Abstract
Inhibition of mTOR signaling by rapamycin has been demonstrated to activate ERK1/2 and Akt in various types of cancer cells, which contributes to rapamycin resistance. However, the downstream effect of rapamycin-activated ERKs and Akt on survival or death substrate(s) remains unclear. We discovered that treatment of human lung cancer cells with rapamycin results in enhanced phosphorylation of Bad at serine (S) 112 and S136 but not S155 in association with activation of ERK1/2 and Akt. A higher level of Bad phosphorylation was observed in rapamycin-resistant cells compared to parental rapamycin-sensitive cells. Thus, Bad phosphorylation may contribute to rapamycin resistance. Mechanistically, rapamycin promotes Bad accumulation in the cytosol, enhances Bad/14-3-3 interaction and reduces Bad/Bcl-XL binding. Rapamycin-induced Bad phosphorylation promotes its ubiquitination and degradation, with a significant reduction of its half-life (i.e. from 53.3 h to 37.5 h). Inhibition of MEK/ERK by PD98059 or depletion of Akt by RNA interference blocks rapamycin-induced Bad phosphorylation at S112 or S136, respectively. Simultaneous blockage of S112 and S136 phosphorylation of Bad by PD98059 and silencing of Akt significantly enhances rapamycin-induced growth inhibition in vitro and synergistically increases the anti-tumor efficacy of rapamycin in lung cancer xenografts. Intriguingly, either suppression of Bad phosphorylation at S112 and S136 sites or expression of the non-phosphorylatable Bad mutant (S112A/S136A) can reverse rapamycin resistance. These findings uncover a novel mechanism of rapamycin resistance, which may promote the development of new strategies for overcoming rapamycin resistance by manipulating Bad phosphorylation at S112 and S136 in human lung cancer.
Introduction
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase and functions as a central regulator of cell growth, cell proliferation and survival (1, 2). mTOR has been identified as a downstream target of the PI3K/Akt survival pathway. The discovery and clinical development of the highly specific and potent mTOR inhibitor rapamycin and its derivatives (i.e. RAD001, CCI-779 and AP23573) as anticancer agents has further enhanced our ability to elucidate mTOR biological function (3-5). Inhibition of the mTOR pathway has been proposed to represent a promising therapeutic approach for lung cancer (5-7). However, it is increasingly recognized that the clinical activity of rapalog(s) as a single agent is insufficient for achieving a broad and robust anticancer effect (1, 8). The molecular mechanisms underlying resistance of some cancer cells to mTOR inhibition are not fully understood. We and others have previously discovered that, in addition to inhibiting the kinase activity of mTOR, rapamycin and RAD001 can also activate Akt and ERK1/2 survival signaling pathways, this effect contributes to resistance of lung and breast cancer cells to mTOR inhibition (6, 9-11). However, the downstream survival or death substrates of rapamycin-activated Akt and ERK signaling pathways remain unclear.
Bcl2 family members are key regulators of apoptosis at the decision phase and share homology clustered within four conserved Bcl2 homology (BH) domains: BH1, BH2, BH3 and BH4. Only the antiapoptotic proteins, such as Bcl2, Bcl-XL, Bcl-w and A1 bear the NH2-terminal BH4 domain (12). The proapoptotic family members are divided into two subgroups based on the presence of BH domains, including the BH123 multidomain proteins (i.e. Bax and Bak) and the BH3-only molecules (i.e. Bad, Bid, Bim, Bik, Nix, Noxa, PUMA, etc.) (13, 14). The BH3-only proapoptotic proteins are upstream sensors of cellular damage that selectively respond to specific, proximal death and survival signals (15). BH3-only proteins exert their proapoptotic activity by hierarchical and tightly choreographed interactions with other Bcl-2 family members. The BH3-only proapoptotic proteins share homology within a single amphipathic BH segment, the BH3 domain, which is also known as the minimum death domain. BH3-only proteins are cell death initiators and their post-translational modifications (i.e. phosphorylation), proteolytic processing and lipid modification, are potential mechanisms that integrate extracellular survival and death signals with the core apoptotic machinery (15). These molecules are also being explored as possible tools for cancer therapy, based on the expectation that molecules mimicking the BH3 domain of these proteins could selectively and efficiently cooperate with chemotherapeutic drugs in cell killing (16). Bad is one of the BH3-only proapoptotic members and can couple death signals to mitochondria and promote apoptosis by quelling the protective action of Bcl-XL (17). Phosphorylation of Bad at serine (S) 112, S136 and S155 has been demonstrated to inactivate its proapoptotic function (18, 19) through a mechanism involving binding to 14-3-3 scaffold proteins that results in sequestering Bad from mitochondria and dissociation of Bad from mitochondrial Bcl2 and/or Bcl-XL (20-22). The active Bad exists in a dephosphorylated form that localizes to the mitochondria and interacts with Bcl-XL to neutralize its antiapoptotic function. Akt and the MAPKs ERK1/2 are reported to function as physiologic Bad kinases (23-25). Here we report that inhibition of mTOR by rapamycin stimulates Bad phosphorylation at S112 and S136 through activation of ERK1/2 and Akt, which results in inactivation of the proapoptotic function of Bad and decreased sensitivity of lung cancer cells to mTOR inhibition. Blockage of rapamycin-induced Bad phosphorylation significantly sensitizes lung cancer cell lines and lung tumors to mTOR inhibition.
Materials and Methods
Materials
Rapamycin was purchased from LC Laboratories (Woburn, MA). PD98059 was purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Phospho-Bad (S136), phospho-Bad (S112), phospho-Bad (S155), phospho-Akt (S473), Akt, ERK1/2, mTOR, p-mTOR, p-p70S6K (T389), p70S6K, p-4EBP1 (T37/46) and 4EBP1 antibodies were purchased from Cell Signaling Technology (Beverly, MA). 14-3-3, pERK1/2, PCNA, prohibitin and β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Bad and Bcl-XL were purchased from Epitomics, Inc. (Burlingame, CA). NanoJuice transfection Kit was obtained from EMD Chemical, Inc. (Gibbstown, NJ). TumorTACS™ In Situ Apoptosis Detection Kit was purchased from Trevigen, Inc. (Gaithersburg, MD). Murine WT-Bad and mutant Bad S112A/S136A (AA) cDNAs in pcDNA3 plasmids were obtained from Addgene (Cambridge, MA). All other reagents used were obtained from commercial sources unless otherwise stated.
