Abstract
Advances in nanoparticle contrast agents for molecular imaging have made magnetic resonance imaging a promising modality for noninvasive visualization and assessment of vascular and cardiac disease processes. This review provides a description of the various nanoparticles exploited for imaging cardiovascular targets. Nanoparticle probes detecting inflammation, apoptosis, extracellular matrix, and angiogenesis may provide tools for assessing the risk of progressive vascular dysfunction and heart failure. The utility of nanoparticles as multimodal probes and/or theranostic agents has also been investigated. Although clinical application of these nanoparticles is largely unexplored, the potential for enhancing disease diagnosis and treatment is considerable.
INTRODUCTION
Atherosclerosis is a chronic inflammatory response of arterial blood vessels where deposits of fatty substances, cholesterol, cellular waste products, calcium, and other substances build up in the inner lining of an artery, forming an atherosclerotic plaque. The plaque may further progress and ultimately rupture, leading to thrombus formation, which can occlude arteries and cause serious clinical events such as myocardial infarction and stroke when the coronary circulation supplying the heart and the cerebral circulation supplying the brain, respectively, are blocked.1
In order to assess atherosclerotic plaques and myocardial infarctions, several invasive (e.g., X-ray angiography, intravascular ultrasound, and angioscopy) and noninvasive (surface B-mode ultrasound and ultrafast computed tomography) imaging techniques have been developed.2 Among the many imaging techniques, high-resolution magnetic resonance (MR) has emerged as a valuable imaging modality for characterization of atherosclerotic arteries and infarct regions in a noninvasive and nondestructive way with high soft tissue contrast. The soft tissue contrast in MR imaging is generated by biophysical and biochemical parameters such as chemical composition and concentration, water content, physical state, molecular motion, and diffusion.3 Although MR imaging can noninvasively produce high spatial resolution images rich in anatomical information, it has significantly lower sensitivity to contrast agents than positron emission tomography (PET), single photon emission computed tomography (SPECT), and fluorescence techniques. On the other hand, SPECT, PET, and fluorescence do not provide anatomical information.4 Therefore, a multimodal imaging approach is often necessary where the strengths of several modalities are exploited to fully validate the results of molecular imaging.
During the development of atherosclerotic plaques, many potential biomarkers, such as adhesion molecules [vascular cell adhesion molecules (VCAMs), intercellular adhesion molecules (ICAMs), selectins], macrophages and their scavenger receptors, matrix metalloproteinases (MMPs), oxidized low-density lipoprotein (oxLDL), αvβ3 integrin, extracellular matrix, and fibrin (summarized in a review by Lipinski et al.5), are upregulated. In the infarcted myocardium, inflammation, angiogenesis, MMPs, thrombin-activated factor XIII (FXIII), apoptosis/necrosis, and extracellular matrix are important factors in the processes of remodeling after injury.6-11 It is important to point out that these molecules are often not unique to cardiovascular diseases, but they are present at increased levels under these disease conditions as compared to disease-free conditions. Moreover, these molecular targets are often present at relatively low levels (10−9 to 10−13 M/g tissue). To overcome sensitivity issues, high payload contrast agent vehicles are desired for molecular MR imaging in order to generate sufficient signal change. Nanoparticle-facilitated imaging is the most promising approach for molecular MR imaging purposes, since nanoparticles exhibit the possibility to include a high contrast agent payload, may be of relatively small size to facilitate penetration into tissue, and have a tunable circulation half-life, a large surface area available for conjugation of functional groups, and the potential to function as both imaging and therapeutic (i.e. ‘theranostic’) agents.
Two categories of contrast agents are frequently used for molecular MR imaging of atherosclerotic plaques and myocardial infarctions: (1) superparamagnetic iron oxide (SPIO) nanoparticles and (2) nanoparticles that incorporate gadolinium (Gd) chelates. The contrast agents change the longitudinal and transverse relaxation times (T1 and T2, respectively), and thus affect relaxation rates (R1 = 1/T1; R2 = 1/T2). The efficiency of contrast agents is described by relaxivity (r1 for longitudinal relaxivity, r2 for transverse relaxivity), which is defined as , where i = 1 or 2 and [CA] is the concentration of contrast agents. SPIO nanoparticles have r2/r1 ratios much higher than 1 (usually higher than 10) and therefore are more suitable for T2- and T2*-weighted MR imaging, while Gd chelates have r2/r1 ratios close to 1 (typically between 1.1 and 2) and are exploited to create MR signal enhancement in T1-weighted imaging. Several nanoparticle platforms have been developed for target-specific imaging of cardiac disease and include dextran-coated iron oxides,12 micelles,13 liposomes,14,15 oil-in-water emulsions,16,17 and lipoproteins18-21 as illustrated in Figure 1. In this review, we will focus on recent work that used nanoparticles for molecular MR imaging of vascular and cardiac diseases.
FIGURE 1.
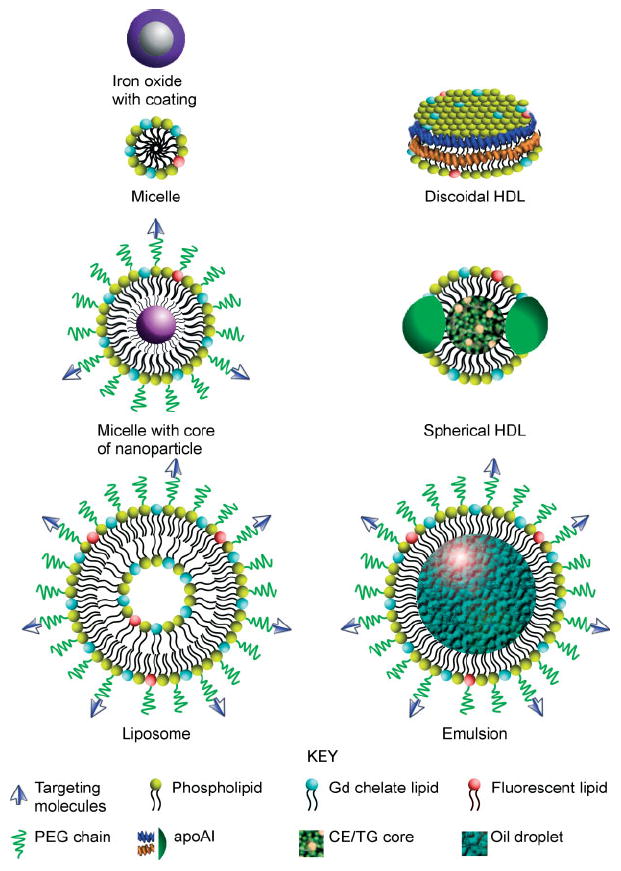
Schematic structures for several nanoparticles: iron oxide with surface coating, micelle, micelle with core of nanoparticles, liposome, discoidal high-density lipoprotein (HDL), spherical HDL, and oil-in-water emulsion. (Targeting molecules: ligands, peptides, and antibodies; apoAI: apolipoprotein A I; CE: cholesteryl ester; TG: triglyceride).
SUPERPARAMAGNETIC IRON OXIDE NANOPARTICLES
SPIO nanoparticles are composed of iron oxide nanocrystals and a coating material such as dextran that prevents aggregation of the iron oxide cores and improves biocompatibility. There are four different categories of SPIO nanoparticles based on their size: large (>200 nm), standard (60–150 nm), ultrasmall (USPIO, 10–40 nm), and monocrystalline (MION, 10–30 nm) agents.22 The association of iron oxide particles with cells can occur via passive or active targeting. Dextran-coated USPIO nanoparticles are inherently sequestered by monocytes/macrophages because of phagocytosis, which is therefore referred as passive targeting to monocytes/macrophages.23,24 This is valuable as high macrophage content is considered a hallmark of plaque vulnerable to rupture.1 Ruehm et al. were the first to show that once these particles are internalized within the intraplaque macrophages, the high magnetization associated with the iron oxide crystal core induces significant T2*-weighted MR signal loss, allowing the detection of plaques that are macrophage rich.25 Several reports have shown that active intraplaque macrophages can readily phagocytose iron oxide particles that diffuse or migrate into the atherosclerotic plaque in preclinical studies,25-34 while dextran-coated USPIOs have also been used clinically to target intraplaque macrophages.29,35-39 In Europe, Combidex (Sinerem/AMI-227, Guerbet) is typically infused (over 30 min) into patients at a dose of 2.6 mg Fe/kg of bodyweight. Iron oxide MR imaging may be used as a surrogate end point in clinical trials designed to test the efficacy of novel cardiovascular therapeutics.40 In these studies signal loss in T2/T2*-weighted MR images is usually observed at late time points (>24 h) after injection of iron oxide. To optimize the detection of low-concentration molecular targets, the relaxivity of iron oxide particles can be further increased by manganese doping.41
The size of iron oxide nanoparticles affects passive targeting to intraplaque macrophages. Feridex has a mean size of ~97 nm and, in a 2008 study, was not observed to accumulate in intraplaque macrophages.42 However, Feridex can be fractionated to isolate ultrasmall particles of 12 nm. After injection of a 4.8 mg Fe/kg of bodyweight dose, significant uptake of this fractionated Feridex was observed in intraplaque macrophages in a rabbit model (Figure 2).42 This size effect can be attributed to easier diffusion of these small nanoparticles into atherosclerotic plaques and their long blood half-life. Small nanoparticles (10–100 nm) are more likely to extravasate from the vasculature and accumulate into diseased tissue via the enhanced permeability and retention (EPR) effect43-47 than large particles (100–500 nm). This is an important aspect to consider because the nonspecific accumulation of nanoparticles contributes to signal attenuation and may result in an overestimation of the actual receptor expression. On the contrary, molecular imaging of an extravascular target requires nanoparticles to escape the blood vessels, which favors the use of small nanoparticles.
