Abstract
Foraging experience is correlated with structural plasticity of the mushroom bodies of the honey bee brain. While several neurotransmitter and intracellular signaling pathways have been previously implicated as mediators of these structural changes, none interact directly with the cytoskeleton, the ultimate effector of changes in neuronal morphology. The Rho family of GTPases are small, monomeric G proteins that, when activated, initiate a signaling cascade that reorganizes the neuronal cytoskeleton. In this study, we measured activity of two members of the Rho family of GTPases, Rac and RhoA, in the mushroom bodies of bees with different durations of foraging experience. A transient increase in Rac activity coupled with a transient decrease in RhoA activity was found in honey bees with 4 days foraging experience compared with same-aged new foragers. These observations are in accord with previous reports based on studies of other species of a growth supporting role for Rac and a growth opposing role for RhoA. This is the first report of Rho GTPase activation in the honey bee brain.
Keywords: Apis mellifera, mushroom bodies, neuroethology, Rac, RhoA
1. Introduction
The honey bee, Apis mellifera, provides a powerful insect model for the study of experience-dependent brain plasticity (Fahrbach and Dobrin, 2009; Giurfa, 2007; Groh and Meinertzhagen, 2010). Studies of neural plasticity in the honey bee have focused on neuropils associated with the mushroom bodies and the antennal lobe. Typical occurrences in the daily lives of honey bees such as foraging or exposure to a queen lead to measureable changes in the structure of the brain.
The first 3 weeks of a worker honey bee’s adult life are spent performing tasks inside the hive (Winston, 1987). Workers then transition to foraging. As foragers, worker honey bees take multiple flights from the hive each day in search of resources: primarily pollen and nectar, but also water and propolis. The neuropils associated with the mushroom bodies are significantly larger in more experienced foragers than in less experienced foragers (Durst et al., 1994; Withers et al., 1993). Foraging experience has also been linked to changes in mushroom body dendritic spine morphology and to changes in the number and volume of areas of synaptic contact in the calyces of the mushroom bodies called microglomeruli (Coss et al., 1980; Krofczik et al., 2008). Studies of precocious foragers indicated that the changes in the mushroom body neuropils reflect primarily foraging experience rather than age (Farris et al., 2001; Withers et al., 1993).
Signaling via neurotransmitter receptors has been linked to changes in neuron structure in honey bees. Pharmacological activation of muscarinic cholinergic receptors in worker honey bees prevented from foraging resulted in growth of the mushroom body neuropil and increases in dendritic complexity that matched changes observed in honey bees foraging under natural conditions (Dobrin et al., 2011; Ismail et al., 2006). Other small molecule neurotransmitters - glutamate, octopamine, acetylcholine, dopamine, and serotonin - have been demonstrated to modulate associative learning in honey bees, and it is possible and even likely that many transmitters regulate experience-induced structural changes in the honey bee brain (Hammer and Menzel, 1998; Locatelli et al., 2005; Lozano and Gauthier, 1998; Maleszka et al., 2000; Müssig et al., 2010; Wright et al., 2010).
Focused analysis of candidate genes in the honey bee brain has led to the identification of several genes with expression modulated by foraging experience, including insulin/insulin-like signaling (IIS), AmUSP, AmFor, and the putative immediate early gene kakusei (Ament et al., 2008; Ben-Shahar et al., 2002; Kiya et al., 2008; Velarde et al., 2006). If the products of these genes do have a role in the regulation of foraging-dependent structural plasticity, they must be upstream of effector molecules directly interacting with the neuronal cytoskeleton. Many other candidate genes related to brain plasticity have been identified but not investigated. The sequencing of the honey bee genome (Honeybee Genome Sequencing Consortium, 2006) offered the opportunity for large scale studies of associations between brain gene expression and behavioral plasticity. Microarray-based comparisons of differences in abundance of specific mRNAs between nurses and foragers revealed thousands of candidate plasticity genes (Whitfield et al., 2003, 2006), nearly 500 of which are correlated with foraging experience (Lutz et al., 2011).
Rho GTPases are small, monomeric G proteins that regulate cellular morphogenesis through direct interactions with actin and microtubule-organizing proteins (Ponimaskin et al., 2007). Because the cytoskeleton determines dendritic morphology, the regulation of microtubule and actin dynamics is the driver of dendritic structural plasticity. Cycling between the inactive GDP-bound and the active GTP-bound forms is controlled by guanine nucleotide exchange factors, GTPase activating proteins, and guanine nucleotide dissociation inhibitors (Nobes and Hall, 1994). Rac and RhoA, two of the best characterized Rho GTPase family members, have been shown to regulate dendritic reorganization in the insect Drosophila melanogaster, the amphibian Xenopus laevis, and other vertebrates including rodents and humans (Bakal et al., 2007; Lee et al., 2000; Li et al., 2000; Nadif Kasri and Van Aelst, 2008; Nakayama et al., 2000; Newey et al., 2005; Sin et al., 2002; Threadgill et al., 1997). Studies in which constitutively-active or dominant negative forms of the GTPases were expressed in D. melanogaster implicated Rho GTPase proteins in the development of neuron morphology, with Rac supporting and RhoA opposing growth (Genova et al., 2000; Hakeda-Suzuki et al., 2002; Lee et al., 2000; Lundquist, 2003).
