Abstract
Background
Administration of an oral cephalosporin allowed advancement of the dosage of oral irinotecan. This study investigates whether administration of an oral cephalosporin increases the maximum tolerated dose (MTD) of intravenous irinotecan.
Procedure
Irinotecan was administered intravenously on Days 1– 5 and Days 8 – 12 of a 21-day cycle with continuous oral cefpodoxime starting 2 days prior to irinotecan. Cohorts of 3 to 6 pediatric patients with refractory solid tumors were enrolled at 4 dosage levels, starting at the single-agent irinotecan MTD of 20 mg/m2/dose.
Results
The 17 evaluable patients received 39 courses of therapy. None of the patients treated with 20 mg/m2/dose experienced dose-limiting toxicity (DLT). One of 6 patients treated at 30 mg/m2/dose experienced dose-limiting neutropenia. Two of 3 patients treated with 45 mg/m2/dose and 2 of 5 treated with 40 mg/m2/dose experienced dose-limiting diarrhea, with associated dehydration and anorexia. Two unconfirmed partial responses were observed after one course in a patient with Ewing sarcoma and one with paraganglioma. A child with refractory neuroblastoma had disease stabilization through 12 courses of therapy. Median (range) systemic exposure to SN-38 at the MTD (30 mg/m2/dose) was 67 ng-h/mL (36 to 111 ng-h/mL).
Conclusions
The MTD of intravenous irinotecan administered on a protracted schedule was increased by 50% from 20 to 30 mg/m2/dose with the addition of oral cefpodoxime. The most prominent DLT remained diarrhea. High interpatient variability in irinotecan pharmacokinetics was observed; however, SN-38 exposure at the MTD was greater than most reported exposures with the 20 mg/m2 dosage.
Keywords: child; adolescent; clinical trial, phase I; irinotecan; cephalosporin
Introduction
Studies in mice bearing human solid tumor xenografts have shown that camptothecin analogs are very active anticancer drugs [1]. In preclinical models, topotecan and irinotecan have shown significant activity in human soft tissue sarcomas [2], rhabdomyosarcoma [1,3], neuroblastoma[4,5], osteosarcoma,[6] and several types of brain tumors [1,7]. Furthermore, studies in these xenograft models have shown that the antitumor activity of camptothecins appears to be dose and schedule dependent; improved responses rates are seen when the agents are given daily at low doses, for protracted periods of time [e.g., daily administration for five consecutive days in two consecutive weeks [(qd × 5) × 2] [1,4,5]]. In some studies, a schedule of prolonged administration induced responses in xenografted tumors that had been unresponsive to intermittent administration of the agents at higher doses [1,7].
Not only is the schedule of irinotecan administration important in preclinical models, there appears to be a dose-response relationship at lower dosages. One study utilizing 4 xenograft models of neruoblastoma investigated the lowest dosages required for tumor response [4]. This study defined minimum irinotecan dosages administered on a protracted schedule that produce partial and complete responses. Pharmacokinetics were also obtained at these dosages to define the exposure levels associated with these levels of response. These exposure levels are higher than what is attained in children treated at the current dosage of 20 mg/m2/dose (qd × 5) × 2 [8,9].
Irinotecan is a pro-drug that requires conversion to its active metabolite SN-38 by carboxylesterases [10]. The majority of irinotecan and its metabolites are excreted in the bile. SN-38 biliary excretion is enhanced by the formation of SN-38 glucuronide (SN-38G), catalyzed primarily by the enzyme UDP-glucuronosyltransferase 1A1 (UGT1A1) [11]. Deconjugation of SN-38G by glucuronidases produced by the intestinal microflora can occur and may result in recycling of SN-38 [12]. A combination of penicillin and streptomycin prevented deglucuronidation of SN-38G in the intestine of rats [13] and ameliorated irinotecan-induced diarrhea [14]. Likewise, two studies of adult patients receiving intravenous irinotecan demonstrated less diarrhea with the addition of oral neomycin [15], or the combination of neomycin with bacitracin [16], to irinotecan-based therapy.
The dose-limiting toxicity in a pediatric phase I study of intravenous irinotecan administered [(qd × 5) × 2] was diarrhea [8]. The mechanism of irinotecan-induced diarrhea is not known, but is thought to be due to the conversion of inactive SN-38G in the intestinal lumen to SN-38. In a study of oral irinotecan in children [17], diarrhea prophylaxis with an oral cephalosporin allowed for a higher MTD that resulted in a median SN-38 lactone exposure similar to one trial of intravenous irinotecan [18] but not as high as in other studies [8,9]. Although it appears that administration of a cephalosporin can ameliorate the diarrhea caused by intravenous irinotecan [15,16], selective intestinal decontamination to allow further escalation of the MTD of intravenous irinotecan has not been prospectively studied. There are preclinical data that demonstrate that the exposures currently being attained in patients are not optimal for drug efficacy [4]. This study investigates whether use of an oral cephalosporin allows escalation of the dosage of intravenous irinotecan administered on a [(qd × 5) × 2] schedule.