FIGURE 2.
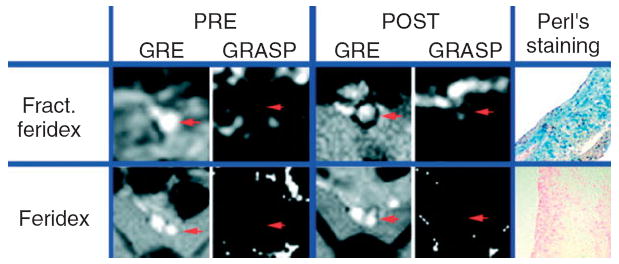
Typical gradient echo (GRE) and GRE acquisition for superparamagnetic particles (GRASP) images of the rabbit aorta (red arrow) pre- and 24 h postinjection of fractionated Feridex or Feridex at 4.8 mg Fe/kg of bodyweight. (Reprinted with permission from Ref 42. Copyright 2008 John Wiley and Sons, Ltd).
In addition to passively targeting monocytes/macrophages, iron oxide particles have also been modified to actively target molecular markers. Nahrendorf et al. functionalized monocrystalline iron oxide nanoparticles with vascular cell adhesion molecule-1 (VCAM-1) targeting peptides.48 In vivo MR imaging revealed that the aortic root of apoE−/− mice became hypointense (dark) after injection of these nanoparticles, which was confirmed through fluorescence imaging to be due to nanoparticle accumulation in cells that overexpressed VCAM-1 (Figure 3). Kang et al. have functionalized cross-linked iron oxide (CLIO) nanoparticles with anti-human E-selectin antibody fragments to detect E-selectin in endothelial cells.49 A three times greater CLIO-induced MR signal decrease on T2*-weighted images was observed in human umbilical vein endothelial cells implanted into mice in response to interleukin-1β (a cytokine that induces E-selectin expression) treatment compared to untreated controls.49 In addition, a dual targeted strategy has been used to image endothelial adhesion molecules in apoE−/− mice,50 where microparticles of iron oxide were conjugated with monoclonal antibodies against VCAM-1 (VCAM-MPIO) and/or P-selectin (P-selectin-MPIO). Using ex vivo MR imaging, dual targeted particles showed higher affinity to the endothelium under flow conditions in comparison to single targeted ones. Smith et al. have decorated SPIO with the protein Annexin V (Anx-SPIO, ~98 nm) to target apoptosis in atherosclerotic plaques.51 In vivo MR imaging revealed that Anx-SPIO induced signal hypointensity in atheromatous lesions, but not in healthy arteries, in a rabbit model. Annexin V decoration was required to produce hypointensity at a low dose (0.05 mg Fe/rabbit, or about 0.012 mg Fe/kg of bodyweight), while a 2000-fold higher dose of untargeted nanoparticles was needed to produce the same negative contrast in this animal model.51 Iron oxide nanocrystals can also be incorporated into nanocarriers, such as micelles,52 liposomes,14 nanoemulsions,53 and high-density lipoproteins (HDLs)54 for active targeting, which will be discussed in the following sections.
FIGURE 3.
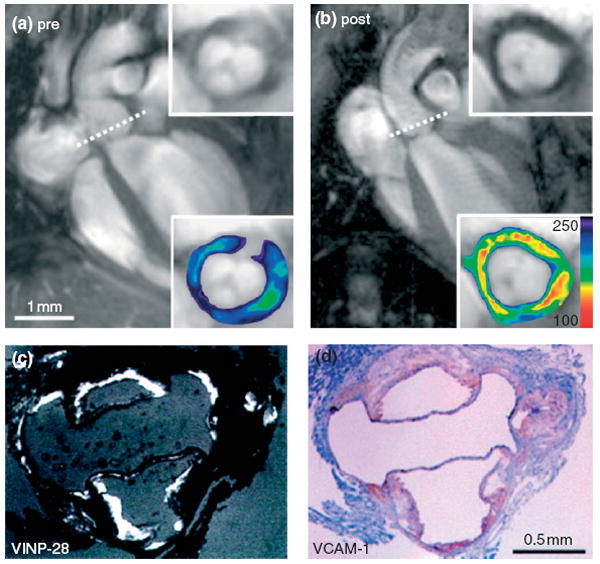
Representative in vivo magnetic resonance (MR) images of apoE−/− heart (a) pre- and (b) 48 h postinjection of VCAM-1 targeting iron oxide nanoparticles (VINP-28). Dotted line shows the location of short-axis view for the insets (lower panel with color-coded signal intensity). (c) The location of VINP-28 under fluorescent microscopy was associated with (d) VCAM-1 expression in immunohistochemistry. (Reprinted with permission from Ref 48. Copyright 2006 American Heart Association, Inc.).
Identifying hypointense regions in MR images of atherosclerotic plaques caused by iron oxide nanoparticles is challenging, since partial volume effects and artifacts may lead to overestimation of the target-rich areas. As an alternative to identifying hypointense regions in T2(*)-weighted imaging of iron oxide contrast agents, positive contrast techniques have been developed by either designing new imaging sequences or tailoring iron oxide particles. The currently available positive contrast imaging sequences are summarized in recent reviews.12,55 T1-weighted MR imaging with iron oxide nanoparticles is usually not performed because of their strong impact on tissue R2 values, even though these nanoparticles often have very high r1 values, comparable with those of nanoparticles that contain Gd chelates. However, some groups have tailored the magnetic properties of iron oxide particles to decrease the r2/r1 ratio, which thus enables T1-weighted MR imaging.56-58 For example, iron oxide nanoparticles have been doped with manganese to form solid-solution nanocrystals (MnxFe1−xO). These nanocrystals showed simultaneous T1 and T2 enhancements in MR imaging of rat liver in vivo.56 Alternatively, magnetite nanoparticles whose surface was coated with Gd chelates were demonstrated to be contrast agents for both T1- and T2-weighted in vivo MR imaging of subcutaneously implanted hydrogels in nude mice.58 Colloidal iron oxide nanoparticles (CION),53 another example of a T1 iron oxide contrast agent, are formed by entrapping magnetite particles within phospholipid nanoemulsion, which will be discussed in detail in the Oil-in-Water Emulsion section below.