Because the functions of these proteins appear to be conserved, the present study tested the prediction that foraging experience alters levels of activated Rac and RhoA in the worker honey bee brain. We used the model of foraging-dependent growth of Kenyon cell dendritic arborizations, hypothesizing that foraging-associated dendritic growth is initiated and/or regulated by Rho family GTPase activity. We predicted that growth-supporting Rac activity would increase in parallel with increasing foraging experience, which is associated with dendritic growth; conversely, growth-opposing RhoA activity would decrease in the mushroom bodies of honey bees with increasing foraging experience. We assayed worker honey bees that had initiated foraging at different ages and at different periods in the summer to examine experience-, age-, and period-based changes in mushroom body Rho GTPase activity.
2. Materials and Methods
2.1 Honey bee collection and experimental design
Honey bees (Apis mellifera) were obtained from research apiaries maintained at Wake Forest University (Forsyth County, NC, USA) using standard commercial techniques. To obtain newly emerged bees, brood comb containing pharate adult workers was removed from multiple field colonies and placed in an incubator (Percival Scientific, Inc., Perry, IA, USA) maintained at 33°C, 35 – 45 % relative humidity. To obtain known-age, known-experience foragers, 6,000 – 8,000 honey bees (< 12 h post-emergence; “focal bees”) were marked individually over the course of two days on the dorsal thorax with a single dot of enamel paint (Testors PLA, Rockford, IL, USA). Marked honey bees were returned to a typical field colony containing a naturally-mated queen, where they were allowed to develop as foragers without further manipulation. Twenty-one days later, when a large number of the marked bees had initiated foraging, the hive entrance was observed for 5 – 7 h. Any focal honey bee observed returning to the hive entrance with a load of pollen or nectar was marked at that time with a second color of paint on the abdomen. These foragers were excluded from the experiment because it is unknown when they initiated foraging. Beginning two days later, 300 – 400 focal foragers (those returning to the hive entrance bearing a single paint dot on the thorax) were painted with a new color on the abdomen (“marked foragers”). These honey bees were allowed to forage under natural conditions in the field for 1, 4, 8, or 12 days before being collected as they returned to the hive entrance from a foraging trip. To collect new foragers for comparison with experienced foragers, the hive entrance was observed for 5 – 7 h the day prior to each collection. Each marked forager with a single paint mark noted (those which initiated foraging 1, 2, or 3 days before) was marked with a second color of paint on the abdomen and excluded from future collections. On the day of collection, bees with a single paint mark (those which initiated foraging that day) were collected as new foragers. This experiment was repeated two times in the summer of 2010, once beginning in May and again in July. A timeline of the studies is provided (Fig. 1).
Fig. 1.
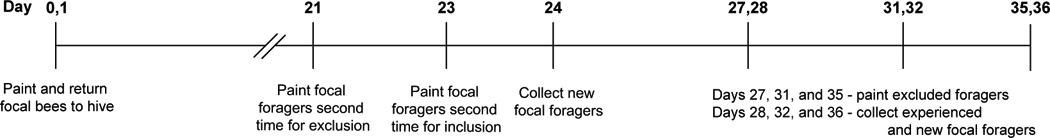
Experimental timeline. Worker honey bees were paint-marked on the dorsal thorax within 12 hours of emergence and returned to a field colony the same day. This was repeated with a second paint color the following day. Beginning 21 days later, the hive entrance was observed and all focal foragers were painted a second time on the abdomen and excluded from the study because the duration of their foraging experience was unknown. Two days later, all focal bees with a single paint mark observed foraging were paint-marked on the abdomen. Collection of foragers for Rho-GTPase assays began the following day and was repeated every 4 days thereafter. On each collection day, focal foragers (those allowed to accumulate 1, 4, 8, or 12 days of natural foraging experience) were captured as they returned to the hive entrance and frozen in liquid nitrogen the field. Additionally, all single-marked foragers observed returning to the hive entrance were painted a second time the day prior to a collection. This meant that, on the day of collection, any focal bee with a single paint mark had less than 24 hours of foraging experience. These workers were collected as age-matched new foragers.
Pollen and nectar foragers were collected as they returned to the hive with full pollen baskets or distended abdomens. To facilitate collections, a wire screen (3 mm spacing) was placed temporarily over the entrance of the hive to prevent bees from entering. Experienced and new marked foragers were collected using forceps and immediately submerged (within seconds of collection) into liquid nitrogen. Immediate freezing was required because inactivation of the Rho family of GTPases can occur extremely rapidly (Murakoshi et al., 2011). Once collections for a day had been completed, the frozen bees were individually transferred to a bed of dry ice and decapitated. The frozen heads (containing the brains) were then immediately transferred to individual prechilled microcentrifuge tubes, placed in a bath of liquid nitrogen, and finally stored at −80°C until preparation of mushroom body lysates. It took approximately 1 – 2 minutes per bee from removing the whole bee from liquid nitrogen to placing the microcentrifuge tube in liquid nitrogen, and approximately 30 – 45 minutes to process all foragers and store the frozen heads at −80°C.
2.2 Group definitions
The experiment was run twice, once beginning in May with forager collections in June (June collection) and again beginning in July with forager collections in August (August collection). Summer in the region of North America where this study was performed can be separated into 3 periods, each associated characteristic honey bee behaviors. Early summer is primarily a period of growth – brood production is paramount, resulting in large strong colonies. Mid-summer is when the colony size and foraging intensity is greatest. In the late summer, the weather remains warm but is typically drier. As a consequence, resource availability decreases and honey bees begin to undergo physiological and behavioral changes in preparation for the winter. The bees collected in June are considered representative of the end of early-summer and those collected in August as representative of the end of mid-summer.
