Abstract
The fatal CNS demyelinating disease, progressive multifocal leukoencephalopathy (PML), is rare and appears to occur almost always as a consequence of immune dysfunction. Thus it is associated with HIV/AIDS and also as a side-effect of certain immunomodulatory monoclonal antibody therapies. In contrast to the rarity of PML, the etiological agent of the disease, the polyomavirus JC (JCV) is widespread in populations worldwide. In the forty years since JCV was first isolated, much has been learned about the virus and the disease from laboratory and clinical observations. However, there are many aspects of the viral life cycle and the pathogenesis of the disease that still remain unclear and our understanding is constantly evolving. In this review, we will discuss our current understanding of the clinical features of PML and molecular characteristics of JCV and how they relate to each other. Clinical observations can inform molecular studies of the virus and likewise molecular findings concerning the life cycle of the virus can guide the development of novel therapeutic strategies.
Keywords: Progressive multifocal leukoencephalopathy (PML), Polyomavirus JC (JCV)
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a rare CNS demyelinating disease that appears to occur almost exclusively in the context of severe impairment of the immune system, such as HIV/AIDS or treatment with new immunomodulatory monoclonal antibody therapies such as Natalizumab, Rituximab and Efalizumab [1–3]. PML was first described in 1958 [4] and the etiological agent, polyomavirus JC (JCV) isolated in 1971 [5]. Interest in PML remains high as shown by the publication of a number of recent reviews [1–3,6–9]. Even so, PML remains an incurable and often fatal disease. The directions of molecular studies of the virus may benefit from guidance from clinical observations from PML patients while the results of molecular studies may yield new insights into the life cycle of the virus, which may aid the development of novel therapeutic strategies [1,2,6–10].
EPIDEMIOLOGY OF PML
In the pre-cART (combination antiretroviral therapy) era, the incidence (rate of new cases of PML) varied from 0.3–8% [11–13] but wide use of antiretroviral treatments led to a significant reduction from 0.7 cases/100 patient-years (PY) to 0.7 case per 1000/person-years at risk [14]. Importantly, the Swiss HIV cohort study prospectively analyzed the incidence and outcome of PML in 226 cases from 1988 through 2007 [15]. The incidence of PML decreased from 0.24 cases/100 PY before 1996 (pre-cART) to 0.06 cases/100 PY from 1996 onward. In this study, the PML-attributable 1-year mortality rate was found to decrease from 82.3 cases/100 PY during the pre-cART era to 37.6 cases/100 PY during the cART era [15]. HIV infection is still the most frequent immunodeficiency setting for PML, ~80% of cases, followed by hematologic malignancies (~8%), solid cancers (~3%), organ transplants and autoimmune disease treated with immunomodulators [16].
DIAGNOSTIC CRITERIA FOR PML
The expanding spectrum of clinical conditions favoring PML and the frequent occurrence of atypical cases, led to the need of diagnostic criteria reported as follows [17]. Definitive (causative) diagnosis can only be made when PML is confirmed by histopathology. This is known as tissue-confirmed PML where there is evidence of consistent neuropathology in brain (biopsy or autopsy) with JCV DNA or protein detected by in situ techniques. CSF-confirmed PML is also known as probable or laboratory-confirmed PML and has a lower degree of diagnostic certainty given the difficulties with sensitivity and specificity of PCR assays in different laboratories. The third and lowest level of diagnostic certainty is presumptive or possible PML and is based on typical clinical and MRI findings consistent with the disease diagnosis and brain biopsy and lumbar puncture either not performed or JCV DNA not detected in CSF [17].
CLINICAL FEATURES OF PML
PML, as classically described in literature, is a demyelinating disease of the CNS with a tumultuous disease course and poor prognosis within a few months [1,2]. The introduction of cART has dramatically modified the HIV-related CNS diseases scenario, and new forms with different clinical presentations (see below) and better prognosis [18] have been described. The onset of the classical form of PML is usually multisymptomatic. Symptoms largely vary depending on location and size of lesions, but most frequent clinical presentation is characterized by motor deficits, altered consciousness, gait ataxia and visual symptoms [1,11]. Some authors have also reported the occurrence of epileptic seizures in 18% (16/89) of patients affected with PML. Epilepsy is usually related to the presence of lesions adjacent to the cortex and does not affect survival [19]. Atypical presentations include pure cerebellar syndrome, reflecting a productive infection of granule cell neurons [20], meningitis [21], meningoencephalitis [22,23], progressive myoclonic ataxia [24] and muscle wasting associated to extrapyramidal signs [25]. Although cerebellar atrophy was reported before in PML patients, the relationship between JCV and involvement of the cerebellum in PML was demonstrated for the first time in 2003 [26]. The authors demonstrated the presence of a productive infection of granule cell neurons by JCV, which had led to tissue loss in the internal granule cell layer. Shortly after, the first case of PML manifesting purely with cerebellar atrophy without white matter lesions was described [20], confirming the presence of selective infection of the cerebellar granular cell layer. JCV was also detected in CSF of a few patients presenting with clinical symptoms compatible with the diagnosis of meningitis or meningoencephalitis. No biopsy was performed in any of these cases, but the positivity for JCV in CSF and the absence of any other etiological agent seem to suggest a causative role for JCV in promoting an inflammatory process at the level of the meninges [21–23].