Iron oxide nanoparticles have also been developed to target biomarkers of interest for cardiovascular diseases other than atherosclerosis, such as myocardial infarction.27,59-64 Dextran-coated iron oxide nanoparticles (~50 nm) induced significant negative contrast in infarcted, but not normal, myocardium on T2-weighted images from 5 to 48 h postinfarction.63 In another example, an annexin-based iron oxide nanoparticle AnxCLIO-Cy5.5 was used to target apoptotic cardiomyocytes in a mouse model of acute myocardial ischemia.61 MR imaging revealed that AnxCLIO-Cy5.5 was most prominently localized in the mid-myocardium, in which apoptosis was observed most frequently using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) ex vivo.61 Weissleder et al. used MION (~3 nm) attached to antimyosin antibody fragments (Fab) for MR imaging of cardiac infarcts by targeting myosin. A significant decrease in the signal intensity of infarcted myocardium was observed by MR imaging at 1 h postinjection, indicating successful uptake of this contrast agent in the target tissue.64
MICELLES
As mentioned above, the trapping of iron oxide nanocrystals into micelles can serve as one of several methods that facilitate active targeting of biomarkers. A typical micelle in aqueous solution is an aggregate of amphiphilic surfactants (formed when above the critical micelle concentration), which has a structure where the hydrophilic ‘head’ regions of the amphiphile molecules are in contact with the surrounding water molecules, and the hydrophobic tail regions are sequestered in the core (Figure 1). As a result, micelle cores can incorporate hydrophobic materials such as oleic-acid-coated iron oxide nanocrystals. These micellular iron oxide nanoparticles sometimes possess polyethylene glycol (PEG) incorporated at the lipid headgroups to increase biocompatibility and to facilitate the introduction of targeting groups. For example, van Tilborg et al. conjugated human recombinant Annexin A5 to pegylated micellular iron oxide nanoparticles, which were able to target apoptotic cells.52
Gadolinium (Gd) chelates are another category of MR contrast agents that are frequently incorporated into micelles. Unlike iron oxide, Gd-chelate-based MR contrast agents speed up longitudinal relaxation by shortening tissue T1 values, resulting in positive enhancement in T1-weighted MR imaging. Micelles containing Gd chelates have been developed for targeting specific epitopes in atherosclerotic plaque, allowing detection of their expression by MR imaging.65 For instance, immunomicelles, i.e. micelles conjugated with antibodies, have been used to assess macrophage burden in atherosclerotic plaques by in vivo MR imaging.65-68 To demonstrate this concept, the macrophage scavenger receptor (MSR) CD204 was chosen as a target for molecular MR imaging.66 In vivo MR imaging revealed that at 24 h postinjection, immunomicelles with MSR antibody (anti-CD204) caused significant enhancement of atherosclerotic aortas in apoE−/− mice compared to untargeted micelles (Figure 4). This enhancement was found to be related to the macrophage content of the atherosclerotic vessel areas imaged. Immunomicelles may thus aid in the detection of high macrophage content, which is associated with plaques vulnerable to rupture.66
FIGURE 4.
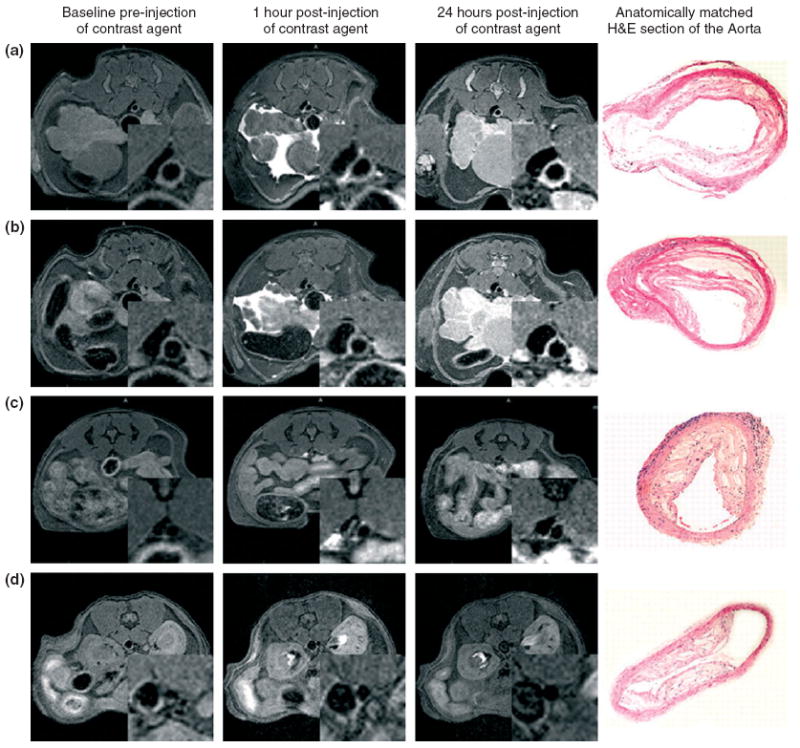
Typical in vivo magnetic resonance (MR) images pre- and postinjection of macrophage-targeted immunomicelles (a and b), untargeted micelles (c), and Gd-DTPA (d) in apoE−/− mice (insets are enlargements of the aortas). The right side of (a)–(d) shows H&E staining of the aorta at the identical anatomic level as the MR images from the same animal. (Reprinted with permission from Ref 9. Copyright 2007 National Academy of Sciences, USA).
Oxidized low-density lipoprotein (oxLDL) plays a key role in the initiation, progression, and destabilization of atherosclerotic plaques and is present in macrophages and the lipid pool. Immunomicelles containing murine (MDA2 and E06) or human (IK17) antibodies that bind unique oxidation-specific epitopes (i.e. oxLDL) induced enhancement in T1-weighted MR images of the aorta wall of apoE−/− mice.67
Micelles have also been used to detect myocardial infarctions. Lukyanov et al. reported that long circulating micelles accumulated in the infarction zone in greater quantities as compared to a non-damaged part of the heart muscle, as evidenced by ex vivo gamma camera imaging.69 The micelle accumulation was primarily due to the EPR effect in the infarct areas. This study demonstrated that untargeted micelles have the potential for the delivery of therapeutic or diagnostic agents to an area of myocardial infarction.
LIPOSOMES
Liposomes are spherical, self-closed structures composed of natural and/or synthetic amphiphilic lipids with diameters in the range of 50–500 nm and can be functionalized with targeting ligands to allow molecular imaging (Figure 1). These biocompatible particles can be further coated with polymers (e.g. PEG) to increase stability and to prolong their blood circulation half-life. A wide range of amphiphilic and hydrophobic molecules can be incorporated in the bilayer of liposomes, such as phospholipids whose headgroup is modified with a Gd chelate or a fluorophore. The aqueous interior can be loaded with water-soluble drugs, proteins, or other therapeutics for treating diseases or with contrast-generating materials such as Gd-DTPA. Untargeted liposomal nanoparticles have been demonstrated to deliver Gd chelate contrast agents to detect lipid-rich atherosclerotic plaques by MR imaging in a study by Mulder et al.70 Neointimal lesions were induced in apoE−/− mice by placing a constrictive collar around the right carotid artery. After injection of paramagnetic liposomes (mean size = 90 nm) that include amphiphilic Gd chelates in the lipid bilayer, a pronounced MR signal enhancement of the lesions was observed, while injection of the control agent Gd-DTPA did not result in signal enhancement.70 This result is likely due to accumulation of the liposomes in plaque via the EPR effect.
Apart from MR imaging exploiting nonspecific uptake of liposomes, targeted imaging of molecular markers, such as E-selectin, apoptosis, and collagens, has also been explored.52,71,72 Paramagnetic liposomes enriched with phosphatidylserine (PS) have been used to target macrophages and enable molecular MR imaging of atherosclerosis.73 In this case, the targeting exploits the fact that PS residues on the plasma membrane are exteriorized in apoptotic cells, triggering rapid phagocytosis by macrophages, so that macrophages would also take up these PS-rich liposomes. A rapid and significant enhancement of the aortic wall was observed in vivo after injection of PS-enriched liposomes in apoE−/− mice. The colocalization of the liposomes with macrophages in mouse atherosclerotic plaques was revealed by confocal microscopy.73
Collagen is an important component of the extracellular matrix and plays important roles in atherosclerosis and myocardial infarction. The degradation of collagen in fibrous caps correlates with rupture of atherosclerotic plaques.1 In myocardial infarctions, the deposition of collagen heals injury in the infarct area on one hand, and contributes to ventricular stiffness and dysfunction in the uninfarcted region on the other hand.74 A collagen-specific, bimodal liposomal MR contrast agent (Figure 5) has been developed by functionalizing liposomes with CNA35, a collagen adhesion protein of the Staphylococcus aureus bacterium. This CNA35 functionalized liposomal contrast agent decreased the T1 value of collagen coated on plate by 1000 ± 50 ms compared to controls, which indicates the potential of this technology for molecular imaging of collagen.72 In addition to CNA35, peptides75-79 have also been investigated as collagen-specific targeting ligands for MR contrast agents. These peptides have been successfully used for molecular imaging of fibrosis and postinfarction myocardial scars.78,79
FIGURE 5.
