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 50.von Heijne G. Life and death of a signal peptide. Nature. 1998;396:111–113. doi: 10.1038/24036. [DOI] [PubMed] [Google Scholar]
- 51.Lill R, Stuart RA, Drygas ME, Nargang FE, Neupert W. Import of cytochrome c heme lyase into mitochondria: a novel pathway into the intermembrane space. EMBO J. 1992;11:449–456. doi: 10.1002/j.1460-2075.1992.tb05074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen JW, Ferguson SJ. The Escherichia coli cytochrome c maturation (Ccm) apparatus can mature cytochromes with an extra cysteine within or adjacent to the CXXCH motif. Biochem Soc Trans. 2006;34:91–93. doi: 10.1042/BST0340091. [DOI] [PubMed] [Google Scholar]
- 53.Hartshorne S, Richardson DJ, Simon J. Multiple haem lyase genes indicate substrate specificity in cytochrome c biogenesis. Biochem Soc Trans. 2006;34:146–149. doi: 10.1042/BST0340146. [DOI] [PubMed] [Google Scholar]
- 54.Kern M, Eisel F, Scheithauer J, Kranz RG, Simon J. Substrate specificity of three cytochrome c haem lyase isoenzymes from Wolinella succinogenes: unconventional haem c binding motifs are not sufficient for haem c attachment by NrfI and CcsA1. Mol Microbiol. 2010;75:122–137. doi: 10.1111/j.1365-2958.2009.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosell FI, Mauk AG. Spectroscopic properties of a mitochondrial cytochrome C with a single thioether bond to the heme prosthetic group. Biochemistry. 2002;41:7811–7818. doi: 10.1021/bi016060e. [DOI] [PubMed] [Google Scholar]
- 56.Benning MM, Meyer TE, Holden HM. X-Ray structure of the cytochrome c2 isolated from Paracoccus denitrificans refined to 1.7-A resolution. Arch Biochem Biophys. 1994;310:460–466. doi: 10.1006/abbi.1994.1193. [DOI] [PubMed] [Google Scholar]
- 57.Benning MM, Wesenberg G, Caffrey MS, Bartsch RG, Meyer TE, Cusanovich MA, Rayment I, Holden HM. Molecular structure of cytochrome c2 isolated from Rhodobacter capsulatus determined at 2.5 A resolution. J Mol Biol. 1991;220:673–685. doi: 10.1016/0022-2836(91)90109-j. [DOI] [PubMed] [Google Scholar]
- 58.Holden HM, Meyer TE, Cusanovich MA, Daldal F, Rayment I. Crystallization and preliminary analysis of crystals of cytochrome c2 from Rhodopseudomonas capsulata. J Mol Biol. 1987;195:229–231. doi: 10.1016/0022-2836(87)90341-x. [DOI] [PubMed] [Google Scholar]
- 59.Kadokura H, Katzen F, Beckwith J. Protein disulfide bond formation in prokaryotes. Annu Rev Biochem. 2003;72:111–135. doi: 10.1146/annurev.biochem.72.121801.161459. [DOI] [PubMed] [Google Scholar]
- 60.Gleiter S, Bardwell JC. Disulfide bond isomerization in prokaryotes. Biochim Biophys Acta. 2008;1783:530–534. doi: 10.1016/j.bbamcr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inaba K. Disulfide bond formation system in Escherichia coli. J Biochem. 2009;146:591–597. doi: 10.1093/jb/mvp102. [DOI] [PubMed] [Google Scholar]
- 62.Sambongi Y, Ferguson SJ. Mutants of Escherichia coli lacking disulphide oxidoreductases DsbA and DsbB cannot synthesise an exogenous monohaem c-type cytochrome except in the presence of disulphide compounds. FEBS Lett. 1996;398:265–268. doi: 10.1016/s0014-5793(96)01256-2. [DOI] [PubMed] [Google Scholar]
- 63.Vertommen D, Depuydt M, Pan J, Leverrier P, Knoops L, Szikora JP, Messens J, Bardwell JC, Collet JF. The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner. Mol Microbiol. 2008;67:336–349. doi: 10.1111/j.1365-2958.2007.06030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goddard AD, Stevens JM, Rao F, Mavridou DA, Chan W, Richardson DJ, Allen JW, Ferguson SJ. c-Type cytochrome biogenesis can occur via a natural Ccm system lacking CcmH, CcmG, and the heme-binding histidine of CcmE. J Biol Chem. 2010;285:22882–22889. doi: 10.1074/jbc.M110.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Depuydt M, Leonard SE, Vertommen D, Denoncin K, Morsomme P, Wahni K, Messens J, Carroll KS, Collet JF. A periplasmic reducing system protects single cysteine residues from oxidation. Science. 2009;326:1109–1111. doi: 10.1126/science.1179557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.