Marked foragers were collected every 4 days beginning at 24 days old. On each collection day, experienced foragers (those with more than one day of foraging experience) and new foragers (those with less than 24 hours of foraging experience) of the same age were collected. When referring to groups within a collection, the age of the bee will be followed by the number of days of foraging experience. For example, 32/1 indicates a 32 day old new forager whereas 32/8 indicates a 32 day old forager with 8 days of foraging experience.
2.3 Mushroom body lysate collection
Whole heads were lyophilized using a VirTis BenchTop 2K freeze dryer (SP Industries, Warminster, PA, USA) for 45 min at 50 mTorr and −100°C condenser temperature. The lyophilized heads were then stored 1 – 3 days at −80°C before dissection. Both mushroom bodies from each brain were dissected in chilled absolute ethanol and placed into chilled microcentrifuge tubes containing 80 µl phosphate buffered saline (PBS; Sigma, St. Louis, MO, USA) with 1% protease inhibitor (Cytoskeleton, Denver, CO, USA). After the tubes were centrifuged for 1 min at 4,400 rpm at 4°C in a 5415 R centrifuge (Eppendorf, Hauppauge, NY, USA), the PBS was removed and replaced with 50 µl lysis buffer (Cytoskeleton) containing 1% protease inhibitor. The cells were then lysed by vigorous trituration with a 200 µl pipette tip for 2 – 3 min. After tubes were centrifuged for 2 min at 13,200 rpm at 4°C, 30 µl of supernatant were removed, transferred to a fresh chilled tube, and immediately frozen in liquid nitrogen. This supernatant constituted the sample on which the GTPase activation assay was run. The original tube containing the remaining supernatant and pellet remained on ice until mushroom body lysates were collected from all heads in a batch (8 – 12 heads/batch). From the start of the dissection to freezing of the tubes containing the samples took approximately 5 – 7 min.
Total protein concentration of the samples was determined by spinning the unfrozen tubes containing the remaining supernatant and pellet at 13,200 rpm and 4°C for 2 min before adding 10 µl of supernatant to 500 µl Precision Red Protein Assay Reagent (Cytoskeleton). The absorbance was read 1 min later at 600 nm on a BioPhotometer (Eppendorf).
2.4 pan-Rac and RhoA activation assays
For measurement of pan-Rac (Rac1, Rac2, and Rac3) and RhoA activity, commercially available pan-Rac and RhoA Activation Assay Biochem kits (Cytoskeleton) were used according to the manufacturer’s instructions and with the buffers supplied by the manufacturer. While designed to recognize activated vertebrate GTPases, these kits were chosen for the study of honey bee GTPase activity because there is 94 and 89% identity between rodent and honey bee Rac1 and RhoA proteins respectively (SD, unpublished) and the kits had been previously used successfully to study Rho GTPases in D. melanogaster neurons (Bakal et al., 2007). In these absorbance-based assays, a membrane containing the binding domain of a Rac or RhoA effector molecule coats the bottom of a 96-well plate; after sample addition, only the activated form of the GTPase will bind and hence be detected in subsequent steps.
To begin the assay, cell lysates were partially thawed in a room temperature water bath for 30 sec. Tubes were then transferred to ice and fresh lysis buffer (containing 1% protease inhibitor) was added to equalize total protein concentration in each tube (0.5 mg/ml) based on the previous protein concentration assays. An equal volume of binding buffer was also added to each tube. After mixing this solution in the tip of a 200 µl pipette, 50 µl of each sample was added to a well of the binding plate. Control wells (on each plate run) were filled with lysis buffer and binding buffer (negative control) or with a non-hydrolyzable form of Rac or RhoA (appropriate to the assay) in lysis buffer and binding buffer (positive control). The plate was then placed on a 400 rpm orbital shaker at 4°C for 30 min before being washed twice with 200 µl of wash buffer. After removal of the wash buffer, 200 µl of antigen-presenting buffer were added to each well at room temperature for 2 min. The wells were then washed three times with 200 µl of wash buffer before incubating with the appropriate primary antibody (Rac: diluted 1:200 in antibody dilution buffer; RhoA: diluted 1:250 in antibody dilution buffer) for 45 min at room temperature. After this incubation, the wells were washed 3 times with 200 µl of wash buffer and then incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Rac: diluted 1:100 in antibody dilution buffer; RhoA: diluted 1:62.5 in antibody dilution buffer) for 45 min at room temperature. Following 3 washes with 200 µl of wash buffer, 50 µl of HRP detection reagent was added to each well followed by 50 µl of HRP detection reagent; pan-Rac assays were allowed to incubate for 20 min at room temperature before addition of the detection reagent and RhoA assays were incubated for 15 min at 37°C. Absorbance at 500 nm was immediately recorded using a 1420 Multilabel Counter microplate reader (Perkin Elmer, San Jose, CA, USA). Higher absorbance readings indicate higher levels of pan-Rac or RhoA activity.
2.5 Statistical analysis
For each sample, the negative control value from the same plate as the sample was subtracted from the absorbance value. Data were grouped appropriately before analysis in Prism 5 (GraphPad Software, La Jolla, CA, USA) utilizing one-way ANOVAs with Newman-Keuls multiple comparisons post-test to assess differences in GTPase activity between same-age foragers of varying experience. Where appropriate, t-tests were used for pairwise comparisons. Unless otherwise indicated, groups from the June and August collections have been pooled.