Methods
Eligibility
Criteria included age ≤ 21 years, histologically verified solid tumor refractory to conventional therapy, Karnofsky or Lansky score ≥ 50%, life expectancy > 8 weeks, recovery from the acute toxic effects of prior therapy, normal bone marrow without transfusion [ (ANC) ≥ 1000/μl, platelet count ≥ 75,000/μl, and hemoglobin ≥ 8 g/dL)]; renal (creatinine level ≤ 3× normal for age); and liver function (serum bilirubin ≤ 3× normal, and ALT elevation ≤ 3× normal). For patients with CNS disease, any deficits must have been stable for at least 2 weeks with a stable or decreasing dexamethasone dosage. Pregnant or breastfeeding patients were excluded. Patients receiving anticonvulsants that interact with CYP3A (i.e., phenytoin, carbamazepine, oxcarbazepine, phenobarbital) were excluded. Additional exclusion criteria were uncontrolled infection and previous allergic reaction to penicillin or cephalosporins. Written informed consent was obtained from patients, parents, or legal guardians, with assent as appropriate. The protocol was approved by the St. Jude Institutional Review Board (IRB) and registered at ClinicalTrial.gov (NCT00143533). The IRB later approved review of the St. Jude medical records of the study participants to obtain and report data regarding those who continued with an oral cephalosporin and intravenous irinotecan after withdrawing from protocol therapy. Data obtained include (1) reason for withdrawal from protocol therapy, (2) further toxicity data, and (3) continued response information.
Drug administration and study design
Irinotecan (Camptosar™, Pfizer, New York, NY) (purchased and supplied by our institution under IND # 67,457) was diluted in 5% dextrose or 0.9% NaCl to a final concentration of 0.12– 2.8 mg/ml prior to intravenous administration at our institution over 1 hour on days 1–5 and 8–12 of a 21-day course. Oral cefpodoxime [10 mg/kg/day divided BID] was administered starting 2 days prior to the beginning of the first course and continued for as long as the patient was on study. Guidelines were provided for treating acute irinotecan-associated diarrhea with atropine and late diarrhea with loperamide. Filgrastim was not administered during the first course of therapy.
The starting dosage level of irinotecan, 20 mg/m2/dose, was the previous MTD of the intravenous [(qd × 5) × 2] schedule [8]. Subsequent planned dose levels were 30, 45, and 60 mg/m2/dose. After the 45 mg/m2/dose was found not to be tolerated, the study was amended to add an intermediate dose level of 40 mg/m2/dose. Toxicity assessment during the first course was used to assess DLT and determine the MTD. Patients without DLT nor disease progression could receive further courses at the same dosage level after recovery from non-hematologic toxicity and with ANC> 1,000/mm3 and platelets > 75,000/mm3. No intra-patient dose escalation was permitted.
At least 3 evaluable patients were treated at each dose level. If 1 of 3 patients at a given level experienced a DLT during course #1, 3 more were accrued at that level. If 2 or more of these patients experienced DLT, the maximum tolerated dose (MTD) was exceeded and the next 3 patients were treated at the next lower dose level. The MTD was the dose level at which no more than 1 patient experienced DLT and was one level less than the dose level at which 2 or more of 3–6 patients experienced DLT. At least 6 patients were treated at the MTD.
Patient evaluation
Patient medical histories and physical examinations with routine laboratory evaluations (CBCs, serum electrolytes, renal and liver function tests) were obtained before enrollment, weekly during course 1 of therapy, and before each subsequent course. Pregnancy tests (if applicable) and urinalysis were obtained prior to each course. Patients who failed to complete one course of therapy due to toxicity at least possibly attributable to protocol therapy were evaluated for toxicity but not response. Patients not completing one course of therapy for reasons unrelated to toxicity were replaced.
Adverse events were assessed using the NCI Common Terminology Criteria (CTC), version 2.0 [19]. Nonhematologic DLT was defined as any grade 3 or 4 nonhematologic toxicity attributable to the investigational drug, with the exclusion of (1) grade 3 nausea or vomiting; (2) grade 3 hepatic toxicity resolving before the second course; (3) grade 3 fever (with or without neutropenia); (4) grade 3 or 4 stomatitis lasting <72 hours; (5) grade 3 diarrhea persisting for <72 h, (6) grade 3 or 4 electrolyte abnormalities and grade 3 dehydration associated with diarrhea, vomiting or renal abnormalities. Hematologic DLT was defined as grade 4 neutropenia or thrombocytopenia for >7 days.
Patients underwent disease-appropriate evaluations within 2 weeks prior to study entry, after course 1 of therapy, and after every other course thereafter. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) [20].
Irinotecan Pharmacokinetic Studies
Irinotecan, SN-38, and SN-38G pharmacokinetics were evaluated in patients who consented to pharmacokinetic studies as previously described [21]. Pharmacokinetic studies were performed on the first and last days of irinotecan therapy during the first course. Whole blood (3 mL) samples were collected in sodium heparin tubes from a site contralateral to the irinotecan infusion site prior to the infusion, at the end of the infusion, and 0.25, 1, 2, 4, and 6 hours after the end of the infusion. The sample was centrifuged to isolate plasma and an aliquot of plasma extracted by addition to cold methanol. Irinotecan (IRN), SN-38, and SN-38G plasma concentrations were determined using high-performance liquid chromatography with fluorescence detection, which allowed for measurement of lactone and carboxylate forms.
A multicompartment model was fit to plasma concentration-time data of the lactone forms of irinotecan, SN-38, and SN-38G using the importance sampling expectation maximization algorithm in NONMEM 7. Irinotecan was modeled with a central and peripheral compartment and metabolites were modeled with a single compartment each. As previously described, elimination of irinotecan in this model was through the formation of SN-38, and elimination of SN-38 was through glucuronidation and subsequent formation of SN-38G. All data were modeled simultaneously with concentrations in molar units, although data were converted to ng/mL for plots for comparison with previous literature. Area under the plasma concentration versus time curves (AUC) for irinotecan, SN-38, and SN-38G from the beginning of the infusion to 7 hours were calculated by integration of the simulated concentration-time data from model estimates. As a measure of net conversion, ratios of the AUCs (in units of μM*h) were calculated for SN-38/irinotecan and SN-38G/SN38.