Cell lines and cell culture
H460, H157 and A459 were purchased from the American Type Culture Collection (ATCC, Manassas, VA), and no authentication for these cell lines was done by the authors. H460 and H157 cells were maintained in RPMI 1640 with 10% fetal bovine serum. A549 cells were maintained in F-12K medium with 10% fetal bovine serum. The rapamycin-resistant A549 cell line (A549-RR) was established as described previously (6). Briefly, A549-RR was established by exposing the rapamycin-sensitive A549 parental cells (A549-P) to gradually increasing concentrations of rapamycin from the initial 1 nM to the final 20 μM over a 6-month period as described (6).
Preparation of cell lysate and Western blot
Cells were washed with cold PBS and resuspended in ice-cold EBC buffer (0.5% Nonidet P-40, 50 mM Tris, pH 7.6, 120 mM NaCl, 1 mM EDTA, and 1 mM-β-mercaptoethanol) containing protease inhibitor mixture set I. Following cell lysis by sonication and centrifugation at 14,000 × g for 15 min at 4 °C, the resulting supernatant was collected as the total cell lysate. As previously described, Western blot was performed by loading 50μg of protein per lane on an 8–12% SDS-PAGE, followed by protein transfer to nitrocellulose membrane for analysis of specific protein(s) (26).
RNA interference, plasmids and transfection
Human Akt shRNA plasmid is a target-specific lentiviral vector plasmid encoding a 19-25 nt (plus hairpin) shRNA designed to knock down gene expression. The control shRNA plasmid-A encodes a scrambled shRNA sequence that will not lead to the specific degradation of any cellular message. Both Akt shRNA and control shRNA plasmids were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Hairpin sequence: GAT CCT GCC CTT CTA CAA CCA GGA TTC AAG AGA TCC TGG TTG TAG AAG GGC ATT TTT. Corresponding siRNA sequences: Sense: 5'- UGC CCU UCU ACA ACC AGG Att -3'; Antisense: 5'- UCC UGG UUG UAG AAG GGC Att -3. Transfection of shRNA, Bad cDNA or HA-tagged ubiquitin plasmid (27, 28) was performed using NanoJuice transfection Kit according to the manufacturer's instructions (EMD Chemical, Inc.).
Subcellular fractionation
Subcellular fractionation was performed as previously described (29). Briefly, H460 cells (2-3×107) were washed with cold 1× PBS and resuspended in isotonic mitochondrial buffer (210 mM mannitol, 70 mM sucrose, 1 mM EGTA, 10 mM Hepes, pH 7.5) containing protease inhibitor mixture set I, homogenized with a Polytron homogenizer operating for 4 bursts of 10s each at a setting of 5 and then centrifuged at 2000 × g for 3 min to pellet the nuclei and unbroken cells. The supernatant was centrifuged at 13,000 × g for another 10 min to pellet mitochondria as described (30). The second supernatant was further centrifuged at 150,000 × g to pellet light membranes. The resulting supernatant containing cytosolic fraction was collected. The mitochondrial pellet was washed with mitochondrial buffer twice and resuspended in 1% NP-40 lysis buffer and rocked for 60 min, then centrifuged at 17,530 × g for 10 min at 4 °C. The resulting supernatant consists of mitochondrial proteins. For nuclear fractionation, the nuclear pellet collected in the first step was washed with 1× PBS and suspended in 2 ml of Buffer A (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.03% Nonidet P-40 with protease inhibitor mixture set I), then incubated on ice until more than 95% of cells could be stained by trypan blue, then centrifuged at 500 × g at 4 °C for 5 min. The resulting pellet was washed with Buffer B (50 mM NaCl, 10 mM Hepes, pH 8.0, 25% glycerol, 0.1 mM EDTA, 0.5 mM spermidine, 0.15 mM spermine) and then resuspended in 150 μl of Buffer C (350 mM NaCl, 10 mM Hepes, pH 8.0, 25% glycerol, 0.1 mM EDTA, 0.5 mM spermidine, 0.15 mM spermine) and rocked at 4 °C for 30 min. After centrifugation at 14, 000 × g at 4 °C, the supernatant (nuclear fraction) was collected (31).
Sulforhodamine B (SRB) assay
Cells were seeded at 6 × 103 - 8 × 103 per well in 96-well plates and allowed to grow overnight. Cells were treated with rapamycin or other agent(s) for 48h. The surviving cell fraction was determined using the sulforhodamine B (SRB) assay as described (32, 33). The percentage of growth inhibition was calculated by using the equation: % growth inhibition = (1 - At / Ac) × 100, where At and Ac represent the absorbance in treated and control cultures, respectively, as described previously (9).
Colony formation assay
A549-P and A549-RR cells were trypsinized (single-cell suspension) and plated in 6-well-plate (1000 cells/well), then treated with rapamycin (10nM). Every 3 days, the medium was replaced with fresh medium containing the rapamycin. After 10 days of treatment, the medium was removed and cell colonies were stained with crystal violet (0.1% in 20% methanol). Pictures were taken using a digital camera to record the results prior to colony counting as described previously (6).