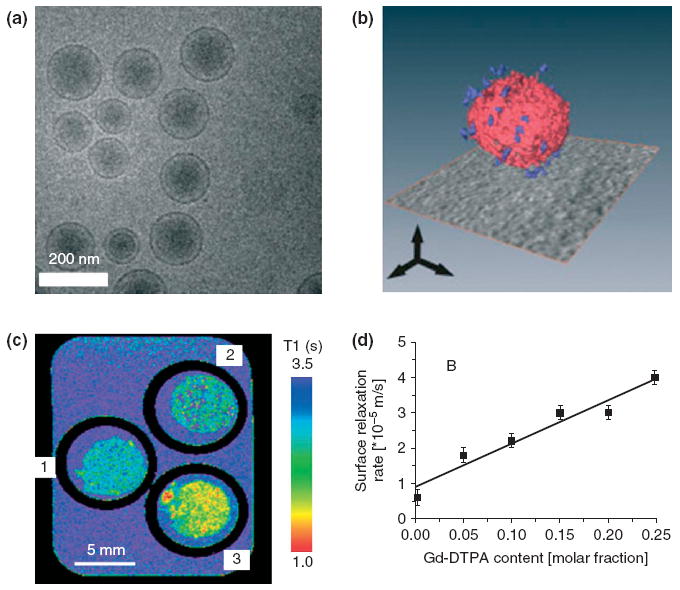
(a) Cryo-TEM image of bare liposomes. (b) 3D reconstruction of CNA35-functionalized liposomes from a series of TEM images. Red: lipid bilayer; Blue: individual CNA35 proteins. (c) T1-map of bovine collagen type I matrices treated with (1) buffer, (2) bare liposomes and (3) CNA35-functionalized liposomes. (d) Surface relaxation rate of bovine collagen type I showed a linear correlation with the molar fraction of Gd-DTPA-BSA in the CNA35-functionalized liposomes. (Reprinted with permission from Ref 72. Copyright 2009 John Wiley and Sons, Ltd).
Hiller et al. used liposomes that include Gd chelates and are targeted with Annexin V to assess apoptosis in myocardial infarctions.15 When these liposomes were used in perfused hearts, a significant increase in signal intensity was visible in MR images of the heart regions where apoptotic cardiomyocytes were present. Consequently, these Annexin-V-enriched liposomes have potential applications in MR imaging of apoptotic cells in the ischemic and reperfused myocardium, enabling visualization of apoptotic cell death noninvasively.
OIL-IN-WATER EMULSIONS
Oil-in-water emulsions are composed of hydrophobic oil cores coated with amphiphilic molecules, and are dispersed in water (Figure 1). Emulsions are capable of carrying both a high payload of hydrophobic materials in their core and an amphiphilic payload in their surfactant corona. There have been many reports from the group of Lanza, and from others, of submicron size emulsions (around 250 nm in diameter) formulated using liquid perfluorocarbons as a core. Some of the formulations include Gd-chelating lipids in the surfactant corona.80-84 Morawski et al. have shown that sufficient MR imaging quality can be achieved at picomolar concentration with these high payload emulsion nanoparticles.85 The lipid surface of these emulsions has been functionalized with several different ligands as targeting moieties. For example, anti-fibrin antibody fragments were conjugated to the lipids in the emulsion surface, allowing target-specific MR imaging of thrombi. This is of interest in cardiovascular disease not only because they are usually formed after atherosclerotic plaque rupture and can occlude the arteries80 but also because they occur before occlusive rupture, serving as an early indicator for severe clinical events.
When small molecules mimicking the RGD-peptide were coupled to the lipid membrane, the resulting emulsions can target the αvβ3 integrin, which is overexpressed in angiogenic endothelial cells. An enhancement was therefore observed when these nanoparticles were used for in vivo MR imaging of the abdominal aortic wall of atherosclerotic rabbits, due to angiogenesis occurring in their plaques.84 A theranostic agent has been developed by loading fumagillin, a water-insoluble angiostatic drug, in αvβ3-integrin-targeted emulsions.82 When these theranostic nanoemulsions were applied in atherosclerotic rabbits, MR signal enhancement was observed at baseline. Seven days after the first injection, a lesser MR signal enhancement was observed in the aortic wall of the rabbits treated with αvβ3-integrin-targeted fumagillin loaded emulsions, while the contrast enhancement for targeted emulsions without fumagillin was unchanged.82 This result indicated the anti-angiogenic effect of the fumagillin loaded emulsions. In a subsequent report on these αvβ3-targeted fumagillin emulsions it was revealed that their anti-angiogenic effects are acute, but can be prolonged when combined with atorvastatin, resulting in a marked and sustainable anti-angiogenic effect.83
Perfluorocarbon emulsions have also been used in 19F MR imaging.86 The MR-active isotope of fluorine, 19F, is 100% naturally abundant. However, there is no 19F background in the body, as fluorine is not used in biological processes. Therefore, significant signals observed in 19F MR can only originate from exogenous, injected 19F-containing compounds. Flögel et al. used 19F MR imaging to show that the accumulation of an injected untargeted perfluorocarbon nanoemulsion into the border of infarcted myocardium in a myocardial ischemia mouse model increased from 1 to 6 days postinjection, due to nonspecific accumulation (Figure 6).86
FIGURE 6.
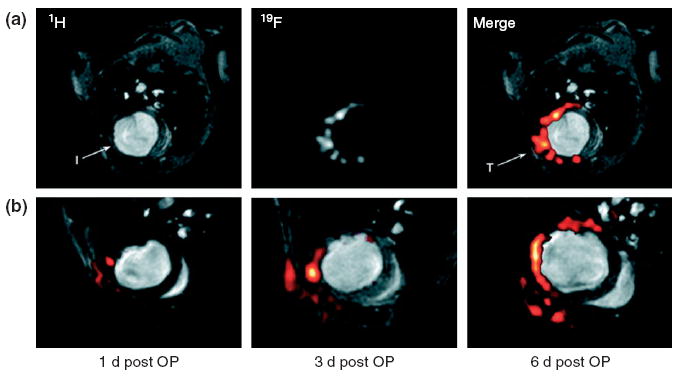
Typical in vivo 19F magnetic resonance (MR) images of myocardial infarction after injection of perfluorocarbon emulsions. (a) 1H and 19F MR images at the same position of a mouse thorax recorded 4 days after ligation of the left anterior descending artery.19F signal is near the infarcted region (I) and at the location of surgery (T). (b) Sections of 1H images superimposed with 19F images (red) at same position acquired 1, 3, and 6 days after surgery. (Reprinted with permission from Ref 86. Copyright 2008 American Heart Association, Inc.).
Jarzyna et al. have recently developed stable nanoemulsions whose size can be controlled in the 30-to 100-nm size range.17 These nanoemulsions were loaded with oleic-acid-coated iron oxide nanocrystals in the core and Cy5.5 fluorophores were attached to the distal ends of PEG chains that were anchored in the phospholipid coating to create a multifunctional nanoplatform for MR/optical imaging.17 This contrast agent was employed for tumor imaging in a mouse model of cancer, with the nanoparticles accumulating in the tumors because of the EPR effect. As the EPR effect occurs in atherosclerotic plaque also, these nanoemulsions could also be used for plaque imaging or targeted to epitopes of interest in plaque. As mentioned before, Senpan et al. have used CIONs as T1-weighted MR contrast agents, which are nanoemulsions whose strength of magnetic flux experienced by surrounding protons is reduced due to cross-linking of the shell of the particles.53 The shielding effect of nanoemulsions resulted in a lengthening of T2 and also higher r1 values, which permitted the T1 contrast to be detected. CIONs with fibrin-specific functional groups caused signal enhancement in T1-weighted MR ex vivo imaging of fibrin in ruptured atherosclerotic plaques from human carotid endarterectomy specimens without blooming artifacts (Figure 7). Fumagillin was incorporated into these CIONs in an in vitro demonstration of their potential as theranostic agents.53
FIGURE 7.
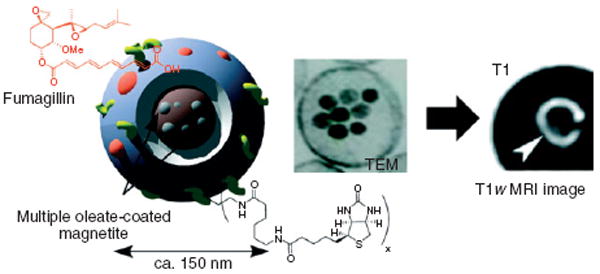
Schematic illustration (left) and TEM image (middle) of colloidal iron oxide nanoparticles (CIONs), and a T1-weighted magnetic resonance (MR) image of a human carotid endarterectomy specimen (right). (Reprinted with permission from Ref 53. Copyright 2009 American Chemical Society).
LIPOPROTEINS
Lipoproteins are nanoparticulate aggregates of lipids and proteins that are responsible for the transport of water insoluble nutrients through the vascular and extravascular spaces to cells. Usually, lipoproteins have a spherical shape and consist of a lipid core [triglycerides (TGs) and cholesteryl esters (CEs)] surrounded by a monolayer of phospholipids and cholesterol in which a family of proteins called apolipoproteins are embedded (Figure 1). Traditionally, lipoproteins are classified into five major classes on the basis of ascending density: chylomicrons, very-low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), low-density lipoproteins (LDLs), and high-density lipoproteins (HDLs).