3. Results
3.1 pan-Rac activity transiently increases with foraging experience
Worker honey bees of known age were permitted to accumulate up to 12 days of foraging experience. Experienced foragers and age-matched new foragers were collected every 4 days. Levels of activated pan-Rac differed significantly across groups of foragers [Fig 2; one-way ANOVA (df = 6, 99), F = 2.828; p = 0.014]. When levels of activated pan-Rac were compared in same-age bees with different amounts of foraging experience, a significant difference was found between 28 day old new foragers and 28 day old foragers with 4 days of foraging experience (Newman-Keuls, p < 0.05; unpaired, two-way t-test, p = 0.0008). By contrast, foragers with 8 or 12 days of foraging experience did not differ from age-matched new foragers in the levels of activated pan-Rac.
Fig. 2.
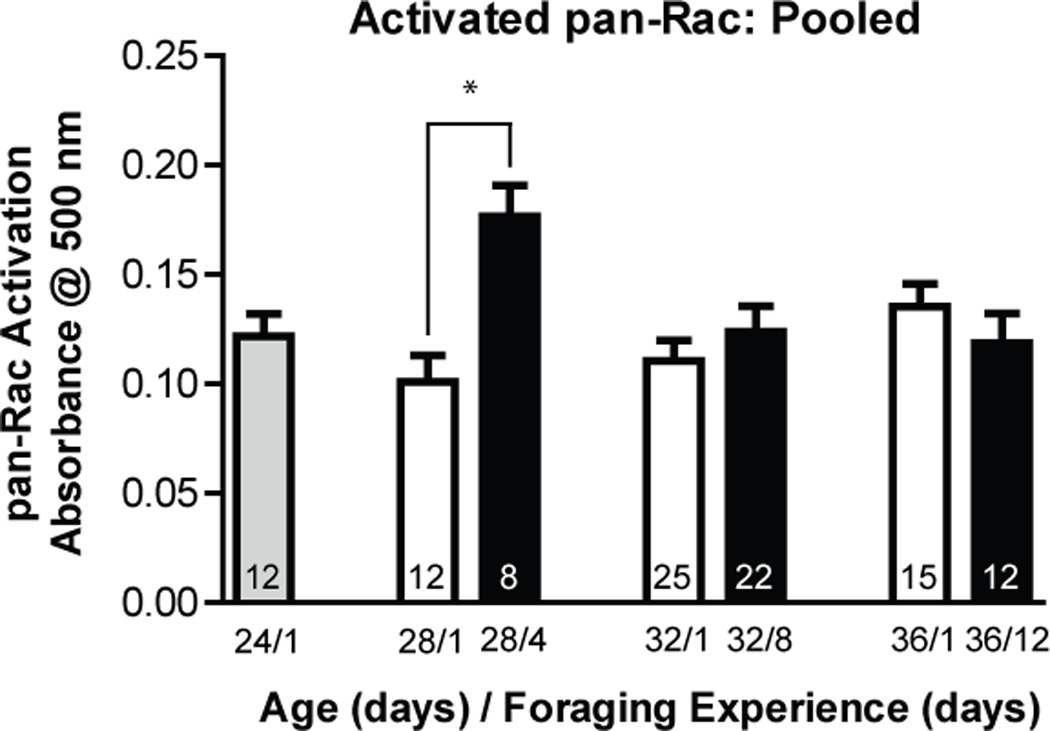
Activated pan-Rac was transiently increased in bees with less than one-week of foraging experience. Foragers of known age that had foraged for different numbers of days were collected and frozen in the field prior to mushroom body isolation and lysate extraction. Statistical analysis of these data used a one-way ANOVA model. Numbers represent the sample size for each group. * indicates statistically significant differences between groups.
Levels of activated pan-Rac were not correlated with age of foraging onset, but instead were transiently raised shortly after the onset of foraging. We examined foragers with one day of foraging experience aged 24, 28, 32, and 36 days and found no difference in pan-Rac expression [one-way ANOVA (df = 3, 60), F = 1.743; p = 0.169]. Comparison of bees with increasing foraging experience revealed a significant difference between the groups [one-way ANOVA (df = 3, 50), F = 3.080; p = 0.036], with 28 day old bees with 4 days of foraging experience having higher levels than all other groups (Newman-Keuls, p < 0.05). These data suggest that a transient increase in mushroom body pan-Rac activity occurred as a result of the first days of foraging. Raised levels were no longer evident in highly experienced foragers.
We compared the effect that the period of the summer had on pan-Rac activation in foraging worker honey bees. The experiment was repeated twice during the summer of 2010, in June and August. Owing to rain on days 28 and 29 of the August collection, no 28 day old bees (new or experienced) were collected. Comparisons of same-age, same experience foragers collected in June versus August revealed no differences in levels of activated pan-Rac in the mushroom bodies (24/1: unpaired, two-way t-test, p = 0.457; 32/1: unpaired, two-way t-test, p = 0.828; 32/8: unpaired, two-way t-test, p = 0.232; 36/1: unpaired, two-way t-test, p = 0.914; 36/12: unpaired, two-way t-test, p = 0.418).
3.2 RhoA activity is regulated with experience, age, and period of summer
On average, bees collected in August had lower levels of activated RhoA than bees collected in June. Because foragers used for the pan-Rac and RhoA analyses were collected simultaneously, no 28 day old bees were available for analysis in the August cohort owing to the same spell of inclement weather. No differences were found between any of the August collected groups [Fig 3B; one-way ANOVA (df = 4, 30), F = 1.068; p = 0.390]. However, a one-way analysis of variance indicated significant differences in the levels of activated RhoA in all groups collected in June [Fig 3A; one-way ANOVA (df = 6, 54), F = 3.699; p = 0.003].