Results
Between December 2003 and December 2008, 20 patients were enrolled; three who enrolled at dosage level 3 were deemed not evaluable due to failure to complete the first course of therapy (rapid disease progression in one; toxicity unrelated to protocol therapy in two). Two of these patients were replaced. The third was not replaced because two of the five evaluable patients experienced DLT, thus ending enrollment at that level. Table I shows characteristics for the 17 evaluable patients. Ten patients received only 1 course of therapy, 2 received two courses, 3 received 3 courses, 1 patient received 4 courses, and 1 patient received 12 courses. The number of patients enrolled at each dosage level is provided in Table II.
Table I.
Characteristics of evaluable patients
| Patient Characteristics (N=17) | ||
|---|---|---|
| Characteristics | No. | % |
| Age at Protocol Enrollment, years | ||
| Median | 12.3 | |
| Range | 4.3 20.0 | |
| Sex | ||
| Male | 14 | 82.4 |
| Female | 3 | 17.6 |
| Race | ||
| White | 12 | 70.6 |
| Black | 3 | 17.6 |
| Other | 2 | 11.8 |
| Diagnosis | ||
| Neuroblastoma | 5 | 29.4 |
| Wilms Tumor | 3 | 17.6 |
| Ewing Sarcoma | 2 | 11.8 |
| Nasopharyngeal carcinoma | 2 | 11.8 |
| Malignant Peripheral Nerve Sheath Tumor | 1 | 5.9 |
| Paraganglioma | 1 | 5.9 |
| Renal cell carcinoma | 1 | 5.9 |
| Chordoma | 1 | 5.9 |
| High-grade sarcoma | 1 | 5.9 |
| Prior Therapy | ||
| Chemotherapy Regimens (16 patients with prior chemotherapy) | ||
| Median | 3 | |
| Range | 1 – 5 | |
| Radiation | 14 | 82.4 |
Table II.
Dose Levels and DLTs
| Dosage Level | Irinotecan dose (mg/m2/dose) | Pts. Enrolled (Pts. Evaluable) | DLTs | DLTs (# patients) |
|---|---|---|---|---|
| 1 | 20 | 3 (3) | 0 | None |
| 2 | 30 | 6 (6) | 1 | Neutropenia (1) |
| 3 | 45 | 8 (5) | 2 | Diarrhea (2), Dehydration (1) |
| 4 | 40 | 3 (3) | 2 | Diarrhea(2), Anorexia (1), Dehydration (1) |
There were 5 patients who continued to receive the combination of irinotecan and cefpodoxime after being removed from the protocol. Two patients were removed according to protocol guidelines for dose-limiting diarrhea during the first course, then received subsequent courses at the single-agent MTD of irinotecan (20 mg/m2/dose) with cefpodoxime until disease progression. One also received vincristine. The other three patients electively withdrew from protocol therapy because they preferred to return home. All continued the same therapy with their local oncologists at the previously assigned dosage of irinotecan with cefpodoxime. They remained in contact with study investigators and provided updates regarding significant toxicities and disease progression.
Toxicity
No DLT was observed in the first 3 patients enrolled at dosage levels 1 (20 mg/m2/dose) and 2 (30 mg/m2/dose). Two of five patients experienced dose-limiting diarrhea at level 3 (45 mg/m2/dose), therefore an additional 3 patients were accrued at level 2. Of these, 1 patient, who had bone marrow involvement with neuroblastoma, had dose-limiting neutropenia. The study was then amended to add an intermediate level of 40 mg/m2/dose. Three additional patients were added at this level 4 (40 mg/m2/dose). Of these 3 patients, 2 experienced dose-limiting diarrhea and the study was closed, thus concluding MTD at level 2 (30 mg/m2/dose). Table II summarizes dosage levels and DLTs.
Table III summarizes all toxicities greater than grade 1 that were possibly, probably, or definitely attributable to protocol therapy. The hematologic toxicities were mild with no patients requiring platelet transfusion and only 2 patients requiring packed red blood cell transfusions during course 1. There were 2 patients with uncomplicated febrile neutropenia during course 1; no patients experienced febrile neutropenia during the 22 later courses. Both of the patients with febrile neutropenia were removed from protocol therapy after the first course, one due to hematologic DLT (prolonged neutropenia) and the other due to progressive disease.
Table III.
