Abstract
The study of lipoproteins, natural nanoparticles comprised of lipids and apolipoproteins that transport fats throughout the body, is of key importance to better understand, treat, and prevent cardiovascular disease. In the current study, we have developed a lipoprotein-based nanoparticle that consists of a quantum dot (QD) core and Cy5.5 labeled lipidic coating. The methodology allows judicious tuning of the QD/Cy5.5 ratio, which enabled us to optimize Förster resonance energy transfer (FRET) between the QD core and the Cy5.5-labeled coating. This phenomenon allowed us to study lipoprotein–lipoprotein interactions, lipid exchange dynamics, and the influence of apolipoproteins on these processes. Moreover, we were able to study HDL-cell interactions and exploit FRET to visualize HDL association with live macrophage cells.
Keywords: Quantum dots, Förster resonance energy transfer (FRET), lipoproteins, lipid-exchange, apolipoprotein A-I, live cell imaging
The development of hybrid nanostructures based on nanocrystals that are stabilized and functionalized by a biocompatible coating has seen rapid progress over the past decade.1–7 Among the different coatings, amphiphilic polymer and lipid coatings have shown unprecedented possibilities since these can be applied in a facile fashion, allow combinations with reactive and/or functionalized amphiphilic molecules, or may be comprised of naturally occurring molecules such as phospholipids and apolipoproteins to create lipoprotein-like nanoparticles.2 In previous studies, we developed lipid-coated quantum dot technology to create targeted nanoparticles for multimodal molecular imaging in animal models of cancer and cardiovascular disease.2,4,8 These nanoparticles are comprised of quantum dots that are stabilized by hydrophobic ligands, which are subsequently coated by a monolayer of phospholipids. Other nanocrystals that have been exploited as contrast agents for various imaging techniques using a similar lipidic coating strategy include gold,9 iron oxide,10 or manganese ferritite.11 Subsequent functionalization of such lipid-coated nanoparticles can be achieved by the conjugation of targeting ligands,5,12 such as peptides,4 or, as we have recently demonstrated, by the adsorption of apolipoproteins, such as apolipoprotein A-I (apoA-I).2 In the latter case the general nanoparticle morphology closely resembles that of high-density lipoprotein (HDL)13 and we have shown it to also exhibit important biological HDL functions, such as macrophage targeting14 and the ability to induce cholesterol efflux.2,15
HDL, popularly known as “good cholesterol”, has a protective role in the development of cardiovascular disease,16,17 a pathology that may ultimately result in clinical events such as myocardial infarction and stroke.18 The study of lipoprotein biology in general, and HDL’s biology in particular, therefore is of key importance to better understand, treat and prevent cardiovascular disease and help reduce mortality and morbidity caused by this pathology.
Both the increasing interest in lipid-coated nanocrystals and the need to better understand HDL biology in detail inspired us to develop a hybrid nanoarchitecture that resembles HDL. The dynamics of lipid exchange and interactions with cells can be studied with Förster resonance energy transfer (FRET) between the quantum dot core and dye-labeled lipids in the coating. In recent reports, FRET between quantum dots and organic fluorophores has been exploited to study fundamental and biological processes,19–22 but this phenomenon has also been applied to create biosensors23 and imaging probes.24 In the majority of these studies the organic fluorophores were adsorbed at the surface of QDs via proteins,25 peptides,26 or polymers.27,28 We sought to develop nanotechnology based on lipid-coated nanocrystals that allows an easier and more precise tuning of the QD/dye ratio to optimize FRET and enables a detailed investigation of the lipid exchange dynamics of HDL nanoparticles with their surroundings as well as their biological interaction with cells.