Fig. 3.
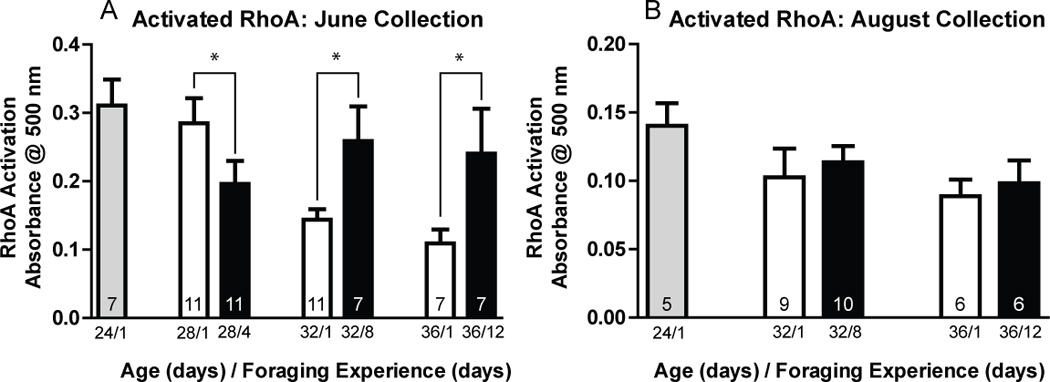
Levels of activated RhoA showed differences in dynamics in different periods of summer. Foragers of known age that had foraged for different numbers of days were collected and frozen in the field prior to mushroom body isolation and lysate extraction. A, Comparison of foragers collected during June 2010. B, Comparison of foragers collected during August 2010. Statistical analysis of these data used a one-way ANOVA model. Numbers represent the samples size for each group. * indicates statistically significant differences between groups.
On the basis of the June collections, it was determined that 28 day old bees with 4 days of foraging experience had significantly lower levels of activated RhoA than same-age new foragers (unpaired, two-way t-test, p = 0.034), while experienced 32 and 36 day foragers had significantly higher levels of activated RhoA than same-age new foragers (32 day: unpaired, two-way t-test, p = 0.019; 36 day: unpaired, two-way t-test, p = 0.027). These data suggest that RhoA activity was reduced transiently during the initial days of foraging (as in 28 day bees with 4 days of foraging experience), but that sustained foraging experience resulted in a return to similar levels of activation of 24 day foragers in the 32 and 36 day experienced foragers. Examination of foragers of increasing age and experience from the June cohort highlighted this transient change in activity correlated with foraging experience. Twenty-eight day old bees that obtained 4 days of foraging experience had significantly lower levels of activated RhoA in their mushroom bodies compared with 24 day old new foragers (unpaired, two-way t-test, p = 0.042).
Age of foraging onset was negatively correlated with RhoA activity in mushroom body in early summer. A statistically significant difference in RhoA activity was found between bees of differing ages collected in June on their first day of foraging [one-way ANOVA (df = 3, 32), F = 10.640; p < 0.0001]. New foragers of all age groups differed from each other, except 24 day olds versus 28 day olds and 32 day olds versus 36 day olds (Newman-Keuls, p < 0.05).
4. Discussion
Here we report two measures demonstrating changes in Rho GTPase activity in the mushroom body of individual honey bees modulated by foraging experience and age. An increase in pan-Rac activity and a decrease in RhoA activity were found in foragers with 4 days of experience compared with bees of the same age collected on their first day of foraging. These changes in activity were transient; the levels of activated Rho GTPases measured in the most experienced foragers were not elevated compared with new foragers. This is the first report of Rho GTPase activity in the honey bee brain.
Foraging experience in honey bees increases the volume of the mushroom body neuropil via increased Kenyon cell complexity. Farris and colleagues (2001) made daily observations outside a hive entrance to record the flight history of individual, number-tagged honey bees. A positive correlation was found between flight experience and mushroom body neuropil volume, suggesting that a gradual expansion of the region results from accumulation of foraging experience. These investigators then went on to show that the mushroom body expansion evident in experienced foragers is associated with increased complexity of Kenyon cell dendritic fields. If, as our data suggest, Rho GTPase signaling mediates foraging-dependent growth, it appears that sustained activation of these pathways is not necessary for continued growth of Kenyon cell dendrites. However, sustained foraging experience is necessary for continued growth; the mushroom body volume of foragers with 1 week of experience which are subsequently caged in the laboratory for an additional week do not differ from the mushroom bodies of 1 week foragers (Ismail et al., 2006). Together with the data presented here, this suggests that an additional factor (or factors) may separately signal persistent foraging and be necessary for the growth found in experienced foragers.
Signaling via Rho GTPases involves numerous effector molecules that function as kinases, transcription factors, and regulators of the cytoskeleton (Ponimaskin et al., 2007). A transient change in Rho GTPase signaling during the first week of foraging can therefore initiate a signaling cascade that leads to continued, gradual mushroom body growth. Most studies of Rho GTPases use constitutively active or dominant negative forms of the proteins, yielding little information on their temporal regulation (Pertz, 2010). Foragers with 3 weeks of foraging experience do not experience mushroom body growth greater than honey bees with 2 weeks experience (SF and G. E. Robinson, unpublished data) and bees with fall flight experience allowed to overwinter also do not experience continued mushroom body growth (Fahrbach et al., 2003); these may reflect the blunting of the signaling cascade initiated by the transient change in activation of Rho GTPases.