Toxicities (possibly, probably or definitely attributed to protocol therapy)
| Course 1 N=17 |
Courses 2 to 12 N=22 |
|||||
|---|---|---|---|---|---|---|
| Grade |
Grade |
|||||
| 2 | 3 | 4 | 2 | 3 | 4 | |
| Hematologic Toxicities | ||||||
|
| ||||||
| Hemoglobin | 2 | |||||
| Leukocytes | 3 | 2 | 9 | |||
| Neutrophils/granulocytes | 5 | 2 | 5 | |||
| Transfusion:pRBCs | 2 | |||||
|
| ||||||
| Non-Hematologic Toxicities | ||||||
|
| ||||||
| Abdominal pain or cramping | 2 | 3 | ||||
| Anorexia | 1 | |||||
| Bone Pain | 1 | |||||
| Dehydration | 1 | 2 | ||||
| Diarrhea (without colostomy) | 6 | 7 | ||||
| Febrile neutropenia | 2 | |||||
| Hyperkalemia | 1 | |||||
| Hypokalemia | 4 | 1 | 2 | |||
| Hyponatremia | 2 | |||||
| Hypophosphatemia | 1 | |||||
| Nausea | 5 | 1 | ||||
| Stomatitis/pharyngitis (oral pharyngeal mucositis) | 1 | |||||
| Vomiting | 5 | |||||
The most common grade 3 nonhematologic toxicities were diarrhea (33.3% of all courses), nausea (15.4% of all courses), and vomiting (12.8% of all courses). Interestingly, although the rate of grade 3 diarrhea did not significantly change between course 1 (35.3%) and subsequent courses (31.8%), there was almost no significant nausea and vomiting in subsequent courses; in contrast, 29% of patients experienced grade 3 nausea and vomiting during course 1. Most of the other non-hematologic toxicities, such as electrolyte disturbances and dehydration, were related to the gastrointestinal symptoms. There were no incidences of Clostridium difficile toxin positive stool. One patient was positive for Clostridium difficile toxin prior to beginning therapy, but never had the toxin detected again despite 12 courses of therapy. This patient also had colonization with vancomycin-resistant enterococci (VRE) prior to starting therapy that cleared while receiving twice-daily cefpodoxime. One of the 5 patients who continued with irinotecan therapy after withdrawal from the protocol experienced diarrhea related to Clostridium difficile that cleared while continuing cefpodoxime and irinotecan.
Antitumor activity
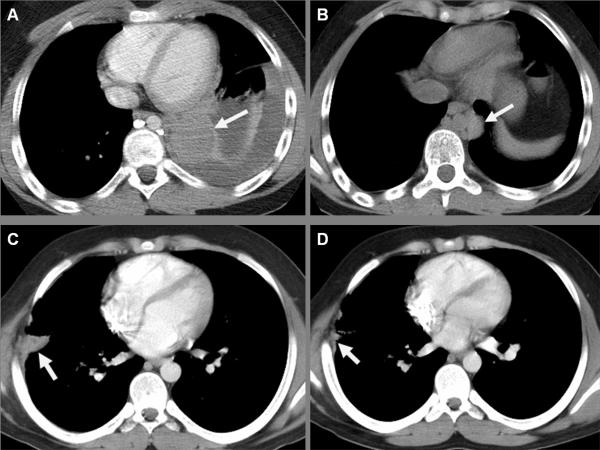
Two patients met the imaging criteria for partial response after course 1 as illustrated in Figure 1, but neither received a second course on protocol and thus the responses were not confirmed. One patient had a partial response by imaging of his Ewing sarcoma and maintained that response through 5 more courses of irinotecan (reduced from 45 mg/m2/dose to 20 mg/m2/dose by the treating physician after removal from protocol therapy for DLT) until choosing to pursue alternative therapy. A patient with a partial response of his metastatic paraganglioma electively withdrew from the protocol after the first course but continued the same treatment (irinotecan 30 mg/m2/dose with cefpodoxime) for 11 more courses until disease progression. All of the subsequent imaging of these 2 patients was performed at our institution. One child with primary refractory neuroblastoma had disease stabilization through 12 courses of therapy at dosage level 2 until electively withdrawing from therapy. Another two patients had disease stabilization while receiving protocol therapy and received the therapy off-protocol until disease progression: one with recurrent neuroblastoma treated at dosage level 1 for a total of 8 courses (4 on protocol) and one with chordoma treated at dosage level 4 for a total of 5 courses (3 on protocol).
Figure 1.
Thoracic CT of partial responses of recurrent metastatic Ewing sarcoma (A and B) and of metastatic paraganglioma (C and D) after one course of therapy. Post-contrast CT images show a pulmonary metastasis of Ewing sarcoma in the left lower lobe (arrow) with associated pleural effusion prior to therapy (A) and a decrease in the size of the solid metastasis with resolution of the effusion after therapy (B). Likewise, a metastatic deposit of paraganglioma in the right chest wall (arrows) evident prior to enrollment (C) decreased in size after the first course (D).
Pharmacokinetics
Pharmacokinetic parameters were analyzed for 11 participants. The other 9 patients either did not consent to pharmacokinetic studies, consented but had poor venous access, or did not have samples obtained due to logistical considerations. The median (range) for the irinotecan systemic clearance and volume of distribution was 11.8 L/h/m2 (9.9 to 16.5 L/hr/m2) and 105.4 L/m2 (102.7 to 107.7 L/m2). The AUC values for irinotecan, SN-38, and SN-38 glucuronide are presented in Table IV. The median (range) relative extent of conversion of irinotecan and relative extent of glucuronidation of SN-38 was 0.09 (0.06 to 0.17) and 2.0 (0.71 to 6.05).
Table IV.
Median (range) AUC values for irinotecan, SN-38 lactone, and SN-38G lactone after intravenous irinotecan administration
| Dosage (mg/m2/d) | N | IRN AUC0→7 (ng*h/mL) | SN-38 AUC0→7 (ng*h/mL) | SN-38G AUC0→7 (ng*h/mL) |
|---|---|---|---|---|
| 20 | 2 | 270, 464 | 14, 40 | 123, 174 |
| 30 | 4 | 814 (491–979) | 67 (36 – 111) | 191 (89 – 268) |
| 40 | 1 | 990 | 62 | 131 |
| 45 | 4 | 1057 (894 – 1354) | 53 (49 – 58) | 127 (73 –171) |
N = number of patients; AUC: area under the concentration-time curve; IRN: irinotecan.