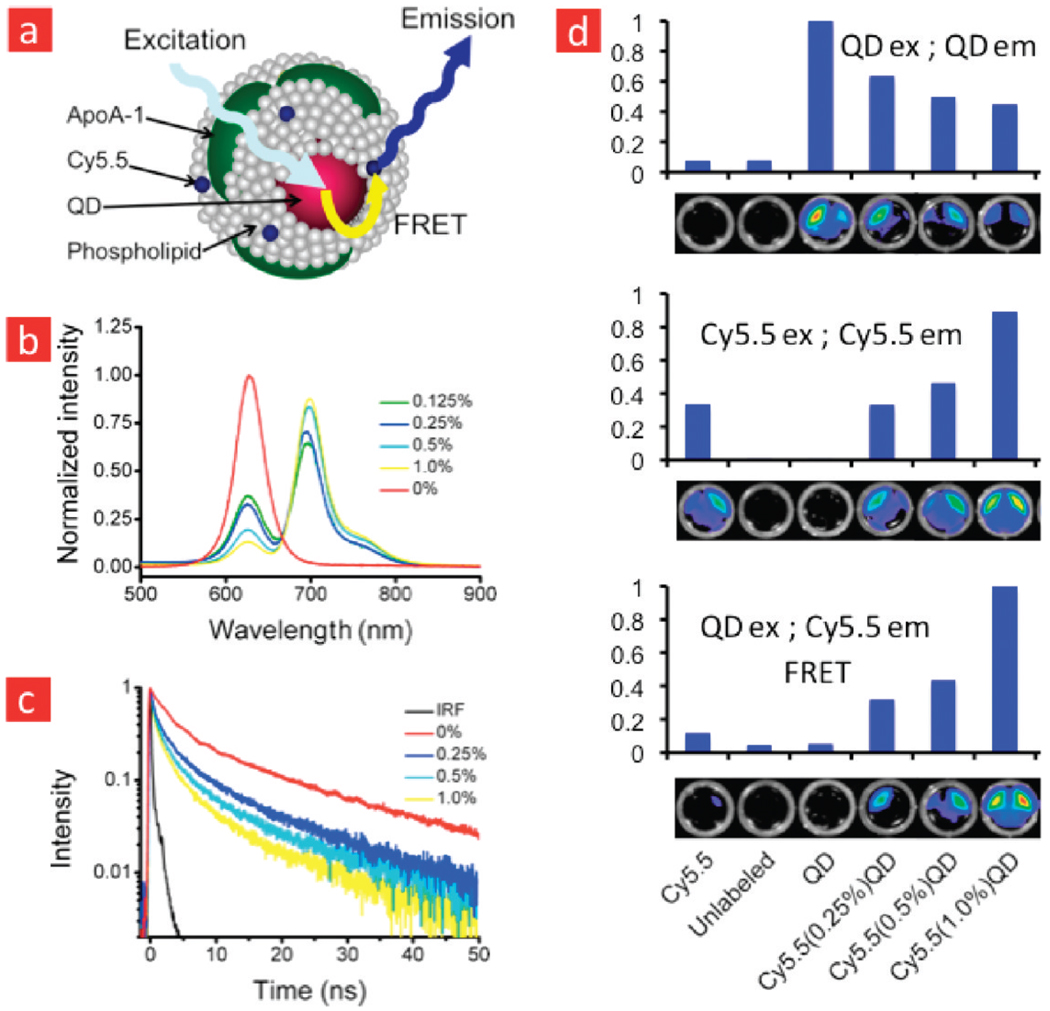
In the present study, we have developed FRET nanoprobes, based on the above-mentioned lipid-coated QD nanoparticle technology, which closely mimics HDL. This nanoparticle has a FRET donor QD in its core and the near-infrared fluorescent dye Cy5.5 FRET acceptor in its lipid corona. The incorporation of the dye in the nanoparticle’s lipid corona was facilitated by its conjugation to DMPE (Figure 1, Supporting Information Methods), a phospholipid that has an amine group available. Exceptionally stable CdSe–CdS–ZnS core–shell–shell (CSS) QDs29 were synthesized and coated with an appropriate mixture of Cy5.5 labeled and unlabeled phospholipids using methods we have described previously.2,4,29 The resulting nanoparticles consist of one QD core per HDL particle and are highly stable in PBS and serum. The emission spectrum of these CSS QDs and the absorption spectrum of the Cy5.5-lipids significantly overlap (Supporting Information Figure 2), which is a pre-requisite for FRET. We further exploited the broad and narrow absorption bands of the QD and Cy5.5 dye, which allowed us to selectively excite the donor QDs. The amount of Cy5.5 in the coating could be easily adjusted by adding more labeled lipid to the mixture. Subsequently, apolipoprotein A-I (apoA-I) was added and left to incubate overnight, after which the product was purified to obtain single QD core and Cy5.5 labeled HDL-like nanoparticles (QD-HDL-Cy5.5), schematically depicted in Figure 1a.
FIGURE 1.
Förster resonance energy transfer (FRET) in quantum dot (QD) core high-density lipoprotein labeled with lipid conjugated fluorophore. (a) Schematic representation of a quantum dot core and Cy5.5-lipid dual labeled high-density lipoprotein (QD-HDL-Cy5.5) nanoparticle. (b) Emission spectra of QD-HDL with varying amounts of Cy5.5-lipid for excitation at 406 nm. (c) Fluorescence decay curves of the QD emission (620 nm, excitation at 406 nm) for QD-HDL with varying amounts of Cy5.5-lipid. The contribution of the instrument response function (IRF) is neglectable. (d) Fluorescence images of different HDL preparations acquired with a QD excitation and emission passband (top), a Cy5.5 excitation and emission passband (middle), and a QD excitation and Cy5.5 emission passband (FRET setup).
To investigate the occurrence of FRET, we acquired emission spectra (Supporting Information Methods) of QD-HDL nanoparticles that contained varying amounts of Cy5.5 in their lipid coating. The emission spectra (Figure 1b), recorded for an excitation wavelength of 400 nm, where the QDs absorb efficiently and the Cy5.5 dye has little or no absorbance, revealed a dramatic decrease in the QD emission intensity with an increasing amount of Cy5.5. This decrease was accompanied by an increase in the intensity of the Cy5.5 emission peak. For QD-HDL particles that contained 1% Cy5.5 labeled lipid in their coating, which corresponds on average to 3 Cy5.5 dye molecules per particle, the QD emission was almost completely quenched. These observations clearly demonstrate the occurrence of FRET from the QDs to the Cy5.5 dye molecules. To further verify that the decrease of the QD emission intensity and increase of Cy5.5 intensity was due to FRET we measured the fluorescence lifetimes of the QD emission for different samples. In Figure 1c the fluorescence decay curves portray a significant reduction in the QD excited state lifetime with increasing amounts of Cy5.5. The increased decay rate of the QD fluorescence observed within the first 10 ns after excitation is ascribed to energy transfer from a QD donor to a Cy5.5 dye molecule acceptor.