Muscarinic signaling has been implicated in foraging-dependent mushroom body plasticity. Honey bees with one week of foraging experience fed the muscarinic acetylcholine receptor agonist pilocarpine for an additional week whilst caged in the laboratory have mushroom body neuropil volumes and Kenyon cell dendritic complexity similar to those of same-age honey bees with 2 weeks of natural foraging experience (Dobrin et al., 2011; Ismail et al., 2006). Can a signaling cascade be envisioned that links muscarinic stimulation to Rho GTPase activation, and ultimately, to reorganization of the cytoskeleton? Like all canonical G-protein coupled receptors, muscarinic receptors undergo a conformational shift upon ligand binding that facilitates release of bound GDP and simultaneous binding of GTP. GTP-binding releases the G protein from the receptor and dissociates the trimer into the α-subunit and βγ complex. The activated α-subunit can then, for example, stimulate PI3-kinase to lead to the formation of PIP3 which activates Tiam, a known Rac activator (Brown et al., 2006; Lanzafame et al., 2003). In addition to an antagonistic interaction between Rac and RhoA (Bustos, et al., 2008), RhoA deactivation can be caused by PI3-kinase/PIP3 signaling leading to the activation of the GTPase activating protein ARAP3, ultimately increasing the rate of endogenous GTPase activity of RhoA (Krugmann et al., 2004). We propose that foraging increases acetylcholine release from synaptic terminals in the mushroom body calyces, which acts on mushroom body muscarinic receptors leading to a transient increase in activation of the growth-supporting Rac protein and a transient decrease in activation of the growth-opposing RhoA protein. These coordinated changes in Rho-GTPase activity initiate a cascade inducing long-term structural plasticity.
Associative learning also results in structural plasticity in specific regions of the honey bee brain. The proboscis extension reflex (PER) is used to study associative learning in the honey bee (Bitterman et al., 1983). Honey bees (and other insects) restrained in small harnesses will learn to extend their proboscis in response to an arbitrary cue, such as an odor or flash of light, after pairing the cue with presentation of a sugar solution. PER training has been linked to increased volume of odor-specific glomeruli in the antennal lobe and increased density of microglomeruli in the olfactory region of the mushroom body calyces (Brown et al., 2002; Hourcade et al., 2009, 2010). In other studies, the volumes of specific antennal lobe glomeruli were found to be correlated with foraging experience and colony queen status (Morgan et al., 1998; Sigg et al., 1997). All of these examples of structural plasticity in the adult honey bee brain – foraging, associative learning, exposure to a queen – share the common feature of dependence upon restructuring of the neuronal cytoskeleton and are candidates for regulation by Rho GTPase activity.
Rho GTPase activity may also contribute to the formation and retrieval of memories in the honey bee. In D. melanogaster and mice, Rac activity enhanced performance on assays measuring aversive olfactory learning, spatial memory, and fear conditioning presumably via dendritic reorganization (Diana et al., 2007; Haditsch et al., 2009; Shuai et al., 2010). We speculate that activation of mushroom body Rho GTPases may also mediate the structural consequences of associative learning through modulation of the actin and microtubules in the dendritic cytoskeleton. A recent report by Pasch and colleagues (2011) identified a sub-population of Kenyon cells with concentrations of activated CaMKII in their dendritic spines. CaMKII is a learning associated protein which becomes activated by an increase in intracellular calcium. While differences between nurse and forager bees were not reported, CaMKII’s ability to link neurotransmission with direct interactions to the cytoskeleton suggests a possible role in Kenyon cell structural plasticity.
Honey bees that initiated foraging at 32 or 36 days old had significantly lower levels of activated RhoA than those which began foraging at 24 or 28 days old. Observational studies have shown that most honey bees initiate foraging near the end of the third week of adult life (for example, see Guzmán-Novoa et al., 1994; Seeley, 1982). Environmental conditions such as persistent bad weather can delay the onset of foraging, but workers that delay foraging until they are 30 or more days old are atypical. Withers and colleagues (1995) created a natural population of overage nurses (21 day old honey bees observed caring for brood) by removing pupae prior to emergence so that there was a deficiency of young adult workers in the hive. The mushroom body neuropil volume of overage nurses was significantly larger than that of normal aged nurses. Possibly the bees sampled in our studies were similar to these overage nurses, and the lower levels of activated RhoA in the bees which initiated foraging at 32 and 36 days old may correspond to the bees’ behavior prior to foraging, which we did not observe in this study. Levels of pan-Rac were not correlated with age of foraging onset.
A difference in levels of activated RhoA, but not pan-Rac, was found between collections made at two different times in the summer. Honey bees used in these studies were collected from multiple source colonies (thus multiple queens) and lived under natural field conditions, undisturbed except for hive entrance observations when painting foragers, in a single host colony. Differences in GTPase activity owing to genetic contributions would be distributed among the sampled groups. But colony demands shift at different points in the season. Early in the summer, brood production is the primary concern, while later in the year the temperate zone colonies begin preparations for overwintering. Levels of activated RhoA were significantly lower in the August collection than those obtained in the first half of the summer. Because the mushroom body lysates used in both the RhoA and pan-Rac assays were obtained from randomly chosen heads from the same pool of frozen foragers and no differences between collections were found in pan-Rac activity, we believe that this difference is biological (as opposed to reflecting fluctuation in the performance of the assay). In addition, lysates from June and August compared directly on the same RhoA plate yielded results similar to previously run samples. Lower levels of activated RhoA in August may indicate an increased likelihood of Kenyon cell growth later in the fall, perhaps as a result of differences in the intensity of foraging activity or in reflection of preparations for overwintering.