Discussion
This phase I study demonstrates that the MTD of intravenous irinotecan administered to children with refractory solid tumors on a [(qd × 5) × 2] schedule could be increased by 50% to 30 mg/m2/dose with the addition of twice daily cefpodoxime given orally. The regimen was tolerated well, with diarrhea (and associated dehydration and anorexia in some patients) as the major dose-limiting toxicity. The most notable other toxicities were nausea and vomiting during course 1 that appeared to improve with subsequent courses. Hematologic toxicity was manageable. Our study is the first prospective trial to demonstrate that concomitant administration of an oral cephalosporin allows advancement in the dosage of intravenous irinotecan similar to findings with oral irinotecan [17].
Irinotecan, as a single-agent or in combination with other chemotherapeutic agents, has been effective in treating a variety of childhood solid tumors [9,22,23] with diarrhea as the predominant toxicity. As reviewed by Wagner et al [24], there have been a variety of approaches to the prevention of irinotecan-associated diarrhea, including attempts to diminish biliary excretion with cyclosporine [25], decrease intestinal absorption with activated charcoal [26], minimize colonic inflammation with budesonide or thalidomide [27,28], and prevent colonic injury with amifostine or glutamine [29,30]. In a randomized study in tumor-bearing rats receiving irinotecan [14], modulation of glucuronidase activity was more effective than activated charcoal or cyclosporine in ameliorating irinotecan-associated diarrhea. Similarly, studies in humans have shown that targeting glucuronidase production [15–17] or activity [31] improves irinotecan-associated diarrhea. Few studies have attempted to actually increase the irinotecan dosage with any of these approaches. Using activated charcoal, investigators were able to deliver more of the prescribed doses of irinotecan [26] and decrease the number of patients who developed diarrhea [32], but neither study attempted to increase the dosage. Two phase I studies in children [33,34] have included an oral cephalosporin when trying to combine intravenous irinotecan with other agents, but neither was able to advance the irinotecan dosage past 20 mg/m2/day. This study also supports that antibiotic use, to presumably decrease the levels of intestinal glucuronidase, is an effective mechanism for ameliorating irinotecan-associated diarrhea and even allows further escalation of the dosage.
Colonization resistance is the concept that the anaerobic bacterial flora in the gastrointestinal tract inhibits the adherence, colonization, and proliferation of potentially pathogenic aerobic flora [35]. The goal of selective intestinal decontamination is to administer an antibiotic that spares the commensal anaerobic flora while eliminating the potential pathogenic aerobic flora (or aerobic glucuronidase-producing flora in this study), thereby preserving colonization resistance. Original work done in patients with neutropenia utilized nonabsorbable antibiotics as prophylactic agents [36]. In recent years, however, the use of broad-spectrum absorbable antibiotics like fluoroquinolones has become a common practice when chemoprophylaxis in neutropenic patients is given [37]. As with any prolonged administration of oral antibiotics, there is an increased risk of gram-positive infections and the potential for antibiotic resistance [37]. Risk of systemic absorption and subsequent renal damage with prolonged use of neomycin (>2 weeks) [38] renders it a less attractive candidate for prolonged selective intestinal decontamination. A third-generation oral cephalosporin has the advantage of being effective against Escherichia coli [39], a known producer of glucuronidase [40], while not eliminating the anaerobes that are important for maintaining colonization resistance. A previous study demonstrated that administration of a cephalosporin decreased stool bacterial beta-glucuronidase [17] without inducing significant rates of colonization by pathogens (such as Clostridium difficile) or resistant organisms such as VRE [17,41,42]. We did not see increased infections with fungi as can occur with prolonged antibiotic use and disruption of colonization resistance. Neither did we observe increased infection with gram-positive organisms. Thus, it appears that selective intestinal decontamination was achieved.
Because all patients were pretreated and had a variety of diagnoses, it is difficult to accurately estimate the activity of this regimen. Pharmacokinetics are sometimes useful to estimate whether patients are being exposed to effective levels of a chemotherapy agent. However, as reviewed by Deeken et al [43], the pharmacokinetics and pharnacodynamics of irinotecan are quite complex, involving many overlapping metabolic pathways. The best-described source of pharmacokinetic variation is the polymorphisms of uridine diphosphate glucuronosyltransferease 1A1 (UGT1A1), but there are many other genes involved in the metabolism of irinotecan currently under study [43]. This unpredictable variability in the pharmacokinetics of irinotecan is depicted in Table V; exposure to the active form of irinotecan, SN-38, varies widely among patients, even when the same dosage is administered by the same route. For the intravenous dose of 20 mg/m2/d, the median SN-38 lactone AUC ranged from 14.8 to 106 ng-h/mL with the absolute range of 7.8 to 421 ng-h/mL. In our study, the median AUC of the 30 mg/m2/d dose was 67 ng-h/mL, which is greater than the median AUC achieved with a dose of 20 mg/m2/d in 3 of the 4 referenced studies. The range of the AUC of SN-38 lactone at the intravenous MTD of this study was greater than that reported when oral irinotecan is administered at a dose of 60 mg/m2/d in one study [17] and similar to that of another [42]. The range of SN-38 lactone AUC associated with partial responses in neuroblastoma xenograft models was 99 to 129 ng-h/mL [4]. Thus, some patients are achieving this level of exposure, but not all. Given this wide interpatient variability, no study has demonstrated clearly that there is a definitive level of SN-38 exposure associated with tumor response. However, it is reasonable to infer that there could be some threshold of exposure that should be achieved for irinotecan to be effective as was demonstrated in the neuroblastoma xenograft models [4]. By increasing the dosage of irinotecan to 30 mg/m2/d, exposure to SN-38 is potentially increased, thus increasing the likelihood that the threshold of effective exposure is reached. A phase II study is required to formally assess the activity of this regimen.