Near infrared fluorescence (NIRF) imaging is a semiquantitative imaging modality30 that is applied to visualize NIRF probes in cells and small laboratory animals, primarily mice. This imaging method is fast, sensitive, and allows multiplex imaging, that is, the visualization of multiple species.31,32 In addition to studying particle–particle and particle–cell interactions, the Cy5.5-coated QD-HDL nanoparticle may also be exploited as a NIRF probe that can be excited by a broad wavelength range via the QD but exhibits the typical Cy5.5 NIRF emission. We tested the ability of fluorescence imaging to observe FRET in samples that contained QD core HDL with varying amounts of Cy5.5 labeled lipids. To that end we applied three different filter set combinations on a black wells plate that contained 100 µL of differently labeled diluted (~1 µMol lipid) HDL samples and measured the photon counts. These were normalized to the maximum value for a certain filter set. In the first QD setup, we aimed to visualize the QD excitation and emission by exciting the samples with an excitation passband of 445–490 nm, while applying an emission passband of 610–630 nm. In the second Cy5.5 setup, a dedicated filter set was applied to directly visualize Cy5.5, that is, an excitation passband of 615–665 and an emission passband of 695–770 nm. The third setup, which we will refer to as the FRET setup, had the QD excitation passband and the Cy5.5 emission passband. In the QD setup, we observed a signal from all the HDL samples that contained a QD core, but quenching from the HDL samples that were also labeled with Cy5.5 was clearly observed (Figure 1d, top). The Cy5.5 setup noticeably showed an increasing photon count for samples that contained a higher Cy5.5 content. In the FRET setup, energy transfer occurred for all the dual-labeled samples with the highest photon count for the sample that contained 1% Cy5.5. Altogether, the experiments presented in Figure 1 convincingly demonstrate the occurrence of FRET in our dual-labeled HDL nanoparticle. Below we will demonstrate how changes in the FRET can be used to probe lipid exchange between nanoparticles and to study the association of the lipoprotein nanoparticles with cells.
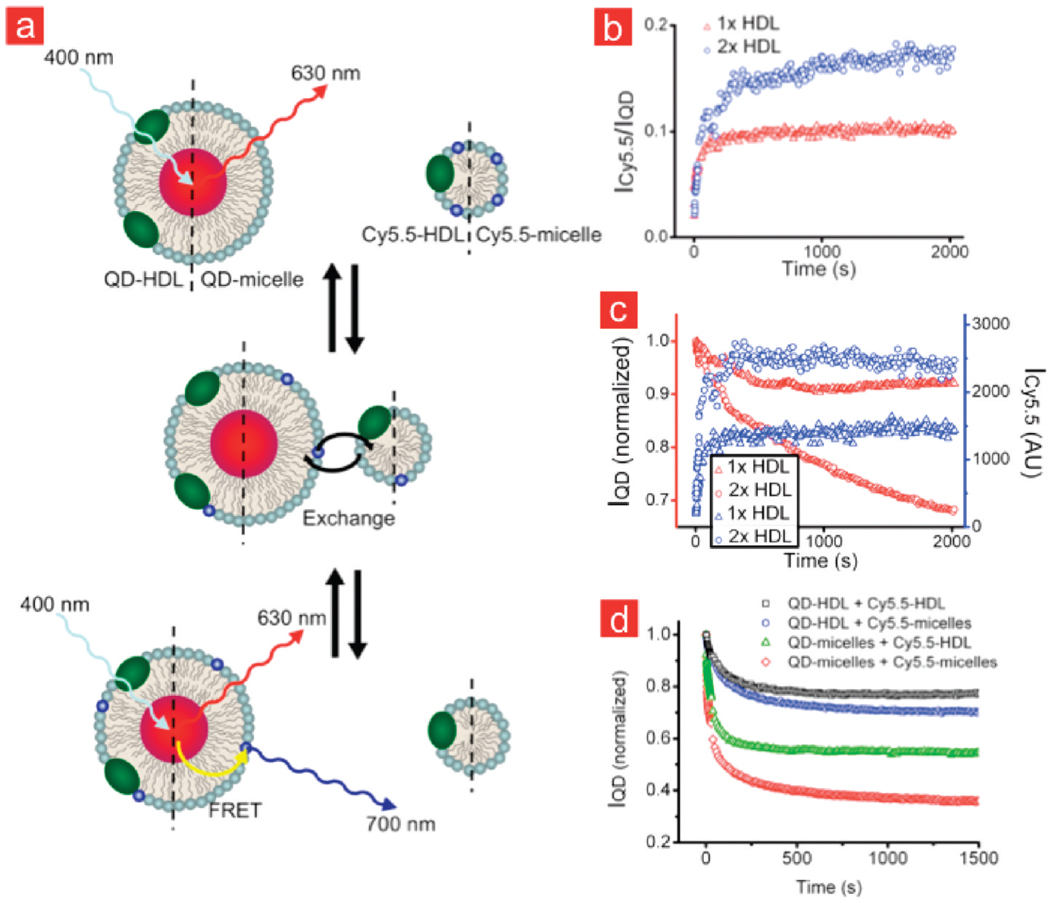
HDL, which measures 7–13 nm in diameter, is comprised of a fatty core of triglycerides and cholesteryl esters that is coated and stabilized by phospholipids and apolipoproteins.33 Besides HDL, many other lipoprotein classes34 exist and several studies have shown that they can take up cholesterol35 and vividly exchange components, including the phospholipids.36 Our FRET QD-HDL-Cy5.5 nanoparticle is very well suited to study lipid-exchange, since the area under the emission peaks in the spectrum of the sample depends on the amount of Cy5.5-lipid present in the particle’s corona, and changes in the emission spectra can be studied with a high temporal resolution of less than 0.4 s. This implies that when a lipid-coated QD sample, which does not contain Cy5.5-lipids, is mixed with lipid nanoparticles that do contain Cy5.5-lipids, and lipid-exchange occurs, the areas under the emission spectra of the QD and Cy5.5 change. In Figure 2a, different exchange processes are schematically depicted. In Figure 2b, the time evolution of the integrated intensity of the Cy5.5 emission divided by the integrated intensity of the QD emission (ICy5.5/IQD) is plotted for QD-HDL samples that were mixed with Cy5.5-labeled HDL, or with Cy5.5-labeled micelles, at the same concentrations of Cy5.5. This ratio was observed to gradually increase after mixing, eventually reaching an equilibrium value. In addition, we found the equilibrium ICy5.5/IQD ratio to be higher when the mixing was done at a higher Cy5.5-HDL concentration. This implies that in the latter case more Cy5.5-labeled lipid is transferred to the QD core HDL nanoparticles. In Figure 2c, the intensities of Cy5.5 and QD are individually depicted, showing that the trends observed in Figure 2b result from a simultaneous decrease in the QD emission intensity and increase in the Cy5.5 emission intensity.