LDL nanoparticles bind to low-density lipoprotein receptors (LDLRs) for cholesterol endocytosis with high selectivity, which makes LDL an interesting carrier for targeted delivery of therapeutic drugs and diagnostic contrast agents. A large numbers of reports can be found in literature that deal with imaging of atherosclerosis using radiolabeled LDL or radiolabeled modified LDL.87 However, reports on LDL or modified LDL as contrast agents for MR imaging of atherosclerosis remains limited, although the in vivo MR efficacy of paramagnetic LDL particles for LDLR targeting has been explored in tumor models.88-90 In the context of atherosclerosis, LDL that is enriched with a hydrophobic contrast agent, manganese-mesoporphyrin, caused reduction in T1 for foam cell pellets that were incubated with this agent.19 Despite the potential of these nanoparticles, in vivo MR imaging of atherosclerotic plaque by LDL nanoparticles has not yet been reported.
In comparison with LDL, HDL nanoparticles have some advantages for atherosclerotic plaque imaging. First, they are the key players in reverse cholesterol transport, which is of potential benefit for regression of atherosclerotic plaque. During reverse cholesterol transport, HDL binds to scavenger receptor B type I (SR-BI) and ATP-binding cassette transporters,91 and thus targets to macrophages expressing these receptors. High HDL cholesterol levels are associated with reduced carotid atherosclerotic plaque burden and lipid content whereas LDL promotes atherosclerosis.1,92 Second, they have a small size (diameter of 7–12 nm), which enables them to penetrate the vascular endothelium more easily than LDL, although the larger size of LDL permits larger payloads to be carried. Third, HDL-like particles can easily be reconstituted, whereas LDL is difficult to reconstitute. HDL-based MR contrast agents were first reported in 2004. They were synthesized via reconstituting HDL nanoparticles with Gd-chelating phospholipids included in the lipid layer (rHDL).20,21 These rHDL nanoparticles are approximately 9 nm in diameter and have longitudinal relaxivity values of about 10 mM−1 s−1. In hyperlipidemic apoE−/− mice, MR imaging revealed significant enhancement of atherosclerotic plaque at 24 h postinjection of paramagnetic rHDL nanoparticles as compared with preinjection images. Confocal microscopy revealed the association of macrophages with these rHDL nanoparticles. The targeting of rHDL to macrophages can be further enhanced by incorporating P2A2, an apolipoprotein-E-derived lipopeptide, into the lipid layers.93 P2A2-modified HDL resulted in an average normalized enhancement ratio (NER) of 93% for MR images of aortic vessel wall at 24 h postinjection, which was significantly higher than HDL (NER of 53%). In addition, HDL nanoparticles can be converted to a versatile platform by rerouting them to other biomarkers than their natural receptors.94-96 For example, conjugation of cyclic RGD peptides to the apoAI component of rHDL redirected these nanoparticles so that they bound to angiogenic endothelial cells, as demonstrated by MR/optical imaging in vitro and in a tumor model.94
This rHDL nanoplatform can also be modified to incorporate hydrophobically coated nanocrystals such as iron oxide (FeO-HDL), quantum dots (QD-HDL), or gold nanoparticles (Au-HDL) in the core. Fluorescent and/or paramagnetic lipids were included in the lipid coating to form multimodal contrast agents.54 A clear increase in MR signal was observed in the aortic wall of apoE−/− mice in vivo 24 hours after injection of the paramagnetic Au-HDL and QD-HDL, while a clear decrease of MR intensity in the aortic wall was observed for the FeO-HDL (Figure 8). Ex vivo computed tomography (CT) and fluorescence imaging confirmed the results found with MR imaging: nanocrystal-core HDL is taken up into the aorta wall to a much greater extent than aspecific control nanoparticles. Immunofluorescence staining revealed these nanoparticles to accumulate in macrophage cells in the plaque.
FIGURE 8.
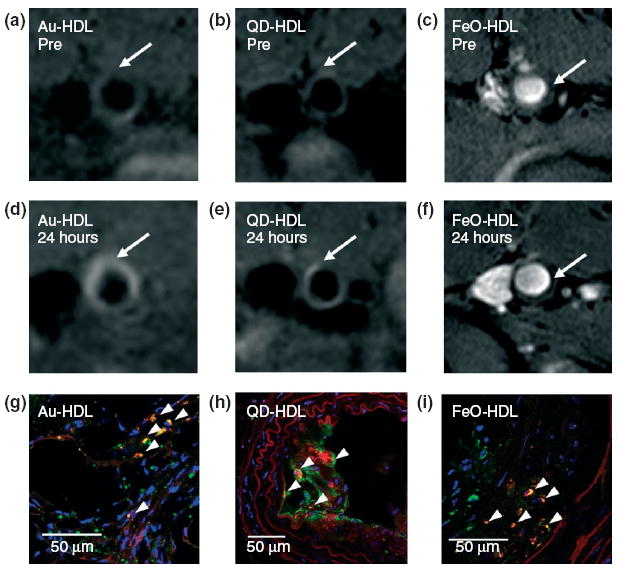
Typical T1-weighted magnetic resonance (MR) images of the apoE−/− mouse aorta (a, b) pre- and (d, e) 24 h postinjection with either Au-HDL or QD-HDL. Arrows point to areas enhanced in the post images. T2*-weighted images of an apoE−/− mouse aorta (c) pre- and (f) 24 h postinjection with FeO-HDL. (g, h, i) Confocal microscopy images of the apoE−/− mouse aortic sections. Red: nanocrystal HDL; Green: macrophages; Blue: nuclei. The arrowheads indicate colocalization of nanocrystal HDL with macrophages. (Reprinted with permission from Ref 54. Copyright 2008 American Chemical Society).
Peptides that mimick apoAI can be used instead of the natural HDL apolipoprotein components to form rHDL contrast agents that target macrophages for MR imaging of atherosclerosis. In 2009 it was reported that two peptides have been explored for this purpose, 18A (an amphiphatic, α-helical peptide with 18 amino acid residues, whose hydrophobic face binds to the acyl chains of the phospholipids) and 37pA (a similar 37 amino acid residue peptide).97,98 In vitro experiments with J774A.1 macrophages indicated that peptide-based rHDL nanoparticles could produce cholesterol efflux from these cells comparable to native HDL and were taken up in a saturable, specific fashion by these cells. Both 18A- and 37pA-based rHDL contrast agents produced enhancements of about 90% in MR imaging of atherosclerotic plaques of apoE−/− mice by specifically targeting macrophages.
QUANTUM DOTS
Quantum dots (QDs) are colloidal nanocrystals with attractive fluorescence features, such as excellent photostability, high molar extinction coefficient, and narrow and tunable emission spectrum.99 As a consequence, QDs have been used in a variety of applications such as fluorescence imaging, immunofluorescence, and fluorescence microscopy.100 QDs have been adapted for use as MR/optical contrast agents by incorporating them into the core of paramagnetic micelles,101 which were composed of CdSe/ZnS core/shell QDs coated with a mixture of paramagnetic Gd-chelate lipids and PEG phospholipids. Via functionalization with cyclic RGD peptides, these paramagnetic micellular QDs have potential for imaging angiogenesis in atherosclerotic plaques by targeting αvβ3 integrin.101 These micellular QDs have also been conjugated with MSR CD204-specific antibodies for molecular imaging of macrophages in atherosclerosis.68 These CD204-specific micellular QDs caused pronounced signal enhancement for MR imaging of aortic vessel wall in apoE−/− mice. The QDs in the micelles allowed fluorescence microscopy and optical imaging of the excised aorta, which identified the regions with high macrophage content under ultraviolet (UV) illumination.68
Paramagnetic QDs with Gd chelates have also been utilized as multimodal contrast agents for noninvasive in vivo MR imaging of angiogenesis in a mouse model of myocardial infarction. The QDs were functionalized with cyclic NGR peptides (cNGR-pQDs), which are specific for CD13, an aminopeptidase that is strongly upregulated during myocardial angiogenesis. Injection of cNGR-pQDs resulted in strong negative contrast of MR images mainly in the infarcted myocardium, although paramagnetic agents usually result in positive contrast (Figure 9).102
FIGURE 9.