Table V.
Comparison of published median (range) AUC values for SN-38 lactone.
| Dosage (mg/m2/d) | N | Route | SN-38 AUC 0→7 (ng*h/mL) | SN-38G AUC0→7 (ng*h/mL) | Reference |
|---|---|---|---|---|---|
| 30 | 4 | IV | 67 (36 – 111) | 191 (89 – 268) | Current study |
| 20 | 8 | IV | 106 (41 – 421) | [8] | |
| 20 | 4 | IV | 14.8 (7.8–42.8) | 73.1 (39.5–85.5) | [9] |
| 20 | 10 | IV + VCR | 32.9 (25.5–77.6) | 46.1 (24.2–234.9) | [9] |
| 20 | 21 | IV | 28.4 (9–119.1) | 80.8 (42.3–160.9) | [21] |
| 40 | 7 | PO | 11.1 (3.8–17.9) | 14.2 (8.9–20.3) | [17] |
| 60 | 7 | PO+CFX | 20.8 (11.2–28.9) | 42.6 (31.1–118.1) | [17] |
| 60 | 5 | PO+TMZ+ CFX | 69.5 (35.2–86.5) | 45.4 (21.8–238.9) | [42] |
N = number of patients; AUC: area-under the concentration-time curve; IV: intravenous; PO: by mouth; VCR:vincristine; CFX: cefixime; TMZ:temozolomide;
Consistent with the fact that irinotecan exerts its effect during the S phase of the cell cycle [44], many of the published responses of pediatric tumors to irinotecan have been from studies using the protracted schedule [8,9,18,22]. More recently, a randomized study in children with relapsed rhabdomyosarcoma [45] demonstrated comparable response rates to vincristine combined with irinotecan 50 mg/m2/day for 5 days [qd × 5] versus irinotecan 20 mg/m2/day on the protracted [(qd × 5) × 2] schedule. Based on this finding, many pediatric studies are transitioning to a 5-day regimen of irinotecan [41]. Since the DLT of the [qd × 5] regimen was diarrhea in less-heavily pretreated children [46], use of a concurrent oral cephalosporin may be useful to escalate the irinotecan dosage on that regimen as well. As was demonstrated with a 5-day oral irinotecan regimen [41], it may be possible to decrease the length of antibiotic administration while still advancing the dosage of the 5-day intravenous irinotecan. A reduction in number of days of cephalosporin administration may decrease the risk of antibiotic-associated toxicity and the emergence of resistance bacteria.
In this study, there was evidence of antitumor activity with irinotecan as a single agent in combination with a cephalosporin. It is important to note that when irinotecan was tested as a single agent in children with rhabdomyosarcoma, there was an unacceptable number of patients with disease progression [9]. When vincristine was added to the regimen, the percentage of patients with disease progression decreased from 32% to 8% [9]. Thus irinotecan is combined with other cytotoxic agents, such as vincristine [9,41] or temozolomide [47,48], or with targeted therapies such as antiangiogenesis agents [49–51] or agents directed against epidermal growth factor signaling [52,53]. In some of these combinations, irinotecan could not be administered at the single agent MTD [18,53]. Diarrhea was one of the dose-limiting toxicities in both of those studies. Addition of a cephalosporin to these and other combinations might allow advancement of the irinotecan dosage, but there is also a risk of new toxicities with addition of another drug. Vigilance will be needed to identify any emerging toxicities from adding another drug to irinotecan combinations.
In summary, the purpose of this study was to investigate if the MTD of irinotecan administered on a protracted intravenous schedule could be increased with selective intestinal decontamination using an oral cephalosporin. We were able to effectively increase the MTD by 50% using this approach, resulting in an increase in SN-38 exposure. There was evidence of antitumor activity as demonstrated by two patients with partial responses of their disease and several others with disease stabilization. Future uses of these results include attempts to advance the irinotecan dose on the same schedule when combined with other agents, such as vincristine or temozolomide, and potentially advancing the dosage of the alternate 5-day irinotecan regimen.
Acknowledgements
This study was supported by Childhood Cancer Solid Tumor Program Project Grant CA23099 and Cancer Center Support Grant CA21765 from the US National Institutes of Health (NIH), and by the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to thank Dana Hawkins, Kathleen Campbell, Debbie Poe, and Amy Sanders for their assistance with the conduct of the study and data collection; Dr. Alberto Pappo for reviewing the manuscript; Brenda Clark for assistance with manuscript preparation; and all of the patients and their parents that participated in the study.
Footnotes
Conflict of Interest Statement The authors have no financial relationships that would impact the design, conduct, or reporting of this study.