FIGURE 2.
Lipid-exchange between different types of lipidic nanoparticles monitored by optical techniques. (a) QD-HDL (or QD-micelle) mixing with HDL-Cy5.5 (or Cy5.5-micelle) results in the occurrence of FRET after Cy5.5-lipids exchange. (b) Intensities of the Cy5.5 emission divided by the intensity of the QD emission (ICy5.5/IQD) for QD-HDL samples that were mixed with Cy5.5 labeled HDL at two different concentrations. (c) The individual intensities ICy5.5 and IQD of the data presented in (b). (d) Temporal course of IQD after mixing QD-HDL and Cy5.5-HDL, QD-HDL and Cy5.5-micelles, QD-micelles and Cy5.5-HDL, and QD-micelles and Cy5.5-micelles.
Besides the targeting function,37,38 apoA-I is known to provide stability to the HDL lipid nanoparticle.39 Studying exchange rates of lipids between HDL particles (and other lipidic aggregates) using the above-mentioned methodology may therefore shed light on the stabilizing properties of apoA-I. To that end, we tested four different situations (Figure 2d) where 0.025 nmol/mL QD core lipid nanoparticles, either with or without apoA-I included in their lipid coating, were mixed with equimolar amounts of either HDL or micellar nanoparticles that contained Cy5.5 labeled lipids. For reasons of clarity, we solely present the intensity of the QD emission for the exchange data in Figure 2d. Mixing HDL-like nanoparticles that are both functionalized with apoA-I resulted in the slowest exchange rates while mixing apoA-I functionalized with unfunctionalized nanoparticles showed intermediate exchange rates. The fastest exchange rates were observed when lipid-coated QDs were mixed with Cy5.5-micelles. In addition, we observed that the exchange rates were temperature dependent. A strong increase in exchange rate is observed between 6 and 37 °C (Supporting Information Figure 3). The experiments confirm that apoA-I has stabilizing properties,40–42 since lipid-exchange was significantly decreased for the lipid nanoparticles that were functionalized with apoA-I, that is, HDL nanoparticles, as compared to the lipid nanoparticles without this apolipoprotein.
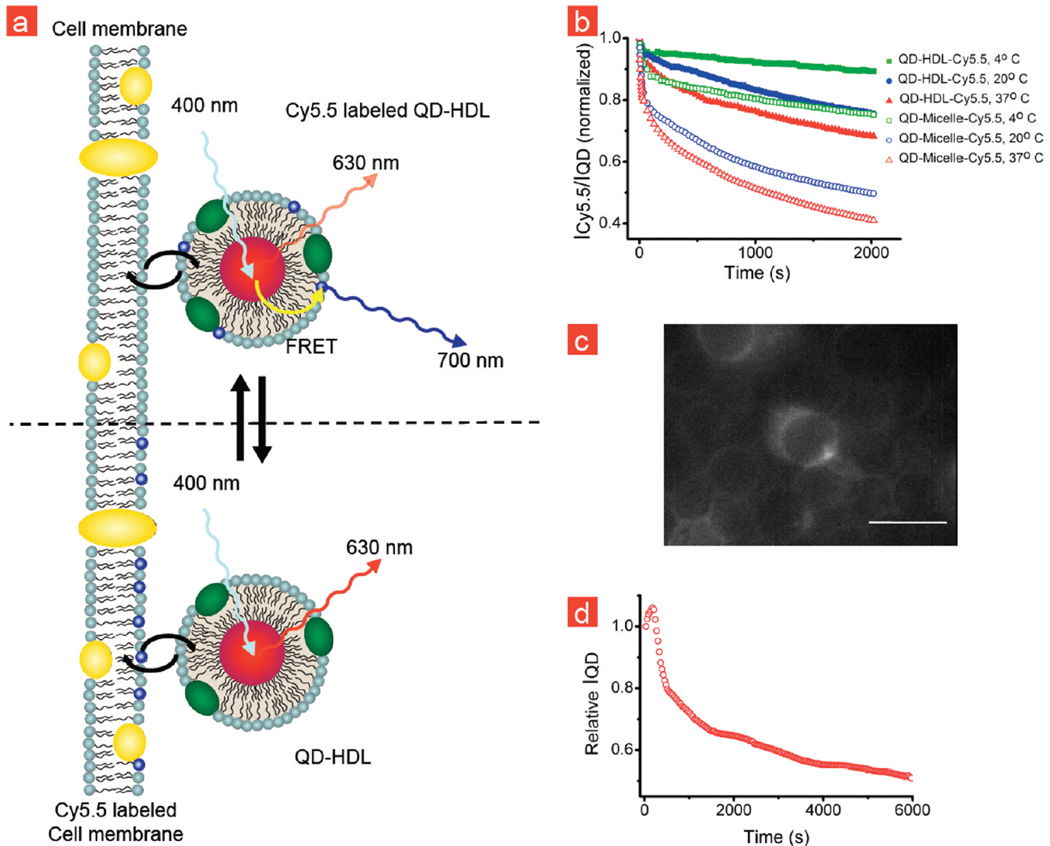
The most important mechanism through which HDL exerts its protective role against cardiovascular disease is the removal of excess cholesterol from peripheral cells, especially from lipid-laden macrophages, and its transport to the liver, a process referred to as reverse cholesterol transport.14,43 FRET measurements can also be applied to investigate interactions between HDL and cells, as schematically illustrated in Figure 3a. To that aim, we incubated THP-1 macrophages, a cell line that grows in suspension and does not adhere, with QD-HDL-Cy5.5. A decrease in ICy5.5/IQD was observed (Figure 3b) for dual labeled lipid nanoparticles, indicative of Cy5.5-lipids