Lung cancer xenografts and treatments
Animal experiments were approved by the Institutional Animal Care and Use Committee of Emory University. Six-week-old Nu/Nu nude mice were purchased from Harlan and housed under pathogen-free conditions in microisolator cages. Xenografts were raised by injecting 5 × 106 of H460 cells or H460 cells expressing Akt shRNA in a balanced salt solution into subcutaneous tissue at the flank region of nude mice. The tumors were allowed to grow to an average volume of 250 mm3 prior to initiation of therapy as described (34). The mice bearing H460 cells or H460 Akt shRNA were randomized into seven various treatment groups (n = 8 per group) as follows: (1) vehicle control (0.5% DMSO, 100 μl/d, i.p.); (2) PD98059 (5mg/kg/d, i.p.), (3) xenografts expressing Akt shRNA; (4) rapamycin (1mg/kg/d, i.p.); (5) PD98059 (5mg/kg/d, i.p.) + rapamycin (1mg/kg/d, i.p.); (6) xenografts expressing Akt shRNA + rapamycin (1mg/kg/d, i.p.); (7) xenografts expressing Akt shRNA + PD98059 (5mg/kg/d, i.p.) + rapamycin (1mg/kg/d, i.p.). Tumor volume (V) was measured by caliper measurements once every two days and calculated with the formula: V=LxW2/2 (L: length; W: width) as described (35). After 14 consecutive days of treatment, all mice were sacrificed by inhaled CO2. The tumors were then removed, weighed, and fixed with formalin for immunohistochemistry.
Immunohistochemistry (IHC) analysis
Harvested tumors were embedded in paraffin and cut into 4-μm sections. Staining was performed using the R.T.U. Vectastain kit following the manufacturer's standard protocol (Vector Laboratories, Burlingame, CA). Tissue sections were incubated with p-Bad (S136), p-Bad (S112) or p-Bad (S155) antibody (dilution 1:50), respectively, overnight at 4°C. The slides were stained with 3,3’-diaminobenzidine (DAB) (Vector Laboratories) and counterstained with hematoxylin (Vector Laboratories), dehydrated, treated with xylene, and mounted. All slides were then examined and representative pictures were taken using an Olympus BX41 microscope (Olympus America, Melville, NY). The semiquantitative evaluation of IHC staining of pBad was performed using immunoscore based on both percentage of stained cells and staining intensity as described (36, 37). The intensity score for p-Bad detection was defined as follows: 0, no appreciable staining; 1, weak intensity; 2, moderate intensity; 3, strong intensity; 4, very strong intensity. The fraction score was based on the proportion of positively staining cells (0-100%). The proportion of cells staining at each intensity was multiplied by the corresponding intensity value and these products were added to obtain an immunoscore ranging from 0 to 400. The mean of the immunoscores was obtained from ten microscopic high power fields.
Statistical analysis
The statistical significance of differences between two groups was analyzed with two-sided unpaired student's t-test. Results were considered to be statistically significant at P < 0.05. Statistical analysis was performed with Graphpad InStat 3 software (San Diego, CA) (10).
Results
Rapamycin induces Bad phosphorylation at S136 and S112 but not S155 via activation of Akt and ERK
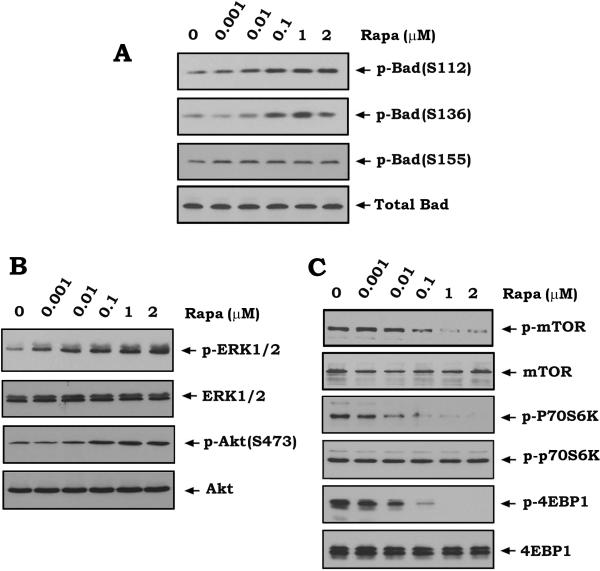
Previous findings reveal that inhibition of mTOR by rapamycin can activate Akt and ERK1/2 (6, 9-11). Because ERK1 and ERK2 are physiological S112 Bad kinases while Akt is an S136 Bad kinase (38-40), it is possible that rapamycin may stimulate Bad phosphorylation via activation of Akt and ERKs. To test this, human lung cancer H460 cells were treated with increasing concentrations of rapamycin for 45 min. Phosphorylation of Bad was analyzed by Western blot using phospho-specific Bad antibodies. Results reveal that inhibition of mTOR by rapamycin results in increased Bad phosphorylation at S112 and S136 but not S155 in association with activation of ERK1/2 and Akt (Fig.1). As expected, decreased phosphorylation of mTOR, p70S6K (the 70-kDa ribosomal S6 kinase) or 4EBP1 (the eukaryotic translation initiation factor 4E–binding protein 1) was observed following rapamycin treatment (Fig.1C), indicating that mTOR kinase activity was inhibited (41). It has already been established that phosphorylation of Bad at S112 or S136 inactivates its proapoptotic function (38). Our findings thus suggest that rapamycin-induced Bad phosphorylation may lead to the loss of death-promoting activity of Bad and thereby contribute to the resistance of human lung cancer cells to rapamycin. Similar results were also obtained in H157 cells (Fig. S1). This confirms that rapamycin-induced Bad phosphorylation is not limited to a specific cell type.
Figure 1. Inhibition of mTOR by rapamycin induces Bad phosphorylation in association with activation of Akt and ERK1/2.
(A, B and C) H460 cells were treated with increasing concentrations of rapamycin (Rapa) for 45 min. Phosphorylation of Bad at S112, S136 or S155 was analyzed by Western blot using the phospho-specific S112, S136 or S155 Bad antibodies (A). Phosphorylation of ERK1/2, Akt, mTOR, p70S6K or 4EBP1 was also analyzed by Western blot (B, C).