References
- 1.Houghton PJ, Cheshire PJ, Hallman JD, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol. 1995;36(5):393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- 2.Jansen WJ, Kolfschoten GM, Erkelens CA, et al. Anti-tumor activity of CPT-11 in experimental human ovarian cancer and human soft-tissue sarcoma. Int J Cancer. 1997;73(6):891–6. doi: 10.1002/(sici)1097-0215(19971210)73:6<891::aid-ijc22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik CA, Houghton PJ, Stewart CF, et al. Effective schedules of exposure of medulloblastoma and rhabdomyosarcoma xenografts to topotecan correlate with in vitro assays. Clin Cancer Res. 1998;4(8):1995–2002. [PubMed] [Google Scholar]
- 4.Thompson J, Zamboni WC, Cheshire PJ, et al. Efficacy of systemic administration of irinotecan against neuroblastoma xenografts. Clin Cancer Res. 1997;3(3):423–31. [PubMed] [Google Scholar]
- 5.Zamboni WC, Stewart CF, Thompson J, et al. Relationship between topotecan systemic exposure and tumor response in human neuroblastoma xenografts. J Natl Cancer Inst. 1998;90(7):505–11. doi: 10.1093/jnci/90.7.505. [DOI] [PubMed] [Google Scholar]
- 6.Houghton PJ, Cheshire PJ, Myers L, et al. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol. 1992;31(3):229–39. doi: 10.1007/BF00685553. [DOI] [PubMed] [Google Scholar]
- 7.Vassal G, Boland I, Santos A, et al. Potent therapeutic activity of irinotecan (CPT-11) and its schedule dependency in medulloblastoma xenografts in nude mice. Int J Cancer. 1997;73(1):156–63. doi: 10.1002/(sici)1097-0215(19970926)73:1<156::aid-ijc24>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17(6):1815–24. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 9.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children's Oncology Group. J Clin Oncol. 2007;25(4):362–9. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 10.Akimoto K, Kawaii M, Ohya K. Kinetic studies of the hydrolysis and lactonization of camptothecin and its derivatives. Chemother Pharmacol Bull. 1994;42:2135–8. [Google Scholar]
- 11.Iyer L, King CD, Whitington PF, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101(4):847–54. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneda N, Nagata H, Furuta T, et al. Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Research. 1990;50(6):1715–20. published erratum appears in Cancer Res 1990 Jul 15;50(14):4451. [PubMed] [Google Scholar]
- 13.Takasuna K, Hagiwara T, Hirohashi M, et al. Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother Pharmacol. 1998;42(4):280–6. doi: 10.1007/s002800050818. [DOI] [PubMed] [Google Scholar]
- 14.Takasuna K, Hagiwara T, Watanabe K, et al. Optimal antidiarrhea treatment for antitumor agent irinotecan hydrochloride (CPT-11)-induced delayed diarrhea. Cancer Chemother Pharmacol. 2006;58(4):494–503. doi: 10.1007/s00280-006-0187-8. [DOI] [PubMed] [Google Scholar]
- 15.Kehrer DF, Sparreboom A, Verweij J, et al. Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res. 2001;7(5):1136–41. [PubMed] [Google Scholar]
- 16.Alimonti A, Satta F, Pavese I, et al. Prevention of irinotecan plus 5-fluorouracil/leucovorin-induced diarrhoea by oral administration of neomycin plus bacitracin in first-line treatment of advanced colorectal cancer. Ann Oncol. 2003;14(5):805–6. doi: 10.1093/annonc/mdg193. [DOI] [PubMed] [Google Scholar]
- 17.Furman WL, Crews KR, Billups C, et al. Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006;24(4):563–70. doi: 10.1200/JCO.2005.03.2847. [DOI] [PubMed] [Google Scholar]
- 18.Wagner LM, Crews KR, Iacono LC, et al. Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res. 2004;10(3):840–8. doi: 10.1158/1078-0432.ccr-03-0175. [DOI] [PubMed] [Google Scholar]
- 19.Common Terminology Criteria (CTC) Cancer Therapy Evaluation Program. version 2.0 U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: Apr 30, 1999. [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Crews KR, Stewart CF, Jones-Wallace D, et al. Altered irinotecan pharmacokinetics in pediatric high-grade glioma patients receiving enzyme-inducing anticonvulsant therapy. Clin Cancer Res. 2002;8(7):2202–9. [PubMed] [Google Scholar]
- 22.Bisogno G, Riccardi R, Ruggiero A, et al. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer. 2006;106(3):703–7. doi: 10.1002/cncr.21629. [DOI] [PubMed] [Google Scholar]
- 23.Shitara T, Shimada A, Tsuchida Y, et al. Successful clinical response to irinotecan in relapsed neuroblastoma. Med Pediatr Oncol. 2003;40(2):126–8. doi: 10.1002/mpo.10104. [DOI] [PubMed] [Google Scholar]
- 24.Wagner LM, Crews KR, Stewart CF, et al. Reducing irinotecan-associated diarrhea in children. Pediatr Blood Cancer. 2008;50(2):201–7. doi: 10.1002/pbc.21280. [DOI] [PubMed] [Google Scholar]
- 25.Desai AA, Kindler HL, Taber D, et al. Modulation of irinotecan with cyclosporine: a phase II trial in advanced colorectal cancer. Cancer Chemother Pharmacol. 2005;56(4):421–6. doi: 10.1007/s00280-005-1020-5. [DOI] [PubMed] [Google Scholar]
- 26.Michael M, Brittain M, Nagai J, et al. Phase II study of activated charcoal to prevent irinotecan-induced diarrhea. J Clin Oncol. 2004;22(21):4410–7. doi: 10.1200/JCO.2004.11.125. [DOI] [PubMed] [Google Scholar]
- 27.Karthaus M, Ballo H, Abenhardt W, et al. Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (CPT-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology. 2005;68(4–6):326–32. doi: 10.1159/000086971. [DOI] [PubMed] [Google Scholar]