exchanging with the cell membrane. A noticeable difference was observed between the apoA-I stabilized HDL particles and the lipid particles that were not functionalized with apoA-I (QD-Micelle-Cy5.5). The much slower exchange observed for the apoA-I particles confirms its stabilizing function. Fluorescence microscopy images of THP-1 macrophages that were incubated with QD-HDL-Cy5.5 and Cy5.5-labeled micelles are presented in Figure 3c and Supporting Information Figure 4, respectively. These data corroborated our hypothesis that the Cy5.5-labeled lipids transferred to the cell membrane, but we cannot rule out the occurrence of other associative processes such as uptake. Alternatively, we prelabeled THP-1 macrophages with Cy5.5-lipid micelles (Supporting Information Figure 4) overnight and subsequently incubated with QD core HDL that was not labeled by Cy5.5-lpids. After mixing, we observed a temporal decrease of IQD, indicative for lipid-exchange from the cells to the nanoparticle (Figure 3d). These results confirm the vivid exchange of lipoprotein components with cells.
FIGURE 3.
Lipid-exchange between lipid nanoparticles and cell membrane. (a) Schematic representation of the QD-HDL-Cy5.5 exchanging Cy5.5-lipids with the cell membrane and the effect on FRET. (b) ICy5.5/IQD of QD-HDL-Cy5.5 and QD-Micelle-Cy5.5 samples that were mixed with THP-1 macrophage cells at different temperatures. (c) Fluorescence microscopy image of THP-1 cells that were incubated with QD-HDL-Cy5.5 showing the incorporation of Cy5.5-lipids in the cell membrane. (d) IQD of QD-HDL that were mixed with THP-1 cells that were prelabeled with Cy5.5-lipids.
Lastly, we performed a proof-of-principle study where we aimed to visualize FRET of QD-HDL-Cy5.5 nanoparticles in living adhered J774A.1 macrophages via fluorescence microscopy. Such experiments can provide information about the mechanism of uptake and the intactness of the lipid nanoparticle after uptake by the cell.
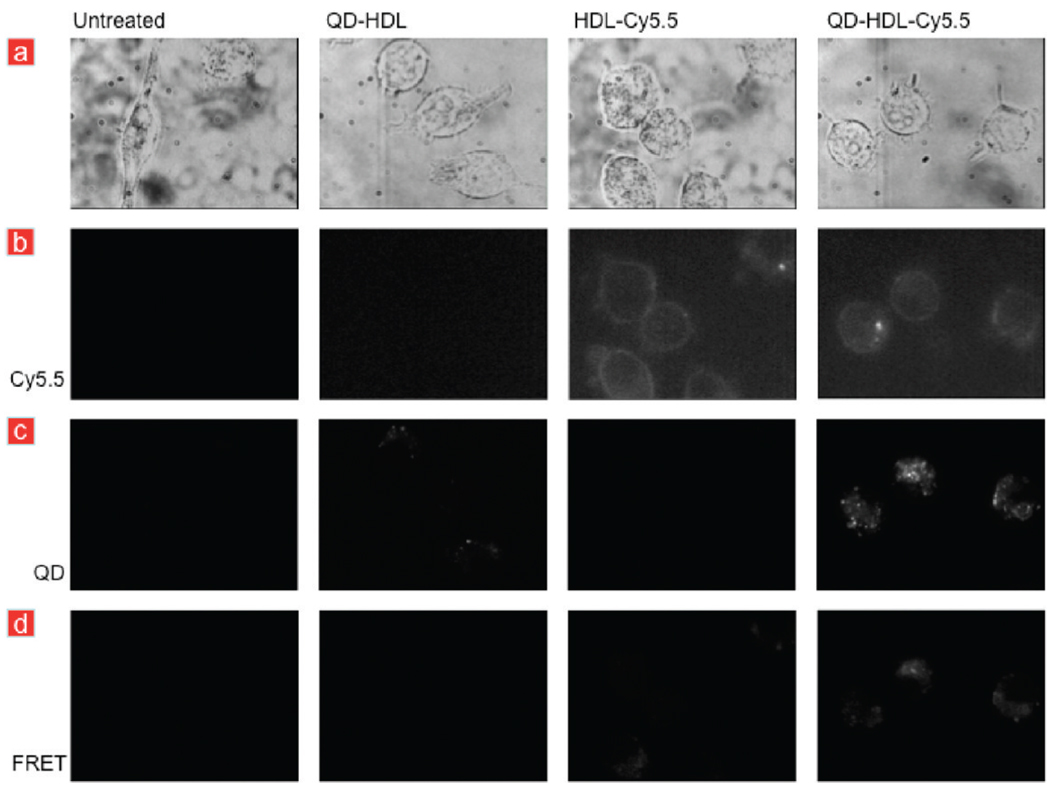
First, we performed experiments where we incubated macrophages with QD-HDL-Cy5.5 and control nanoparticles for 5 min. These were subsequently washed to remove the aforementioned nanoparticles from the medium. Fluorescence microscopy images were recorded with different filter sets to directly visualize QDs and Cy5.5-lipids, as well as to visualize the occurrence of FRET, 10 min after washing the cells. Figure 4a presents brightfield images of untreated and cells that were incubated with HDL-Cy5.5, QD-HDL, and QD-HDL-Cy5.5, respectively. Cy5.5 fluorescence (Figure 4b) was observed in the cell membrane of cells incubated with HDL-Cy5.5 and QD-HDL-Cy5.5, confirming exchange of lipids with the cell membrane, in line with the data presented in Figure 3, but also inside the cell, indicative for