The principal finding in this study was that a transient change in Rho GTPase activity (increase in pan-Rac, decrease in RhoA), occurred in foragers with less than 1 week of experience. Our findings are consistent with the growth-promoting function of Rac and the growth-inhibiting function of RhoA previously reported. Rho GTPases are exciting new targets for the study of molecular regulation in the honey bee brain because their activity does not depend on de novo gene expression. Microarray studies that measure relative levels of mRNA would not directly detect changes in GTPase activity, although it is notable that a recent study of gene expression in forager mushroom bodies identified 11 genes related to small GTPase signal transduction, suggesting that foraging experience also modulates this signaling system on a longer time scale (Lutz et al., 2011).
Highlights.
Mushroom body Rac activity increased transiently in honey bees shortly after the onset of foraging.
Mushroom body RhoA activity decreased transiently in honey bees shortly after the onset of foraging.
RhoA activity was correlated negatively with age of foraging onset.
Differences were observed in RhoA, but not Rac, activity in distinct periods of summer.
Acknowledgements
Thanks to Jeffrey T. Jackson and Erika Vardeman for assistance with beekeeping, Erik C. Johnson for the use of the microplate reader, and members of the Fahrbach laboratory for feedback on the manuscript. S.E.D. was supported by NIH Award GM073644 to S.E.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proceedings of the National Academies of Science, USA. 2008;105:4226–4231. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakal C, Aach J, Church G, Perrimon N. Quantitative morphological signatures define local signaling networks regulating cell morphology. Science. 2007;316:1753–1756. doi: 10.1126/science.1140324. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) Journal of Comparative Psychology. 1983;97:107–119. [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: A decade of hypertrophic signaling hits. Circulation Research. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Brown SM, Napper RM, Thompson CM, Mercer AR. Stereological analysis reveals striking differences in the structural plasticity of two readily identifiable glomeruli in the antennal lobes of the adult worker honeybee. Journal of Neuroscience. 2002;22:8514–8522. doi: 10.1523/JNEUROSCI.22-19-08514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos RI, Forget MA, Settleman JE, Hansen SH. Coordination of Rho and Rac GTPase Function via p190B RhoGAP. Current Biology. 2008;18:1606–1611. doi: 10.1016/j.cub.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss RG, Brandon JG, Globus A. Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experiences. Brain Research. 1980;192:49–59. doi: 10.1016/0006-8993(80)91007-0. [DOI] [PubMed] [Google Scholar]
- Diana G, Valentini G, Travaglione S, Falzano L, Pieri M, Zona C, Meschini S, Fabbri A, Fiorentini C. Enhancement of learning and memory after activation of cerebral Rho GTPases. Proceedings of the National Academy of Sciences, USA. 2007;104:636–641. doi: 10.1073/pnas.0610059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin SE, Herlihy JD, Robinson GE, Fahrbach SE. Muscarinic regulation of Kenyon cell dendritic arborizations in adult worker honey bees. Arthropod Structure and Development. 2011;40:409–419. doi: 10.1016/j.asd.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst C, Eichmüller S, Menzel R. Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behavioral and Neural Biology. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Farris SM, Sullivan JP, Robinson GE. Limits on volume changes in the mushroom bodies of the honey bee brain. Journal of Neurobiology. 2003;57:141–151. doi: 10.1002/neu.10256. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Dobrin SE. The how and why of structural plasticity in the honey bee brain. In: Dukas R, Ratcliffe JM, editors. Cognitive Ecology II. University of Chicago Press; 2009. pp. 27–46. [Google Scholar]
- Farris SM, Robinson GE, Fahrbach SE. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. Journal of Neuroscience. 2001;21:6395–6404. doi: 10.1523/JNEUROSCI.21-16-06395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova JL, Jong S, Camp JT, Fehon RG. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Developmental Biology. 2000;221:181–194. doi: 10.1006/dbio.2000.9671. [DOI] [PubMed] [Google Scholar]
- Giurfa M. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. Journal of Comparative Physiology A. 2007;193:801–824. doi: 10.1007/s00359-007-0235-9. [DOI] [PubMed] [Google Scholar]
- Groh C, Meinertzhagen IA. Brain plasticity in Diptera and Hymenoptera. Frontiers in Bioscience. 2010;2:268–288. doi: 10.2741/s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Novoa E, Page RE, Gary NE. Behavioral and life-history components of division of labor in honey bees (Apis mellifera L) Behavioral Ecology and Sociobiology. 1994;34:409–417. [Google Scholar]
- Haditsch U, Leone DP, Farinelli M, Chrostek-Grashoff A, Brakebusch C, Mansuy IM, McConnell SK, Palmer TD. A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Molecular and Cellular Neuroscience. 2009;41:409–419. doi: 10.1016/j.mcn.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learning and Memory. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade B, Muenz TS, Sandoz JC, Rossler W, Devaud JM. Long-term memory leads to synaptic reorganization in the mushroom bodies: A memory trace in the insect brain? Journal of Neuroscience. 2010;30:6461–6465. doi: 10.1523/JNEUROSCI.0841-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade B, Perisse E, Devaud JM, Sandoz JC. Long-term memory shapes the primary olfactory center of an insect brain. Learning and Memory. 2009;16:607–615. doi: 10.1101/lm.1445609. [DOI] [PubMed] [Google Scholar]