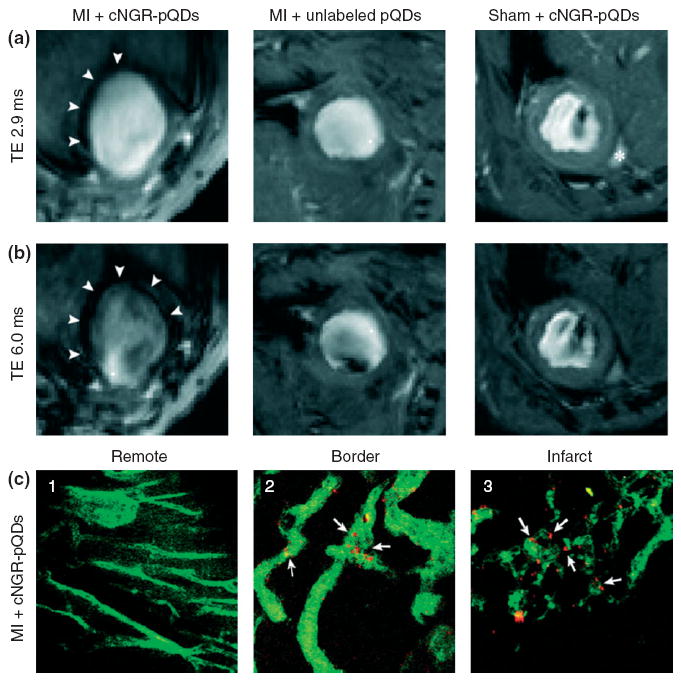
Typical short-axis magnetic resonance (MR) images with an echo time (TE) of (a) 2.9 ms and (b) 6.0 ms for a myocardial infarction (MI) mouse injected with cNGR-pQDs (left), an MI mouse injected with unlabeled QDs (middle), and a sham-operated mouse injected with cNGR-pQDs (right). Arrowheads indicate the hypointense area for MI mouse injected with cNGR-pQDs. (c) Two-photon laser-scanning microscopy revealed that cNGR-pQDs were mainly in the (2) border zone and (3) infarct areas, but not in (1) remote myocardium. Arrows indicate the colocalization of nanoparticles with vasculature. Red: quantum dots; Green: α-CD31-FITC. (Reprinted with permission from Ref 102. Copyright 2010 American Heart Association, Inc.).
DISCUSSION
In this review, we have highlighted several nanoparticle platforms for molecular imaging of cardiovascular diseases. Each system has its own advantages and disadvantages in terms of ease of synthesis, toxicity, payload, and biodistribution.
Among the nanoparticle platforms discussed, lipid-based systems, such as micelles, liposomes, and HDL, are relatively easy to synthesize. Iron oxide nanoparticles are of interest since they can be degraded by the liver and therefore have low toxicity and exhibit good biocompatibility.103 Gd chelates are, however, linked with nephrogenic systemic fibrosis (NSF) in patients with renal disease.104 Many QD formulations are toxic because of the leakage of the heavy metal element Cd, which can partially be reduced by appropriate surface coatings.105 Large particles in general have a higher payload than small particles, which improves their detectability. Small particles, however, can penetrate deeper into tissue, which offers advantages for targeting extravascular biomarkers in cardiovascular diseases.45
Molecular imaging of cardiovascular disease faces several challenges as compared to other pathologies, such as cancer. First, as atherosclerosis is a systemic disease it requires investigators to focus on different structures, which may include the carotids, the aortic root and arch, the abdominal aorta, the renal arteries, the aortic bifurcation and femoral arteries. Moreover, movement, flow effects, the beating of the heart, as well as the small size of vessels hamper molecular imaging sensitivity. Therefore, nanoparticles used for cardiovascular imaging usually need to be designed to allow their detection with superb sensitivity and should strongly and very specifically bind to the targeted biomarker to enable sufficient accumulation of the contrast-generating material.
CONCLUSION
With the advances in nanoparticle contrast agents for cellular and molecular imaging, the roles for different factors in cardiovascular diseases can be investigated in vivo and be better understood. The multimodal capacity of nanoparticle contrast agents can enable visualization of both the anatomical morphology and pathological characteristics of cardiovascular disease at high resolution by combinations of MR with complimentary imaging techniques. The theranostic potential of nanoparticles may aid in the early detection of high-risk patients with cardiovascular diseases as well as in the advancement of therapy, and thus, it is hoped, will contribute to reductions in patient morbidity and mortality rates.
References
- 1.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part II: approaches by noninvasive computed tomographic/magnetic resonance imaging. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi RH, Bradley WG, Lisanti CJ. MRI: The Basics. 2. New York: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 4.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipinski MJ, Fuster V, Fisher EA, Fayad ZA. Technology insight: targeting of biological molecules for evaluation of high-risk atherosclerotic plaques with magnetic resonance imaging. Nat Clin Pract Cardiovasc Med. 2004;1:48–55. doi: 10.1038/ncpcardio0013. [DOI] [PubMed] [Google Scholar]
- 6.Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, Lindner JR, Wu JC, Kraitchman DL, Fayad ZA, et al. Multimodality cardiovascular molecular imaging, part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinusas AJ, Bengel F, Nahrendorf M, Epstein FH, Wu JC, Villanueva FS, Fayad ZA, Gropler RJ. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008;1:244–256. doi: 10.1161/CIRCIMAGING.108.824359. [DOI] [PubMed] [Google Scholar]
- 8.Dobrucki LW, Sinusas AJ. PET and SPECT in cardiovascular molecular imaging. Nat Rev Cardiol. 2010;7:38–47. doi: 10.1038/nrcardio.2009.201. [DOI] [PubMed] [Google Scholar]
- 9.Shirani J, Narula J, Eckelman WC, Narula N, Dilsizian V. Early imaging in heart failure: exploring novel molecular targets. J Nucl Cardiol. 2007;14:100–110. doi: 10.1016/j.nuclcard.2006.12.318. [DOI] [PubMed] [Google Scholar]
- 10.Sutton MGSJ, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 11.Kalinowski L, Dobrucki LW, Meoli DF, Dione DP, Sadeghi MM, Madri JA, Sinusas AJ. Targeted imaging of hypoxia-induced integrin activation in myocardium early after infarction. J Appl Physiol. 2008;104:1504–1512. doi: 10.1152/japplphysiol.00861.2007. [DOI] [PubMed] [Google Scholar]
- 12.Briley-Saebo KC, Mulder WJM, Mani V, Hyafil F, Amirbekian V, Aguinaldo JGS, Fisher EA, Fayad ZA. Magnetic resonance imaging of vulnerable atherosclerotic plaques: current imaging strategies and molecular imaging probes. J Magn Reson Imaging. 2007;26:460–479. doi: 10.1002/jmri.20989. [DOI] [PubMed] [Google Scholar]
- 13.Mulder WJ, Strijkers GJ, van Tilborg GA, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc Chem Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meincke M, Schlorf T, Kossel E, Jansen O, Glueer CC, Mentlein R. Iron oxide-loaded liposomes for MR imaging. Front Biosci. 2008;13:4002–4008. doi: 10.2741/2987. [DOI] [PubMed] [Google Scholar]
- 15.Hiller KH, Waller C, Nahrendorf M, Bauer WR, Jakob PM. Assessment of cardiovascular apoptosis in the isolated rat heart by magnetic resonance molecular imaging. Mol Imaging. 2006;5:115–121. [PubMed] [Google Scholar]
- 16.Doiron AL, Chu K, Ali A, Brannon-Peppas L. Preparation and initial characterization of biodegradable particles containing gadolinium-DTPA contrast agent for enhanced MRI. Proc Natl Acad Sci U S A. 2008;105:17232–17237. doi: 10.1073/pnas.0710205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarzyna PA, Skajaa T, Gianella A, Cormode DP, Samber DD, Dickson SD, Chen W, Griffioen AW, Fayad ZA, Mulder WJ. Iron oxide core oil-in-water emulsions as a multifunctional nanoparticle platform for tumor targeting and imaging. Biomaterials. 2009;30:6947–6954. doi: 10.1016/j.biomaterials.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams KJ, Vallabhajosula S, Rahman IU, Donnelly TM, Parker TS, Weinrauch M, Goldsmith SJ. Low density lipoprotein receptor-independent hepatic uptake of a synthetic, cholesterol-scavenging lipoprotein: implications for the treatment of receptor-deficient atherosclerosis. Proc Natl Acad Sci U S A. 1988;85:242–246. doi: 10.1073/pnas.85.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsumori LM, Ricks JL, Rosenfeld ME, Schmiedl UP, Yuan C. Development of a lipoprotein based molecular imaging MR contrast agent for the noninvasive detection of early atherosclerotic disease. Int J Cardiovasc Imaging. 2004;20:561–567. doi: 10.1007/s10554-004-7020-4. [DOI] [PubMed] [Google Scholar]
- 20.Frias JC, Ma YQ, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6:2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 21.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126:16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 22.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 23.Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 24.von zur Muhlen C, Bode C, Choudhury R. MRI of vulnerable plaque. Curr Cardiovasc Imaging Rep. 2009;2:5–14. [Google Scholar]