- 28.Govindarajan R, Heaton KM, Broadwater R, et al. Effect of thalidomide on gastrointestinal toxic effects of irinotecan. Lancet. 2000;356(9229):566–7. doi: 10.1016/s0140-6736(00)02586-1. [DOI] [PubMed] [Google Scholar]
- 29.Savarese D, Al Zoubi A, Boucher J. Glutamine for irinotecan diarrhea. J Clin Oncol. 2000;18(2):450–1. doi: 10.1200/JCO.2000.18.2.450. [DOI] [PubMed] [Google Scholar]
- 30.Delioukina ML, Prager D, Parson M, et al. Phase II trial of irinotecan in combination with amifostine in patients with advanced colorectal carcinoma. Cancer. 2002;94(8):2174–9. doi: 10.1002/cncr.10432. [DOI] [PubMed] [Google Scholar]
- 31.Mori K, Kondo T, Kamiyama Y, et al. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51(5):403–6. doi: 10.1007/s00280-003-0585-0. [DOI] [PubMed] [Google Scholar]
- 32.Sergio GC, Felix GM, Luis JV. Activated charcoal to prevent irinotecan-induced diarrhea in children. Pediatr Blood Cancer. 2008;51(1):49–52. doi: 10.1002/pbc.21491. [DOI] [PubMed] [Google Scholar]
- 33.McGregor LM, Spunt SL, Furman WL, et al. Phase 1 study of oxaliplatin and irinotecan in pediatric patients with refractory solid tumors: a Children's Oncology Group study. Cancer. 2009;115(8):1765–75. doi: 10.1002/cncr.24175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNall-Knapp RY, Williams CN, Reeves EN, et al. Extended phase I evaluation of vincristine, irinotecan, temozolomide, and antibiotic in children with refractory solid tumors. Pediatr Blood Cancer. 2010;54(7):909–15. doi: 10.1002/pbc.22460. [DOI] [PubMed] [Google Scholar]
- 35.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38(3):409–14. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yates JW, Holland JF. A controlled study of isolation and endogenous microbial suppression in acute myelocytic leukemia patients. Cancer. 1973;32(6):1490–8. doi: 10.1002/1097-0142(197312)32:6<1490::aid-cncr2820320628>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Patrick CC. Use of fluoroquinolones as prophylactic agents in patients with neutropenia. Pediatr Infect Dis J. 1997;16(1):135–9. doi: 10.1097/00006454-199701000-00038. [DOI] [PubMed] [Google Scholar]
- 38.Neomycin sulfate tablet [X-GEN Pharmaceuticals, Inc.] Daily Med. National Library of Medicine (US); Sep 6, 2010. 2010. [Google Scholar]
- 39.Knothe H, Shah PM, Eckardt O. Cefpodoxime: comparative antibacterial activity, influence of growth conditions, and bactericidal activity. Infection. 1991;19(5):370–6. doi: 10.1007/BF01645371. [DOI] [PubMed] [Google Scholar]
- 40.McBain AJ, Macfarlane GT. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J Med Microbiol. 1998;47(5):407–16. doi: 10.1099/00222615-47-5-407. [DOI] [PubMed] [Google Scholar]
- 41.Wagner LM, Perentesis JP, Reid JM, et al. Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children's Oncology Group phase I consortium study. Pediatr Blood Cancer. 2010;54(4):538–45. doi: 10.1002/pbc.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner LM, Villablanca JG, Stewart CF, et al. Phase I trial of oral irinotecan and temozolomide for children with relapsed high-risk neuroblastoma: a new approach to neuroblastoma therapy consortium study. J Clin Oncol. 2009;27(8):1290–6. doi: 10.1200/JCO.2008.18.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeken JF, Slack R, Marshall JL. Irinotecan and uridine diphosphate glucuronosyltransferase 1A1 pharmacogenetics: to test or not to test, that is the question. Cancer. 2008;113(7):1502–10. doi: 10.1002/cncr.23777. [DOI] [PubMed] [Google Scholar]
- 44.Del BG, Lassota P, Darzynkiewicz Z. The S-phase cytotoxicity of camptothecin. Exp Cell Res. 1991;193(1):27–35. doi: 10.1016/0014-4827(91)90534-2. [DOI] [PubMed] [Google Scholar]
- 45.Mascarenhas L, Lyden ER, Breitfeld PP, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2010;28(30):4658–63. doi: 10.1200/JCO.2010.29.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: a pediatric oncology group study. Clin Cancer Res. 2001;7(1):32–7. [PubMed] [Google Scholar]
- 47.Bagatell R, London WB, Wagner LM, et al. Phase II Study of Irinotecan and Temozolomide in Children With Relapsed or Refractory Neuroblastoma: A Children's Oncology Group Study. J Clin Oncol. 2011;29(2):208–13. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48(2):132–9. doi: 10.1002/pbc.20697. [DOI] [PubMed] [Google Scholar]
- 49.Aguilera DG, Goldman S, Fangusaro J. Bevacizumab and irinotecan in the treatment of children with recurrent/refractory medulloblastoma. Pediatr Blood Cancer. 2011;56(3):491–4. doi: 10.1002/pbc.22868. [DOI] [PubMed] [Google Scholar]
- 50.Gururangan S, Chi SN, Young PT, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069–75. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–5. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 52.Furman WL, Navid F, Daw NC, et al. Tyrosine kinase inhibitor enhances the bioavailability of oral irinotecan in pediatric patients with refractory solid tumors. J Clin Oncol. 2009;27(27):4599–604. doi: 10.1200/JCO.2008.19.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trippett TM, Herzog C, Whitlock JA, et al. Phase I and pharmacokinetic study of cetuximab and irinotecan in children with refractory solid tumors: a study of the pediatric oncology experimental therapeutic investigators' consortium. J Clin Oncol. 2009;27(30):5102–8. doi: 10.1200/JCO.2008.20.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]