Rapamycin resistance is associated with increased Bad phosphorylation
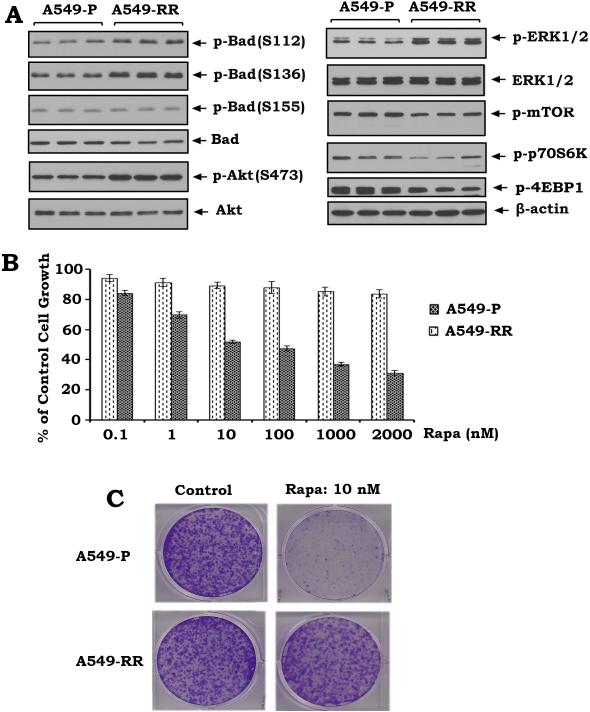
To further demonstrate whether rapamycin resistance involves Bad phosphorylation, rapamycin-sensitive (i.e. A549 parental: A549-P) and rapamycin-resistant lung cancer cells (i.e. A549-RR) were generated as described previously (6). Phosphorylation levels of Bad at S112, S136 and S155 were compared in A549-P and A549-RR cells. Intriguingly, higher levels of Bad phosphorylation at S112 and S136 but not S155 in association with increased activities of ERK1/2 and Akt were observed in A549-RR cells as compared to A549-P cells (Fig. 2A). Importantly, sulforhodamine B (SRB) colorimetric and colony formation assays reveal that A549-P is sensitive but A549-RR is insensitive to rapamycin (Fig. 2BC). These results provide strong evidence that rapamycin-induced Bad phosphorylation at S112 and S136 contributes to rapamycin resistance in human lung cancer cells. To test whether the nonphosphorylatable mutations of Bad at S112 and S136 sites (i.e. S→ A) are sufficient to reverse rapamycin resistance, the non-phosphorylatable S112A/S136A (AA) mutant murine Bad as well as the WT murine Bad were transfected into A549 parental (A549-P) and rapamycin-resistant A549 cells (A549-RR). After transfection, cells were treated with rapamycin (100nM) for 48h. Results reveal that expression of the AA mutant Bad but not WT-Bad reverses rapamycin resistance of A549-RR cells (Fig. S2AB). Previous reports have demonstrated that murine Bad and human Bad have conserved structural homology and function (39, 42). However, the number of amino acids (aa) in murine Bad and human Bad are different (210 aa vs. 168 aa) (43, 44). Human Bad lacks a stretch of 42 amino acids as previously reported (44). This is consistent with our findings that the molecular weight of exogenous murine Bad is larger than the endogenous human Bad (Fig. S2A).
Figure 2. Enhanced Bad phosphorylation is associated with rapamycin resistance in human lung cancer cells.
(A) Phosphorylation of Bad at S112, S136 or S155 and phosphorylation of Akt, ERK1/2, mTOR, p70S6K and 4EBP1 in A549 parental (A549-P) and rapamycin-resistant A549 (A549-RR) cells were analyzed by Western blot. (B) A549-P and A549-RR cells were treated with increasing concentrations of rapamycin for 48h. Cell growth was analyzed by SRB assay. Error bars represent ± S.D. (C) A549-P and A549-RR cells were treated with rapamycin (10 nM) and colony formation assay was performed as described in “Methods”.
Treatment of lung cancer cells with rapamycin results in Bad accumulation in the cytosol, increased Bad/14-3-3 association and decreased Bad/Bcl-XL binding
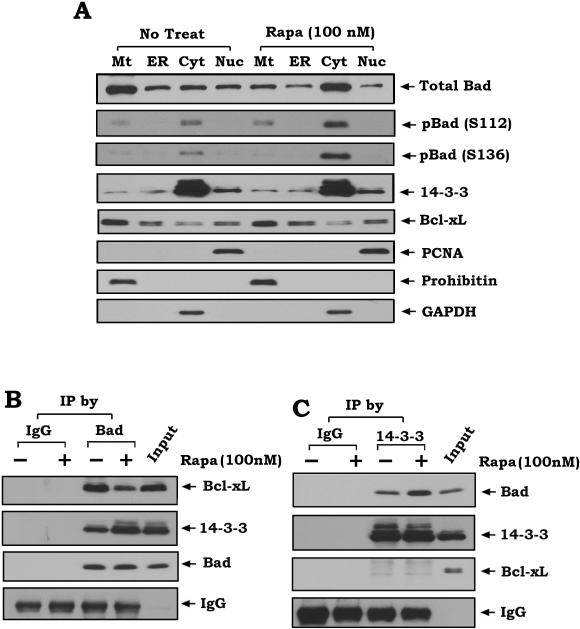
Our results indicate that rapamycin induces phosphorylation of Bad at S112 and S136 (Fig.1A). Phosphorylation has been reported to promote Bad translocation from mitochondria into cytosol, interaction with the scaffold protein 14-3-3 and dissociation from Bcl-XL (18, 21). To test whether rapamycin-stimulated Bad phosphorylation affects its subcellular localization and interactions with 14-3-3 or Bcl-XL, human lung cancer H460 cells were treated with rapamycin (100 nM) for 45 min, and subcellular distributions of total Bad, pBad, 14-3-3 and Bcl-XL were examined by subcellular fractionation analysis as previously described (45). After treatment with rapamycin, Bad was translocated from mitochondria into the cytosol (Fig.3A). Since rapamycin increases the phosphorylated forms of Bad in the cytosol and only the phosphorylated Bad could be observed in the cytosolic fraction (Fig.3A), this indicates that rapamycin-mediated sequestration of Bad from mitochondria may occur through its phosphorylation. By contrast, rapamycin has no significant effect on the subcellular localization of 14-3-3 or Bcl-XL (Fig.3A). To determine the purity of the subcellular fractions obtained, fraction-specific proteins were assessed by probing the same filters. Prohibitin, an exclusively mitochondrial protein (46), was detected only in the mitochondrial fraction (Mt) whereas proliferating cell nuclear antigen (PCNA), a nuclear marker (47), was detected exclusively in the nuclear fraction (Nuc), and GAPDH, the cytosol marker (48), was only observed in the cytosolic fraction (Fig.3A). This determination further confirmed the purity of these subcellular fractions without artifactual cross-contamination. In addition to accumulation of Bad in the cytosol, rapamycin also enhances Bad/14-3-3 interaction in association with decreased Bad/Bcl-XL binding (Fig.3BC). These findings indicate that rapamycin-induced Bad phosphorylation results in sequestering Bad from the mitochondria and functionally blocking its proapoptotic function. Decreased Bad/Bcl-XL binding induced by rapamycin can render Bad less able to suppress the antiapoptotic function of Bcl-XL.