particle uptake. QD fluorescence was observed inside cells that were incubated with QD-HDL or QD-HDL-Cy5.5 (Figure 4c). The QDs were most likely present in vesicular structures, that is, endosomes. In the FRET microscopy setting, we observed pronounced Cy5.5 fluorescence in cells that were incubated with QD-HDL-Cy5.5 (Figure 4d), confirming the occurrence of particle uptake and applicability of our FRET technology in cell fluorescence microscopy experiments. We ascribe the marginal fluorescence observed in cells incubated with HDL-Cy5.5 to direct excitation of Cy5.5, which was also observed when we directly imaged this sample (Figure 1d). Additional live microscopy images of cells incubated with QD-HDL-Cy5.5 nanoparticles HDL-Cy5.5 can be found in Supporting Information Figure 5.
FIGURE 4.
Live cell fluorescence microscopy of macrophages. (a) Brightfield images of J774A.1 macrophages that were left untreated or incubated with differently labeled HDL nanoparticles. (b) Cy5.5 fluorescence was observed in cells that were incubated with HDL-Cy5.5 or QD-HDL-Cy5.5. (c) QD fluorescence was observed in cells that were incubated with QD-HDL or QD-HDL-Cy5.5, while (d) FRET was only observed in cells that were incubated with QD-HDL-Cy5.5.
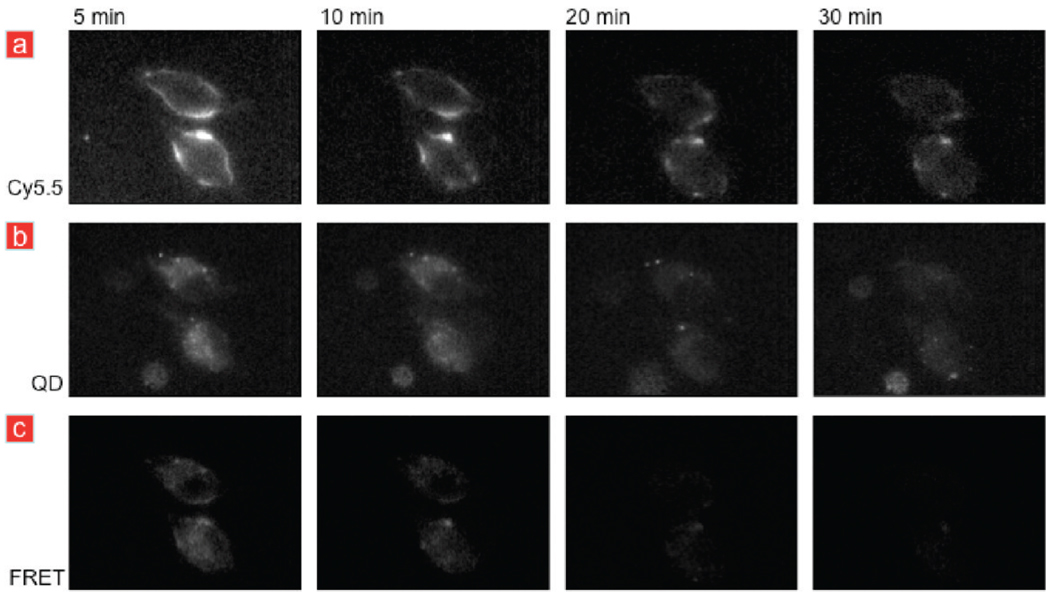
To establish the temporal evolution of QD-HDL-Cy5.5 particles, we acquired fluorescence microscopy images at different time points after washing, using the different filter settings. The Cy5.5 setting allowed us to visualize the Cy5.5-labeled lipids, while the QD setting showed the location of the oleic acid coated QD cores. The FRET microscopy setting was applied to investigate the possible temporal dissociation of the nanoparticle, since the different components, that is, fatty core and lipid/apolipoprotein corona, of HDL are known to behave differently with cells. In general, the consensus is that HDL does not enter the cell as an intact entity, but adheres at the cell surface and can exchange its fatty core. The hydrophobic cholesteryl ester core is transferred from the HDL particle to the cell via scavenger receptor BI.44,45 In Figure 5a, the Cy5.5 fluorescence can be observed to be primarily localized in the cell membrane, especially at early time points, becomes more diffuse in time, but remains visible. At early time points, the QD fluorescence is found primarily in the cytoplasm and becomes more homogenously distributed at later time points (Figure 5b). Figure 5a,b also shows that the HDL labels do not enter the nuclei of the cells. Most interestingly, we found the Cy5.5 fluorescence to decrease over time for the FRET filter set combination, which implies that the Cy5.5 and QD label dissociate in time due to the disassembly of our QD-HDL-Cy5.5 nanoparticle once it is associated with the macrophages.