- 25.Ruehm S, Corot C, Vogt P, Kolb S, Debatin J. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 26.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis emerging applications. J Am Coll Cardiol. 2006;47:1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Sosnovik DE, Nahrendorf M, Deliolanis N, Novikov M, Aikawa E, Josephson L, Rosenzweig A, Weissleder R, Ntziachristos V. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115:1384–1391. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 28.Ala-Korpela M, Sipola P, Kaski K. Characterization and molecular detection of atherothrombosis by magnetic resonance - potential tools for individual risk assessment and diagnostics. Ann Med. 2006;38:322–336. doi: 10.1080/07853890600862418. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz SA, Taupitz M, Wagner S, Wolf KJ, Beyersdorff D, Hamm B. Magnetic resonance imaging of atherosclerotic plaques using superparamagnetic iron oxide particles. J Magn Reson Imaging. 2001;14:355–361. doi: 10.1002/jmri.1194. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz SA, Taupitz M, Wagner S, Coupland SE, Gust R, Nikolova A, Wolf KJ. Iron-oxide-enhanced magnetic resonance imaging of atherosclerotic plaques: postmortem analysis of accuracy, inter-observer agreement, and pitfalls. Invest Radiol. 2002;37:405–411. doi: 10.1097/00004424-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz SA, Coupland SE, Gust R, Winterhalter S, Wagner S, Kresse M, Semmler W, Wolf KJ. Superparamagnetic iron oxide-enhanced MRI of atherosclerotic plaques in Watanabe hereditable hyperlipidemic rabbits. Invest Radiol. 2000;35:460–471. doi: 10.1097/00004424-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi RA, U-King-Im JM, Graves MJ, Cross JJ, Horsley J, Goddard MJ, Skepper JN, Quartey G, Warburton E, Joubert I, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 33.Yancy AD, Olzinski AR, Hu TC, Lenhard SC, Aravindhan K, Gruver SM, Jacobs PM, Willette RN, Jucker BM. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: critical determinants of atherosclerotic plaque labeling. J Magn Reson Imaging. 2005;21:432–442. doi: 10.1002/jmri.20283. [DOI] [PubMed] [Google Scholar]
- 34.Hyafil F, Laissy JP, Mazighi M, Tchetche D, Louedec L, Adle-Biassette H, Chillon S, Henin D, Jacob MP, Letourneur D, et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler Thromb Vasc Biol. 2006;26:176–181. doi: 10.1161/01.ATV.0000194098.82677.57. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi RA, U-King-Im JM, Graves MJ, Kirkpatrick PJ, Gillard JH. Noninvasive imaging of carotid plaque inflammation. Neurology. 2004;63:187–188. doi: 10.1212/01.wnl.0000132962.12841.1d. [DOI] [PubMed] [Google Scholar]
- 36.Tang TY, Patterson AJ, Miller SR, Graves MJ, Howarth SP, UK-I JM, Li ZY, Sadat U, Young VE, Walsh SR, et al. Temporal dependence of in vivo USPIO-enhanced MRI signal changes in human carotid atheromatous plaques. Neuroradiology. 2009;51:457–465. doi: 10.1007/s00234-009-0523-x. [DOI] [PubMed] [Google Scholar]
- 37.Tang TY, Muller KH, Graves MJ, Li ZY, Walsh SR, Young V, Sadat U, Howarth SPS, Gillard JH. Iron oxide particles for atheroma imaging. Arterioscler Thromb Vasc Biol. 2009;29:1001–1008. doi: 10.1161/ATVBAHA.108.165514. [DOI] [PubMed] [Google Scholar]
- 38.Tang TY, Moustafa RR, Howarth SP, Walsh SR, Boyle JR, Li ZY, Baron JC, Gillard JH, Warburton EA. Combined PET-FDG and USPIO-enhanced MR imaging in patients with symptomatic moderate carotid artery stenosis. Eur J Vasc Endovasc Surg. 2008;36:53–55. doi: 10.1016/j.ejvs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Kooi ME, Cappendijk VC, Cleutjens KBJM, Kessels AGH, Kitslaar PJEHM, Borgers M, Frederik PM, Daemen MJAP, van Engelshoven JMA. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 40.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, U-King-Im JM, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Lee J-H, Huh Y-M, Jun Y-W, Seo J-W, Jang J-T, Song H-T, Kim S, Cho E-J, Yoon H-G, Suh J-S, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 42.Briley-Saebo KC, Mani V, Hyafil F, Cornily JC, Fayad ZA. Fractionated Feridex and positive contrast: in vivo MR imaging of atherosclerosis. Magn Reson Med. 2008;59:721–730. doi: 10.1002/mrm.21541. [DOI] [PubMed] [Google Scholar]
- 43.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 44.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 45.Cormode DP, Skajaa T, Fayad ZA, Mulder WJM. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29:992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JQ, Sui MH, Fan WM. Nanoparticles for tumor targeted therapies and their pharmacokinetics. Curr Drug Metab. 2010;11:129–141. doi: 10.2174/138920010791110827. [DOI] [PubMed] [Google Scholar]
- 47.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 48.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 49.Kang HW, Torres D, Wald L, Weissleder R, Bogdanov AA., Jr Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006;86:599–609. doi: 10.1038/labinvest.3700421. [DOI] [PubMed] [Google Scholar]
- 50.McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis. 2008;209:18–27. doi: 10.1016/j.atherosclerosis.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith BR, Heverhagen J, Knopp M, Schmalbrock P, Shapiro J, Shiomi M, Moldovan NI, Ferrari M, Lee SC. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI) Biomed Microdevices. 2007;9:719–727. doi: 10.1007/s10544-007-9081-3. [DOI] [PubMed] [Google Scholar]
- 52.van Tilborg GA, Mulder WJ, Deckers N, Storm G, Reutelingsperger CP, Strijkers GJ, Nicolay K. Annexin A5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjug Chem. 2006;17:741–749. doi: 10.1021/bc0600259. [DOI] [PubMed] [Google Scholar]
- 53.Senpan A, Caruthers SD, Rhee I, Mauro NA, Pan D, Hu G, Scott MJ, Fuhrhop RW, Gaffney PJ, Wickline SA, et al. Conquering the dark side: colloidal iron oxide nanoparticles. ACS Nano. 2009;3:3917–3926. doi: 10.1021/nn900819y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers WJ, Meyer CH, Kramer CM. Technology insight: in vivo cell tracking by use of MRI. Nat Clin Pract Cardiovasc Med. 2006;3:554–562. doi: 10.1038/ncpcardio0659. [DOI] [PubMed] [Google Scholar]
- 56.Choi D, Han A, Park JP, Kim JK, Lee JH, Kim TH, Kim S-W. Fabrication of MnxFe1−xO colloidal solid solution as a dual magnetic resonance contrast agent. Small. 2009;5:571–573. doi: 10.1002/smll.200801258. [DOI] [PubMed] [Google Scholar]
- 57.Tromsdorf UI, Bruns OT, Salmen SC, Beisiegel U, Weller H. A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009;9:4434–4440. doi: 10.1021/nl902715v. [DOI] [PubMed] [Google Scholar]
- 58.Bae KH, Kim YB, Lee Y, Hwang J, Park H, Park TG. Bioinspired synthesis and characterization of gadolinium-labeled magnetite nanoparticles for dual contrast T1- and T2-weighted magnetic resonance imaging. Bioconjug Chem. 2009;21:505–512. doi: 10.1021/bc900424u. [DOI] [PubMed] [Google Scholar]
- 59.Jaffer FA, Sosnovik DE, Nahrendorf M, Weissleder R. Molecular imaging of myocardial infarction. J Mol Cell Cardiol. 2006;41:921–933. doi: 10.1016/j.yjmcc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Sosnovik D, Schellenberger E, Nahrendorf M, Novikov M, Matsui T, Dai G, Reynolds F, Grazette L, Rosenzweig A, Weissleder R, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54:718–724. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 61.Sosnovik DE, Garanger E, Aikawa E, Nahrendorf M, Figuiredo JL, Dai G, Reynolds F, Rosenzweig A, Weissleder R, Josephson L. Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo: potential for midmyocardial salvage in acute ischemia. Circ Cardiovasc Imaging. 2009;2:460–467. doi: 10.1161/CIRCIMAGING.109.859678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapon C, Lemaire L, Franconi F, Marescaux L, Legras P, Denizot B, Le Jeune JJ. Assessment of myocardial viability in rats: evaluation of a new method using superparamagnetic iron oxide nanoparticles and Gd-DOTA at high magnetic field. Magn Reson Med. 2004;52:932–936. doi: 10.1002/mrm.20210. [DOI] [PubMed] [Google Scholar]
- 63.Chapon C, Franconi F, Lemaire L, Marescaux L, Legras P, Saint-Andre JP, Denizot B, Le Jeune JJ. High field magnetic resonance imaging evaluation of superparamagnetic iron oxide nanoparticles in a permanent rat myocardial infarction. Invest Radiol. 2003;38:141–146. doi: 10.1097/01.RLI.0000052979.96332.90. [DOI] [PubMed] [Google Scholar]