Figure 3. Rapamycin-induced phosphorylation facilitates Bad translocation from mitochondria into the cytosol leading to interaction with 14-3-3 and dissociation from Bcl-XL.
(A) H460 cells were treated with rapamycin (100nM) for 45 min. Subcellular fraction was performed to isolate mitochondria (Mt), light membrane (ER), cytosol (Cyt) and nuclear (Nuc) fractions. Total Bad, phospho-Bad, 14-3-3 and Bcl-XL were analyzed by Western blot. Prohibitin, GAPDH and PCNA were used as purity control for various fractions. (B and C), H460 cells were treated with rapamycin (100nM) for 45 min. Co-immunoprecipitation (co-IP) experiments were performed using Bad (B) or 14-3-3 (C) antibodies. Bad-associated Bcl-XL, 14-3-3, total Bad, 14-3-3-associated Bad or total 14-3-3 were analyzed by Western blot. Normal rabbit IgG was used for negative control.
Rapamycin promotes Bad ubiquitination and degradation
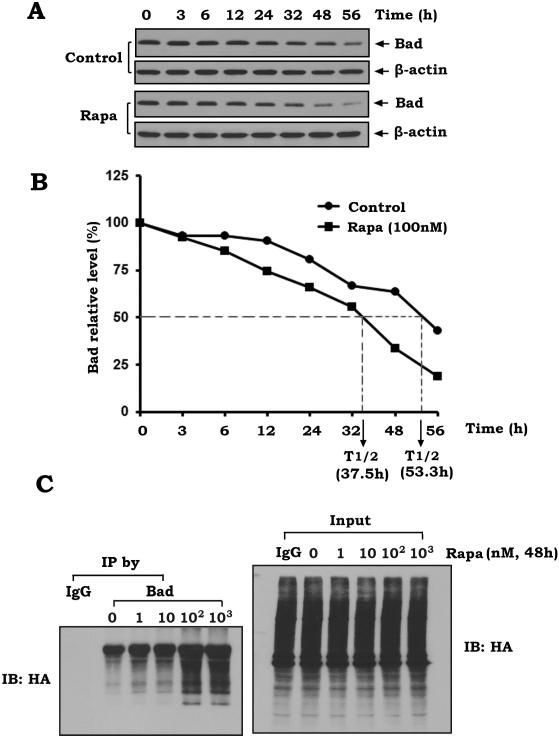
Phosphorylation has been demonstrated to regulate ubiquitination and degradation of the Bcl2 family proteins (49-51). To test whether rapamycin-induced Bad phosphorylation affects its stability in human lung cancer cells, the half-life of Bad was measured using cycloheximide blocking methods as described (52). H460 cells were treated with 100 μg/ml cycloheximide in the absence or presence of rapamycin (100nM) for various times as indicated. Levels of Bad were analyzed by Western blot and further quantified by the ImageJ software (National Institutes of Health, Bethesda, MD) for calculating the half-life as described (53). Results reveal that rapamycin significantly reduces the half-life of Bad from 53.3 h to 37.5 h (Fig. 4AB), indicating that rapamycin-induced Bad phosphorylation may promote Bad degradation. To further uncover the mechanism by which rapamycin reduces Bad stability, ubiquitination was measured following rapamycin treatment as described (27, 28). First, the HA-tagged ubiquitin expression plasmid was transfected into H460 cells. After 24h, cells were treated with increasing concentrations of rapamycin from 1nM to 1μM for 48h. A co-immunoprecipitation (co-IP) was performed using a Bad antibody. Bad ubiquitination was analyzed by Western blot using anti-HA antibody. Results reveal that rapamycin induces a dose-dependent ubiquitination of Bad, which is characterized as the typical higher molecular weight smear of the polyubiquitin chains on Bad protein (Fig. 4C, left panel). Total cell lysate was used as input control before co-IP (Fig. 4C, right panel). These findings suggest that rapamycin-induced reduction in the half-life of Bad may occur through its ubiquitination and degradation.
Figure 4. Rapamycin reduces the half-life of Bad and increases its ubiquitination in human lung cancer cells.
(A and B) H460 cells were treated with 100 μg/ml cycloheximide 5 min prior to starting the indicated time course evaluation (i.e. 0h, 3h, 6h, 12h, 24h, 32h, 48h, 56h) in the absence or presence of rapamycin (100nM). Bad was analyzed by Western blot (A). Western blot bands of Bad were further quantified by the ImageJ software for calculating the half-life of Bad (B). (C) H460 cells were transfected with HA-tagged ubiquitin constructs using NanoJuice transfection Kit. After 24h, cells were treated with increasing concentrations of rapamycin from 1nM to 1μM for 48h. A co-IP was performed using a Bad antibody. Bad ubiquitination was analyzed by Western blot using anti-HA antibody. 50μg of total lysate was used as input control.