FIGURE 5.
Temporal course of the fluorescence signal of macrophages incubated with QD-HDL-Cy5.5. (a) Cy5.5 fluorescence images and (b) QD fluorescence images revealed the presence of Cy5.5 and QDs at all the time points, albeit the signal intensity decreased and became more diffuse in time. (c) FRET was observed to disappear after 10 min, indicative for the dissociation of the QD and Cy5.5-lipid label.
The data presented in Figure 5 reveal that our nanoparticle both exchanges lipids with the cell membrane and deposits the QD core but was also taken up by cells as, at least partially, an intact entity that disassembled in time. Additional live microscopy images of cells incubated with the control nanoparticles HDL-Cy5.5 or QD-HDL can be found in Supporting Information Figure 6. Further studies will be performed to clarify the exact mechanism of uptake, but the value of our dual labeled lipid nanoparticle FRET technology to investigate cell lipoprotein interactions was convincingly demonstrated in these live cell fluorescence microscopy experiments.
In the present study, we have shown QD core and Cy5.5 dual-labeled HDL nanoparticles to be of great value to study lipid exchange dynamics for lipoprotein nanoparticles via FRET. We have demonstrated a methodology to follow lipid-exchange between HDL and other lipidic nanoparticles and confirmed the stabilizing features of the apoA-I.39 In a setup where nonadherent macrophage cells were incubated with QD-HDL-Cy5.5, or Cy5.5-lipid-labeled macrophages with QD-HDL, we were able to measure interactions between the cells and HDL. Lastly, we have shown the value of dual-labeled HDL to visualize, using FRET fluorescence microscopy, the temporal fate of lipoproteins once associated with macrophages. It was observed that the lipids from the HDL nanoparticle exchange with the cell membrane and, once taken up by the cell, in time dissociate from the QD core. Besides labeling the lipids of QD core HDL, we anticipate this FRET effect to occur if apoA-I is labeled with an organic fluorophore, thereby allowing the investigation of its dynamics and cellular fate.
Generally this technology can be applied to investigate the (exchange) behavior of a variety of lipid-coated QDs, such as PEG-lipid coated QDs that are extensively used in the field of target-specific imaging and drug delivery.2,4,8,12,46 In addition, this technology may be applied to other lipidic nanoparticles such as nanoemulsions47 or liposomes,48 as well as to nanoparticles composed of block copolymers.49 Importantly, lipid-coated and dye labeled QDs offer unique possibilities to study fundamental principles of FRET between QDs and organic fluorophores, since the nanotechnology allows facile variation of the number (or type) of dye molecules per QD as well as the manipulation of the QD-dye distance, key for efficient energy transfer, using e.g. lipids with different acyl chain lengths. Lastly, our approach may be applied to create (bio)sensors23 using lipid-coated QDs that are equipped with lipid-dye molecules of which the dye is cleaved, for example, by the presence of enzymes, ions, or at certain pH.
In conclusion, lipid-coated and dye-labeled QDs represent a versatile probe to study FRET as well as fundamental and biological processes via FRET, such as lipid-exchange between nanoparticles and nanoparticle uptake by cells.
Acknowledgment
We thank Yu Zhou for his help with the fluorescence imaging experiments and Bart de Haan and Ton Peters for their help with cell culturing. This work was financially supported by ECHO.06.B.047 (A.M.) and partial support was provided by NIH/NHLBI R01 HL71021, NIH/NHLBI R01 EB009638 (Z.A.F.).
Footnotes
Supporting Information Available. Experimental methods and additional figures. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES AND NOTES
- 1.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler., Thromb., Vasc. Biol. 2009;29:992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cormode DP, et al. Nanocrystal core high-density lipoproteins: A multimodal molecular imaging contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubertret B, et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 4.Mulder WJ, et al. Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 5.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 6.Medintz IL, Mattoussi H, Clapp AR. Potential clinical applications of quantum dots. Int. J. Nanomed. 2008;3:151–167. doi: 10.2147/ijn.s614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruns OT, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Lauterwasser J, Nikolic MS, Mollwitz B, Merkel M, Bigall NC, Sapra S, Reimer R, Hohenberg H, Weller H, Eychmüller A, Adam G, Beisiegel U, Heeren J. Real-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystals. Nat. Nanotechnol. 2009;4(3):193–201. doi: 10.1038/nnano.2008.405. (PubMed PMID: 19265850) [DOI] [PubMed] [Google Scholar]
- 8.Mulder WJ, et al. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis. 2009;12:17–24. doi: 10.1007/s10456-008-9124-2. [DOI] [PubMed] [Google Scholar]
- 9.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. Biomaterials. 2009;30:6065–6075. doi: 10.1016/j.biomaterials.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J. Biol. Inorg. Chem. 2004;9:706–712. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials. 2009;30:2919–2928. doi: 10.1016/j.biomaterials.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Mulder WJ, et al. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols AV, Krauss RM, Musliner TA. Nondenaturing polyacrylamide gradient gel electrophoresis. Method Enzymol. 1986;128:417–431. doi: 10.1016/0076-6879(86)28084-2. [DOI] [PubMed] [Google Scholar]