- 64.Weissleder R, Lee AS, Khaw BA, Shen T, Brady TJ. Antimyosin-labeled monocrystalline iron oxide allows detection of myocardial infarct: MR antibody imaging. Radiology. 1992;182:381–385. doi: 10.1148/radiology.182.2.1732953. [DOI] [PubMed] [Google Scholar]
- 65.Lipinski MJ, Amirbekian V, Frias JC, Aguinaldo JG, Mani V, Briley-Saebo KC, Fuster V, Fallon JT, Fisher EA, Fayad ZA. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn Reson Med. 2006;56:601–610. doi: 10.1002/mrm.20995. [DOI] [PubMed] [Google Scholar]
- 66.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JG, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briley-Saebo KC, Shaw PX, Mulder WJ, Choi SH, Vucic E, Aguinaldo JG, Witztum JL, Fuster V, Tsimikas S, Fayad ZA. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulder WJ, Strijkers GJ, Briley-Saboe KC, Frias JC, Aguinaldo JG, Vucic E, Amirbekian V, Tang C, Chin PT, Nicolay K, et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58:1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 69.Lukyanov AN, Harder WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Controlled Release. 2004;94:187–193. doi: 10.1016/j.jconrel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Mulder WJ, Douma K, Koning GA, van Zandvoort MA, Lutgens E, Daemen MJ, Nicolay K, S GJ. Liposome-enhanced MRI of neointimal lesions in the ApoE-KO mouse. Magn Reson Med. 2006;55:1170–1174. doi: 10.1002/mrm.20883. [DOI] [PubMed] [Google Scholar]
- 71.Mulder WJ, Strijkers GJ, Griffioen AW, van Bloois L, Molema G, Storm G, Koning GA, Nicolay K. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug Chem. 2004;15:799–806. doi: 10.1021/bc049949r. [DOI] [PubMed] [Google Scholar]
- 72.Sanders HM, Strijkers GJ, Mulder WJ, Huinink HP, Erich SJ, Adan OC, Sommerdijk NA, Merkx M, Nicolay K. Morphology, binding behavior and MR-properties of paramagnetic collagen-binding liposomes. Contrast Media Mol Imaging. 2009;4:81–88. doi: 10.1002/cmmi.266. [DOI] [PubMed] [Google Scholar]
- 73.Maiseyeu A, Mihai G, Kampfrath T, Simonetti OP, Sen CK, Roy S, Rajagopalan S, Parthasarathy S. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res. 2009;50:2157–2163. doi: 10.1194/jlr.M800405-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van den Borne SWM, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 75.Spuentrup E, Ruhl KM, Botnar RM, Wiethoff AJ, Buhl A, Jacques V, Greenfield MT, Krombach GA, Gunther RW, Vangel MG, et al. Molecular magnetic resonance imaging of myocardial perfusion with EP-3600, a collagen-specific contrast agent: initial feasibility study in a swine model. Circulation. 2009;119:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.826388. [DOI] [PubMed] [Google Scholar]
- 76.Caravan P. Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc Chem Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 77.Caravan P, Das B, Deng Q, Dumas S, Jacques V, Koerner SK, Kolodziej A, Looby RJ, Sun WC, Zhang Z. A lysine walk to high relaxivity collagen-targeted MRI contrast agents. Chem Commun (Camb) 2009:430–432. doi: 10.1039/b819098d. [DOI] [PubMed] [Google Scholar]
- 78.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed Engl. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 79.Helm PA, Caravan P, French BA, Jacques V, Shen L, Xu Y, Beyers RJ, Roy RJ, Kramer CM, Epstein FH. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology. 2008;247:788–796. doi: 10.1148/radiol.2473070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, Winter P, Sicard GA, Gaffney PJ, Wickline SA, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 81.Winter PM, Caruthers SD, Yu X, Song SK, Chen J, Miller B, Bulte JW, Robertson JD, Gaffney PJ, Wickline SA, et al. Improved molecular imaging contrast agent for detection of human thrombus. Magn Reson Med. 2003;50:411–416. doi: 10.1002/mrm.10532. [DOI] [PubMed] [Google Scholar]
- 82.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 83.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. J Am Coll Cardiol Cardiovasc Imaging. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 85.Morawski AM, Winter PM, Crowder KC, Caruthers SD, Fuhrhop RW, Scott MJ, Robertson JD, Abendschein DR, Lanza GM, Wickline SA. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn Reson Med. 2004;51:480–486. doi: 10.1002/mrm.20010. [DOI] [PubMed] [Google Scholar]
- 86.Flogel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frias JC, Lipinski MJ, Lipinski SE, Albelda MT. Modified lipoproteins as contrast agents for imaging of atherosclerosis. Contrast Media Mol Imaging. 2007;2:16–23. doi: 10.1002/cmmi.124. [DOI] [PubMed] [Google Scholar]
- 88.Li H, Gray BD, Corbin I, Lebherz C, Choi H, Lund-Katz S, Wilson JM, Glickson JD, Zhou R. MR and fluorescent imaging of low-density lipoprotein receptors. Acad Radiol. 2004;11:1251–1259. doi: 10.1016/j.acra.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 89.Zheng G, Chen J, Li H, Glickson JD. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc Natl Acad Sci U S A. 2005;102:17757–17762. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Corbin IR, Li H, Cao WG, Glickson JD, Zheng G. Ligand conjugated low-density lipoprotein nanoparticles for enhanced optical cancer imaging in vivo. J Am Chem Soc. 2007;129:5798–5799. doi: 10.1021/ja069336k. [DOI] [PubMed] [Google Scholar]
- 91.Fitzgerald ML, Mujawar Z, Tamehiro N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010;211:361–370. doi: 10.1016/j.atherosclerosis.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phan BA, Chu B, Polissar N, Hatsukami TS, Yuan C, Zhao XQ. Association of high-density lipoprotein levels and carotid atherosclerotic plaque characteristics by magnetic resonance imaging. Int J Cardiovasc Imaging. 2007;23:337–342. doi: 10.1007/s10554-006-9175-7. [DOI] [PubMed] [Google Scholar]
- 93.Chen W, Vucic E, Leupold E, Mulder WJ, Cormode DP, Briley-Saebo KC, Barazza A, Fisher EA, Dathe M, Fayad ZA. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol Imaging. 2008;3:233–242. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- 94.Chen W, Jarzyna PA, van Tilborg GA, Nguyen VA, Cormode DP, Klink A, Griffioen AW, Randolph GJ, Fisher EA, Mulder WJ, et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 2010;24:1667–1681. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z, Chen J, Ding L, Jin H, Lovell JF, Corbin IR, Cao W, Lo PC, Yang M, Tsao MS, et al. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6:430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 96.Cao W, Ng KK, Corbin I, Zhang Z, Ding L, Chen J, Zheng G. Synthesis and evaluation of a stable bacteriochlorophyll-analog and its incorporation into high-density lipoprotein nanoparticles for tumor imaging. Bioconjug Chem. 2009;20:2023–2031. doi: 10.1021/bc900404y. [DOI] [PubMed] [Google Scholar]
- 97.Cormode DP, Briley-Saebo KC, Mulder WJ, Aguinaldo JG, Barazza A, Ma Y, Fisher EA, Fayad ZA. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4:1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- 98.Cormode DP, Chandrasekar R, Delshad A, Briley-Saebo KC, Calcagno C, Barazza A, Mulder WJ, Fisher EA, Fayad ZA. Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconjug Chem. 2009;20:937–943. doi: 10.1021/bc800520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghasemi Y, Peymani P, Afifi S. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed. 2009;80:156–165. [PubMed] [Google Scholar]
- 101.Mulder WJ, Koole R, Brandwijk RJ, Storm G, Chin PTK, Strijkers GJ, de Mello Donegá C, Nicolay K, Griffioen AW. Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 102.Oostendorp M, Douma K, Wagenaar A, Slenter JM, Hackeng TM, van Zandvoort MA, Post MJ, Backes WH. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation. 2010;121:775–783. doi: 10.1161/CIRCULATIONAHA.109.889451. [DOI] [PubMed] [Google Scholar]
- 103.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, Jacobs PM, Watnick SG. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF) Kidney Int. 2008;75:465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gauden AJ, Phal PM, Drummond KJ. MRI safety; nephrogenic systemic fibrosis and other risks. J Clin Neurosci. 2010;17:1097–1104. doi: 10.1016/j.jocn.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]