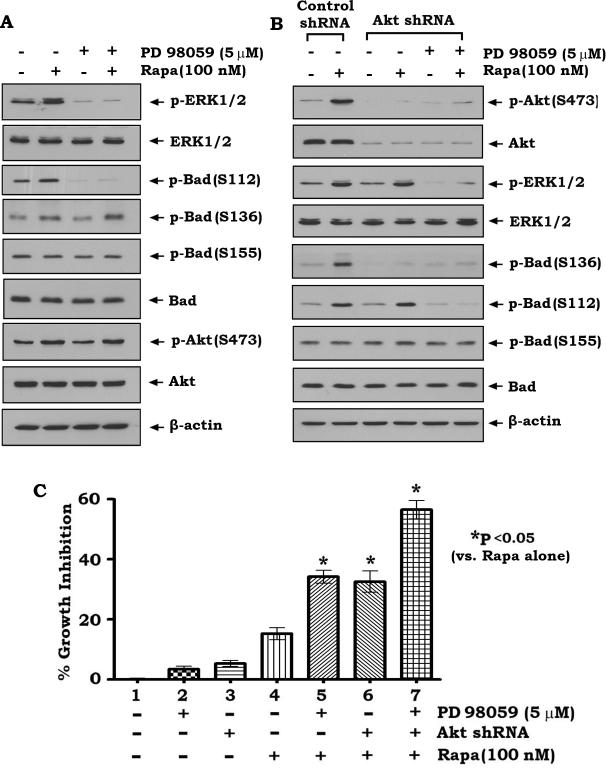
Inhibition of rapamycin-induced Bad phosphorylation by PD98059 or depletion of AKT sensitizes lung cancer cells to rapamycin
Our findings suggest that rapamycin-induced Bad phosphorylation may inactivate its proapoptotic function (Figs. 1, 3 and 4). Inhibition of rapamycin-induced Bad phosphorylation may restore the proapoptotic activity of Bad and sensitize lung cancer cells to rapamycin. To test this hypothesis, H460 parental cells, H460 cells expressing Akt shRNA or control shRNA were treated with rapamycin (Fig. S3) in the absence or presence of PD98059 (Fig. S3). Results reveal that inhibition of MAPK ERK1/2 by PD98059 specifically blocks rapamycin-induced S112 site phosphorylation of Bad but has no significant effect on Bad phosphorylation at S136 or S155 (Fig. 5A). By contrast, depletion of AKT by RNA interference using Akt shRNA specifically blocks rapamycin-induced S136 site phosphorylation and has no effect on Bad phosphorylation at S112 or S155 (Figure 5B). Intriguingly, simultaneous shutdown of MAPK/ERK1/2 and Akt by PD98059 and Akt shRNA blocks rapamycin-stimulated phosphorylation of Bad at both S112 and S136 sites (Fig. 5B), which additively enhances rapamycin-induced growth inhibition of human lung cancer cells (Fig. 5C). To test whether ERK and Akt inhibition is effective to reverse rapamycin resistance, A549-P and A549-RR cells were treated with PD98059 and/or transfected with Akt shRNA in the presence or absence of rapamycin for 48h. Results show that simultaneous inhibition of ERK and Akt not only significantly sensitizes A549-P cells to rapamycin but also reverses rapamycin resistance of A549-RR cells, suggesting that inhibition of Bad phosphorylation at S112 and S136 by blocking ERK and Akt signal pathways can reverse rapamycin resistance (Fig. S2C).
Figure 5. Treatment of cells with PD98059 or depletion of Akt by RNAi blocks rapamycin-induced Bad phosphorylation at S112 or S136 and sensitizes lung cancer cells to rapamycin.
(A) H460 cells were treated with rapamycin in the absence or presence of PD98059 for 45 min. Phosphorylation of Bad at S112, S136 or S155 and phosphorylation of ERK1/2 and Akt were analyzed by Western blot. (B) H460 cells expressing Akt shRNA or control shRNA were treated with rapamycin in the absence or presence of PD98059. Phosphorylation of Bad at S112, S136 or S155, phosphorylation of ERK1/2 and Akt and total Bad, Akt and ERK1/2 were analyzed by Western blot. (C) H460 cells expressing control shRNA or Akt shRNA were treated with rapamycin in the absence or presence of PD98059 for 48h. Cell growth inhibition was analyzed as described in “Methods”. Error bars represent ± S.D.