- 14.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J. Intern. Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 15.Davidson WS, et al. The influence of apolipoprotein structure on the efflux of cellular free cholesterol to high density lipoprotein. J. Biol. Chem. 1994;269:22975–22982. [PubMed] [Google Scholar]
- 16.Gordon DJ, Rifkind BM. High-density lipoprotein - the clinical implications of recent studies. New Engl. J. Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 17.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A propective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N. Engl. J. Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 18.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill R, et al. Probing biocatalytic transformations with CdSe-ZnS QDs. J. Am. Chem. Soc. 2006;128:15376–15377. doi: 10.1021/ja066636t. [DOI] [PubMed] [Google Scholar]
- 20.Gill R, Willner I, Shweky I, Banin U. Fluorescence resonance energy transfer in CdSe/ZnS-DNA conjugates: probing hybridization and DNA cleavage. J. Phys. Chem. B. 2005;109:23715–23719. doi: 10.1021/jp054874p. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, et al. Compact biocompatible quantum dots functionalized for cellular imaging. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clapp AR, et al. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 2004;126:301–310. doi: 10.1021/ja037088b. [DOI] [PubMed] [Google Scholar]
- 23.Gill R, Zayats M, Willner I. Semiconductor quantum dots for bioanalysis. Angew. Chem., Int. Ed. 2008;47:7602–7625. doi: 10.1002/anie.200800169. [DOI] [PubMed] [Google Scholar]
- 24.Koole R, et al. Magnetic quantum dots for multimodal imaging. Nanomed. Nanobiotechnol. 2009;1:475–491. doi: 10.1002/wnan.14. [DOI] [PubMed] [Google Scholar]
- 25.Medintz IL, et al. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat. Mater. 2003;2:630–638. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 26.Medintz IL, et al. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006;5:581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 27.Jiang G, et al. Cascaded FRET in conjugated polymer/quantum dot/dye-labeled DNA complexes for DNA hybridization detection. ACS Nano. 2009;3:4127–4131. doi: 10.1021/nn901324y. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Arguelles MT, et al. Synthesis and characterization of polymer-coated quantum dots with integrated acceptor dyes as FRET-based nanoprobes. Nano Lett. 2007;7:2613–2617. doi: 10.1021/nl070971d. [DOI] [PubMed] [Google Scholar]
- 29.Koole R, et al. Paramagnetic lipid-coated silica nanoparticles with a fluorescent quantum dot core: a new contrast agent platform for multimodality imaging. Bioconjug. Chem. 2008;19:2471–2479. doi: 10.1021/bc800368x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat. Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 31.Choi HS, et al. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi HS. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Briggs MR. HDL: The Metabolism, Function, and Therapeutic Importance. Chem. Rev. 2004;104:119–127. doi: 10.1021/cr020466v. [DOI] [PubMed] [Google Scholar]
- 34.Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J. Lipid Res. 1984;25:1277–1294. [PubMed] [Google Scholar]
- 35.Rothblat GH, et al. Cell cholesterol efflux: integration of old and new observations provides new insights. J. Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- 36.Jonas A. Interaction of bovine serum high density lipoprotein with mixed vesicles of phosphatidylcholine and cholesterol. J. Lipid Res. 1979;20:817–824. [PubMed] [Google Scholar]
- 37.Vedhachalam C, et al. ABCA1-induced cell surface binding sites for apoA-I. Arterioscler., Thromb., Vasc. Biol. 2007;27:1603–1609. doi: 10.1161/ATVBAHA.107.145789. [DOI] [PubMed] [Google Scholar]
- 38.Lu R, Arakawa R, Ito-Osumi C, Iwamoto N, Yokoyama S. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler., Thromb., Vasc. Biol. 2008;28:1820–1824. doi: 10.1161/ATVBAHA.108.169482. [DOI] [PubMed] [Google Scholar]
- 39.Frank PG, Marcel YL, Apolipoprotein A-I. structure-function relationships. J. Lipid Res. 2000;41:853–872. [PubMed] [Google Scholar]
- 40.Tall AR, Small DM, Deckelbaum RJ, Shipley GG. Structure and thermodynamic properties of high density lipoprotein recombinants. J. Biol. Chem. 1977;252:4701–4711. [PubMed] [Google Scholar]
- 41.Pownall HJ, Massey JB, Kusserow SK, Gotto AM., Jr Kinetics of lipid--protein interactions: interaction of apolipoprotein A-I from human plasma high density lipoproteins with phosphatidylcholines. Biochemistry. 1978;17:1183–1188. doi: 10.1021/bi00600a008. [DOI] [PubMed] [Google Scholar]
- 42.Frank PG, Marcel YL. Apolipoprotein A-I: structure-function relationships. J. Lipid Res. 2000;41:853–872. [PubMed] [Google Scholar]
- 43.Rye K-A, Barter PJ. Antiinflammatory actions of HDL: a new insight. Arterioscler., Thromb., Vasc. Biol. 2008;28:1890–1891. doi: 10.1161/ATVBAHA.108.173575. [DOI] [PubMed] [Google Scholar]
- 44.Acton S, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigueza WV, et al. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J. Biol. Chem. 1999;274:20344–20350. doi: 10.1074/jbc.274.29.20344. [DOI] [PubMed] [Google Scholar]
- 46.van Tilborg GA, et al. Annexin A5-conjugated quantum dots with a paramagnetic lipidic coating for the multimodal detection of apoptotic cells. Bioconjug. Chem. 2006;17:865–868. doi: 10.1021/bc0600463. [DOI] [PubMed] [Google Scholar]
- 47.Lanza GM, et al. Nanomedicine opportunities for cardiovascular disease with perfluorocarbon nanoparticles. Nanomedicine (London, U.K.) 2006;1:321–329. doi: 10.2217/17435889.1.3.321. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 49.O’Reilly RK, Hawker CJ, Wooley KL. Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]