- Ismail N, Robinson GE, Fahrbach SE. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proceedings of the National Academy of Sciences, USA. 2006;103:207–211. doi: 10.1073/pnas.0508318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiya T, Kunieda T, Kubo T. Inducible- and constitutive-type transcript variants of kakusei, a novel non-coding immediate early gene, in the honeybee brain. Insect Molecular Biology. 2008;17:531–536. doi: 10.1111/j.1365-2583.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- Krofczik S, Khojasteh U, Hempel de Ibarra N, Menzel R. Adaptation of microglomerular complexes in the honeybee mushroom body lip to manipulations of behavioral maturation and sensory experience. Developmental Neurobiology. 2008;68:1007–1017. doi: 10.1002/dneu.20640. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Williams R, Stephens L, Hawkins PT. ARAP3 Is a PI3K- and Rap-Regulated GAP for RhoA. Current Biology. 2004;14:1380–1384. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors and Channels. 2003;9:241–260. [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Li Z, Van Aelst L, Cline HT. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nature Neuroscience. 2000;3:217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Bundrock G, Muller U. Focal and temporal release of glutamate in the mushroom bodies improves olfactory memory in Apis mellifera. Journal of Neuroscience. 2005;25:11614–11618. doi: 10.1523/JNEUROSCI.3180-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano VC, Gauthier M. Effects of the muscarinic antagonists atropine and pirenzepine on olfactory conditioning in the honeybee. Pharmacology Biochemistry and Behavior. 1998;59:903–907. doi: 10.1016/s0091-3057(97)00524-8. [DOI] [PubMed] [Google Scholar]
- Lundquist EA. Rac proteins and the control of axon development. Current Opinion in Neurobiology. 2003;13:384–390. doi: 10.1016/s0959-4388(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Lutz CC, Rodriguez-Zas SL, Fahrbach SE, Robinson GE. Transcriptional Response to foraging experience in the honey bee mushroom bodies. Developmental Neurobiology. 2011 doi: 10.1002/dneu.20929. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleszka R, Helliwell P, Kucharski R. Pharmacological interference with glutamate re-uptake impairs long-term memory in the honeybee, Apis mellifera. Behavioural Brain Research. 2000;115:49–53. doi: 10.1016/s0166-4328(00)00235-7. [DOI] [PubMed] [Google Scholar]
- Morgan SM, Butz Huryn VM, Downes SR, Mercer AR. The effects of queenlessness on the maturation of the honey bee olfactory system. Behavioural Brain Research. 1998;91:115–126. doi: 10.1016/s0166-4328(97)00118-6. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müssig L, Richlitzki A, Rössler R, Eisenhardt D, Menzel R, Leboulle G. Acute disruption of the NMDA receptor subunit NR1 in the honeybee brain selectively impairs memory formation. Journal of Neuroscience. 2010;30:7817–7825. doi: 10.1523/JNEUROSCI.5543-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Van Aelst L. Rho-linked genes and neurological disorders. European Journal of Physiology. 2008;455:787–797. doi: 10.1007/s00424-007-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. The Journal of Neuroscience. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. Journal of Neurobiology. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Current Opinion in Genetics and Development. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? Journal of Cell Science. 2010;234:1841–1850. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- Pasch E, Muenz TS, Rö ssler W. CaMkII is differentially localized in synaptic regions of Kenyon cells within the mushroom bodies of the honeybee brain. Journal of Comparative Neurology. 2011;519:3700–3712. doi: 10.1002/cne.22683. [DOI] [PubMed] [Google Scholar]
- Ponimaskin E, Voyno-Yasenetskaya T, Richter DW, Schachner M, Dityatev A. Morphogenic signaling in neurons via neurotransmitter receptors and small GTPases. Molecular Neurobiology. 2007;35:278–287. doi: 10.1007/s12035-007-0023-0. [DOI] [PubMed] [Google Scholar]
- Seeley TD. Adaptive significance of the age polyethism schedule in honeybee colonies. Behavioral Ecology and Sociobiology. 1982;11:287–293. [Google Scholar]
- Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y. Forgetting is regulated through Rac activity in Drosophila. Cell. 2010;140:579–589. doi: 10.1016/j.cell.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Sigg D, Thompson CM, Mercer AR. Activity-dependent changes to the brain and behavior of the honey bee, Apis mellifera (L.) Journal of Neuroscience. 1997;17:7148–7156. doi: 10.1523/JNEUROSCI.17-18-07148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- Velarde RA, Robinson GE, Fahrbach SE. Nuclear receptors of the honey bee: annotation and expression in the adult brain. Insect Molecular Biology. 2006;15:583–595. doi: 10.1111/j.1365-2583.2006.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield CW, Ben-Shahar Y, Brillet C, Leoncini I, Crauser D, Leconte Y, Rodriguez-Zas S, Robinson GE. Genomic dissection of behavioral maturation in the honey bee. Proceedings of the National Academy of Sciences of the USA. 2006;103:16068–16075. doi: 10.1073/pnas.0606909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- Winston ML. The biology of the honey bee. Boston, MA., USA: Harvard University Press; 1987. [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees. Journal of Neurobiology. 1995;26:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]
- Wright GA, Mustard JA, Simcock NK, Ross-Taylor AAR, McNicholas LD, Popescu A, Marion-Poll F. Parallel reinforcement pathways for conditioned food aversions in the honeybee. 2010;20:2234–2240. doi: 10.1016/j.cub.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