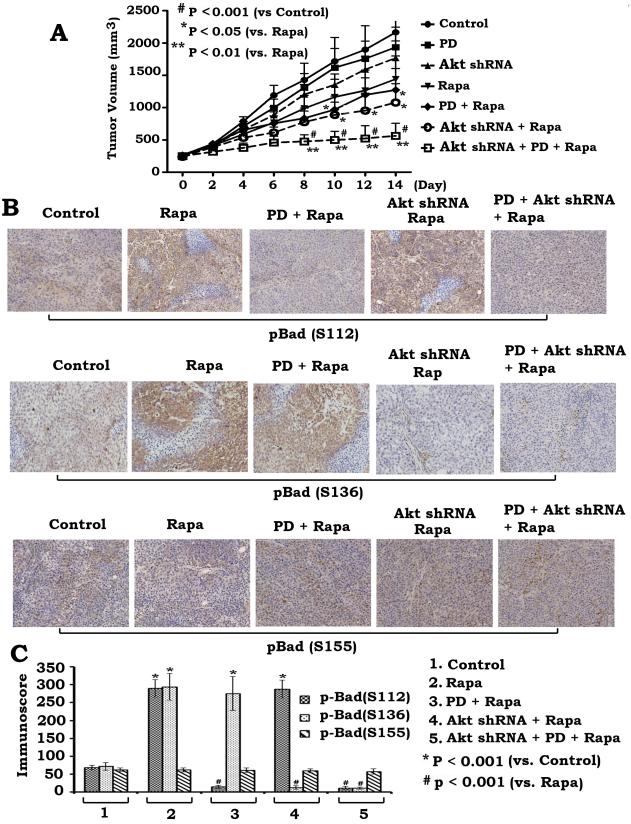
Suppression of rapamycin-induced Bad phosphorylation by PD98059 or depletion of Akt enhances anti-tumor efficacy of rapamycin in lung cancer xenografts
To further test whether blockage of rapamycin-enhanced Bad phosphorylation increases rapamycin's anti-tumor efficacy in vivo, we generated lung cancer xenografts using H460 cells or H460 cells expressing Akt shRNA. Xenograft mice were randomly grouped and treated with rapamycin or PD98059 or the combination for two weeks as described in “Materials and Methods”. Results indicate that either treatment with PD98059 or silencing of Akt using shRNA in lung cancer xenografts significantly enhances the anti-tumor efficacy of rapamycin in association with inhibition of Bad phosphorylation at S112 or S136 in tumor tissues (Fig. 6). Importantly, PD98059 plus Akt shRNA block rapamycin-stimulated Bad phosphorylation at both S112 and S136 sites in tumors (Fig. 6BC), and more efficiently represses lung tumor growth than either PD98059 or Akt shRNA alone (Fig. 6A). Consistent with in vitro results, PD98059 and Akt shRNA have no significant effect on S155 site phosphorylation of Bad in vivo (Fig. 6BC). These findings suggest that blockage of rapamycin-induced Bad phosphorylation at both S112 and S136 sites may not only sensitize cancer cells to rapamycin but also can overcome rapamycin resistance leading to increased anti-tumor activity in vivo. To evaluate the role of apoptosis in tumor growth, a TUNEL assay was employed for measuring apoptosis in tumor tissues using a “Tumor TACS™ In Situ Apoptosis Detection Kit” (Trevigen, Inc). Results reveal that inhibition of Bad phosphorylation by PD98059 and Akt shRNA significantly enhances apoptosis in tumor tissues (Fig. S4).
Figure 6. Inhibition of rapamycin-induced Bad phosphorylation by PD98059 or depletion of Akt enhances anti-tumor efficacy of rapamycin in vivo.
(A) Seven groups of Nu/Nu nude mice with H460 or Akt shRNA H460 xenografts were treated as indicated. Each group includes 8 mice. After 14 days, the mice were sacrificed and the tumors were removed. Tumor sizes were measured once every 2 days. (B and C), Phosphorylation of Bad at S112, S136 or S155 in tumor tissues from various groups was detected with immunohistochemistry using phospho-specific Bad antibodies (B) and quantified by analyzing immunoscore as described in “Methods” (C). Error bars represent ± S.D.
Discussion
Lung cancer, a major cigarette smoke-related cancer, is the primary cause of cancer-related mortality in the United States, accounting for more deaths than breast, prostate and pancreatic cancer combined (54). mTOR inhibitors, such as rapamycin (Fig.S4) and everolimus, have been evaluated as lung cancer therapeutics but with limited success (1). Previous reports indicate that rapalog-activated Akt and MAPK ERK1/2 may contribute to the development of resistance to these agents (6, 9, 10). However, the downstream mediators of the survival consequence of rapamycin-activated AKT and ERK1/2 remain unclear. Here we discovered that rapamycin, in addition to mTOR inhibition, potently stimulates Bad phosphorylation at S112 and S136 sites via activation of AKT and MAPK ERK1/2 (Figs. 1 and 5), which can lead to rapamycin resistance since increased levels of Bad phosphorylation were observed in rapamycin-resistant cells (Fig. 2). Intriguingly, either blockage of Bad phosphorylation at S112 and S136 sites or expression of the non-phosphorylatable Bad mutant (S112A/S136A) can reverse rapamycin resistance (Fig. S2), suggesting that manipulation of Bad phosphorylation at these two sites should be an effective approach for overcoming rapamycin resistance. Because PKA is the physiological S155 Bad kinase (55) and a previous study has demonstrated that rapamycin does not influence the activity of PKA (56), this helps explain why rapamycin has no effect on S155 Bad phosphorylation in vitro and in vivo (Figs. 1, 5 and 6).
It is known that phosphorylation of Bad at one or more sites (S112, S136 or S155) can inactivate the proapoptotic function of Bad (18, 19, 39). Therefore, we expect that rapamycin-induced Bad phosphorylation at S112 or S136 will abolish the death-promoting activity of Bad. In support of this, rapamycin facilitates Bad translocation from mitochondria into the cytosol, and promotes Bad interaction with 14-3-3 and its dissociation from Bcl-XL (Fig. 3). The overall outcome of this series of effects results in the inability of Bad to overcome the antiapoptotic function of Bcl-XL in the mitochondria. Additionally, treatment of lung cancer cells with rapamycin promotes Bad ubiquitination and degradation, leading to a reduced half-life (Fig. 4) and ultimately a loss of function.
Because Bad is a potent BH3-only proapoptotic protein that is ubiquitously expressed in both SCLC and NSCLC cells (38), blocking rapamycin-induced Bad phosphorylation may represent a novel therapeutic strategy for improving the anti-tumor efficacy of rapamycin. Although rapamycin can induce Bad phosphorylation at two sites (i.e. S112 and S136; Fig. 1A), PD98059 blocks Bad phosphorylation only at the S112 site while depletion of Akt blocks Bad phosphorylation only at the S136 site in either lung cancer cells or in lung tumor tissues (Figs. 5 and 6). These findings provide strong evidence that rapamycin-induced S112 site phosphorylation occurs through the MEK/ERK1/2 signaling pathway while rapamycin-induced S136 site phosphorylation occurs through the Akt pathway (Figs. 1, 5 and 6). Abrogation of rapamycin-stimulated phosphorylation of Bad at S112 and S136 led to increased growth inhibition of lung cancer cells in vitro and synergistic enhancement of rapamycin activity against lung cancer tumor xenografts in vivo (Figures. 5 and 6).
In summary, our studies identify a novel rapamycin survival signal transduction pathway that depends on phosphorylation of Bad at S112 and S136 but not S155 through activation of MAPKs ERK1/2 and Akt. Rapamycin-induced double-site phosphorylation results in translocation of Bad from the mitochondria, sequestration in the cytosol where it interacts with 14-3-3, dissociation from Bcl-XL in mitochondria and reduced stability via ubiquitination, which leads to loss of the apoptotic function of Bad and rapamycin resistance. Our findings have established Bad as a new signaling target of rapamycin in human lung cancer cells. Therefore, activation of Bad by reducing or blocking its phosphorylation may represent a new therapeutic strategy to overcome resistance to mTOR inhibition in patients with lung cancer.
Supplementary Material
Acknowledgments
This work was supported by a Scholarship Award from the China Scholarship Council (CSC) (YL), by Flight Attendant Medical Research Institute Clinical Innovator Awards (XD), by NCI, National Institutes of Health Grants R01CA112183 (XD), R01CA136534 (XD), R01CA118450 (SYS) and NIH lung cancer P01 CA116676 (FRK and SYS), and by a Kennedy Seed Grant from the Winship Cancer Institute (XD).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011 doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caron E, Ghosh S, Matsuoka Y, Ashton-Beaucage D, Therrien M, Lemieux S, et al. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 4.Legrier ME, Yang CP, Yan HG, Lopez-Barcons L, Keller SM, Perez-Soler R, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–8. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BE, Jackman D, Janne PA. Rationale for a phase I trial of erlotinib and the mammalian target of rapamycin inhibitor everolimus (RAD001) for patients with relapsed non small cell lung cancer. Clin Cancer Res. 2007;13:s4628–31. doi: 10.1158/1078-0432.CCR-07-0717. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–18. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wangpaichitr M, Wu C, You M, Kuo MT, Feun L, Lampidis T, et al. Inhibition of mTOR restores cisplatin sensitivity through down-regulation of growth and anti-apoptotic proteins. Eur J Pharmacol. 2008;591:124–7. doi: 10.1016/j.ejphar.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831–7. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 9.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, et al. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther. 2008;7:1952–8. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–30. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 13.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci U S A. 2005;102:17975–80. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 16.Ghiotto F, Fais F, Bruno S. BH3-only proteins: the death-puppeteer's wires. Cytometry A. 2010;77:11–21. doi: 10.1002/cyto.a.20819. [DOI] [PubMed] [Google Scholar]
- 17.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–42. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 18.Hirai I, Wang HG. Survival-factor-induced phosphorylation of Bad results in its dissociation from Bcl-x(L) but not Bcl-2. Biochem J. 2001;359:345–52. doi: 10.1042/0264-6021:3590345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275:25865–9. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann A. Survival signaling goes BAD. Dev Cell. 2002;3:607–8. doi: 10.1016/s1534-5807(02)00328-3. [DOI] [PubMed] [Google Scholar]
- 21.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 22.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell. 1996;87:619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 23.Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–13. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 24.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–94. [PubMed] [Google Scholar]
- 26.Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem. 1997;272:11671–3. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Liu X, Yue P, Benbrook DM, Berlin KD, Khuri FR, et al. Involvement of c-FLIP and survivin down-regulation in flexible heteroarotinoid-induced apoptosis and enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol Cancer Ther. 2008;7:3556–65. doi: 10.1158/1535-7163.MCT-08-0648. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein down-regulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–9. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- 29.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–19. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 30.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–23. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 31.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 32.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–9. [PubMed] [Google Scholar]
- 33.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 34.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 35.Liu AW, Cai J, Zhao XL, Jiang TH, He TF, Fu HQ, et al. ShRNA-targeted MAP4K4 inhibits hepatocellular carcinoma growth. Clin Cancer Res. 2011;17:710–20. doi: 10.1158/1078-0432.CCR-10-0331. [DOI] [PubMed] [Google Scholar]
- 36.Jonat W, Maass H, Stegner HE. Immunohistochemical measurement of estrogen receptors in breast cancer tissue samples. Cancer Res. 1986;46:4296s–8s. [PubMed] [Google Scholar]
- 37.Morote J, Fernandez S, Alana L, Iglesias C, Planas J, Reventos J, et al. PTOV1 expression predicts prostate cancer in men with isolated high-grade prostatic intraepithelial neoplasia in needle biopsy. Clin Cancer Res. 2008;14:2617–22. doi: 10.1158/1078-0432.CCR-07-4987. [DOI] [PubMed] [Google Scholar]
- 38.Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–44. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 39.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 40.Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63:8330–7. [PubMed] [Google Scholar]
- 41.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 42.Jeong SY, Gaume B, Lee YJ, Hsu YT, Ryu SW, Yoon SH, et al. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 2004;23:2146–55. doi: 10.1038/sj.emboj.7600225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taghiyev AF, Guseva NV, Harada H, Knudson CM, Rokhlin OW, Cohen MB. Overexpression of BAD potentiates sensitivity to tumor necrosis factor-related apoptosis-inducing ligand treatment in the prostatic carcinoma cell line LNCaP. Mol Cancer Res. 2003;1:500–7. [PubMed] [Google Scholar]
- 44.Ottilie S, Diaz JL, Horne W, Chang J, Wang Y, Wilson G, et al. Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins. J Biol Chem. 1997;272:30866–72. doi: 10.1074/jbc.272.49.30866. [DOI] [PubMed] [Google Scholar]
- 45.Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci U S A. 2000;97:1578–83. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikonen E, Fiedler K, Parton RG, Simons K. Prohibitin, an antiproliferative protein, is localized to mitochondria. FEBS Lett. 1995;358:273–7. doi: 10.1016/0014-5793(94)01444-6. [DOI] [PubMed] [Google Scholar]
- 47.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–90. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 48.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci U S A. 2000;97:3850–5. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–44. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer. 2011 doi: 10.1002/cncr.26011. [DOI] [PubMed] [Google Scholar]
- 54.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 55.Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. 2000;275:25046–51. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
- 56.de Groot RP, Ballou LM, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.