JCV has also been reported in human cancers, such as gioblastomas, astrocytomas, olygodendrogliomas and GI cancers and an extensive review of this was recently published [27]. The oncogenic features of JCV reside in viral proteins, T-antigen, agnoprotein and t-antigen, to disrupt cell cycle, prevent apoptosis and promote cell growth. While association between JCV and tumors in animal models and transformation of cultured cells is indisputable, a role of JCV in human cancers is still controversial, due to the difficulties involved with performing epidemiological studies and technical issues [27].
IMAGING FEATURES OF PML
While histopathology is still the gold standard diagnostic criterion for high sensitivity and specificity, imaging and laboratory tools are non-invasive, reliable and suitable in daily clinical practice. Typically, conventional MRI shows multifocal, bilateral, asymmetrical lesions involving subcortical white matter (WM) [28] in early stages of disease, subsequently spreading to deep periventricular WM. An MRI of a PML patient is shown in Figure 1. Lesions are principally located in supratentorial regions, at edge between cortex and WM, sometimes with involvement of U-fibers [29,30]. The parieto-occipital lobe is preferentially involved, followed by the frontal lobe [31]. Pathological processes may also involve deep WM, with periventricular distribution and corpus callosum. Infratentorial WM is commonly affected, with lesions located in middle cerebellar peduncles and adjacent regions, e.g., pons and mid-cerebellum [29,32]. PML may affect the GM, usually in association with WM involvement. Thalamus and basal ganglia are most frequently involved [29,32].
Figure 1. MRI of a case of PML.
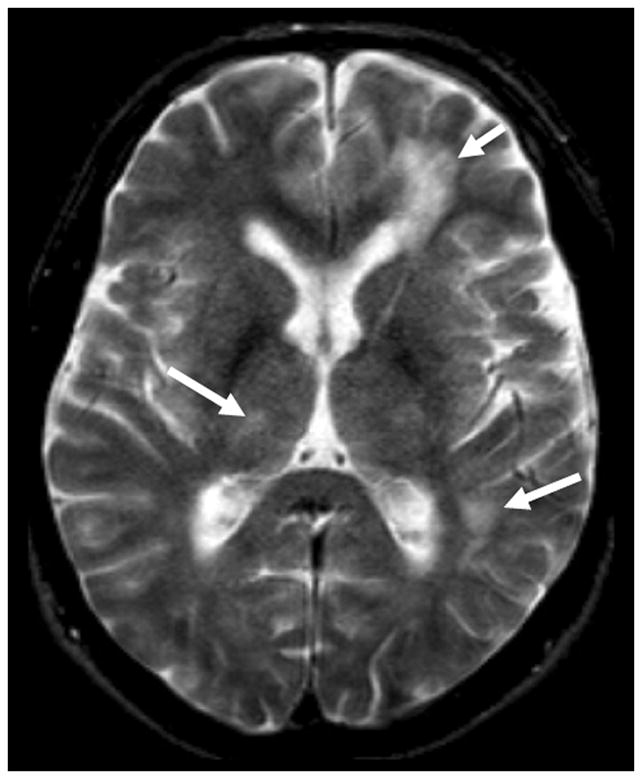
Shown is an axial T2 weighted sequence showing multiple hyperintese lesions involving both the superficial and the deep white and the grey matter (arrows).
PML lesions are well demarcated and appear as hyperintense in T2-weighted sequences and hypointense in T1-weighted sequences. Sometimes T1-weighted hyperintensity may be seen at the edge of the expanding lesions; the histopathological correlate is not clear, but might be related to presence of foamy macrophages in response to myelin breakdown, which has been frequently reported [17]. As there is very little or no inflammation, neither mass effect nor contrast enhancement are typical features of PML lesions. With progression of disease, lesions may coalesce and the central lesion may become necrotic with an increase of signal hypointensity in T1-weighted sequences [33]. Advanced MRI techniques give useful information on the pathogenetic mechanisms and allow non-invasive monitoring of disease evolution. Diffusion imaging studies have reported an increase of the mean diffusivity of water molecules in the center of evolving lesions and in old, inactive lesions, due to tissue injury and loss [34]. Instead, newly forming lesions and the edges of expanding lesions show a restriction of water mobility due to the reduction of extracellular space reflecting the presence of cellular infiltrates, large “bizarre” astrocytes, swollen oligodendrocytes and some infiltrating macrophages [4,34,35]. Lytic infection of oligodendrocytes results in diffuse, profound demyelination, whose MRI correlates are increased choline peaks in MR spectroscopy and a markedly reduced magnetization transfer ratio [36]. A decrease in N-acetylaspartate, a marker of neuronal damage, has also been reported [37], and is more pronounced in the older, central part of lesions [38].
LABORATORY FINDINGS FOR PML
Detection of JCV DNA in CSF by PCR had a sensitivity of 72–92% and specificity of 92–100% in the pre-cART era [39] but dropped to 58% after the introduction of cART. This is probably related to better immune control of virus and higher CSF clearance of virus by immune cells [40]. The problem of false PCR negatives, due to mutations in targeted regions, can be overcome by use of the highly conserved N-terminus of T-antigen rather than the VP1 region [41,42].
The occurrence of PML in patients treated with novel immunomodulant therapies prompted clinicians and scientists to look for biological markers that are more easily accessible, reliable and detectable than CSF for the early phase of the disease [3,8–10]. So far, no blood biomarkers of JCV activity have been identified; JCV DNA was detected in plasma of 0.3–0.6% of multiple sclerosis patients treated with natalizumab, similar to general population [43,44], in PBMCs of 40–60% of PML cases and in 40% of HIV infected patients with no PML [45]. The biological meaning of these findings is still unknown.
MOLECULAR BIOLOGY OF JCV
JCV is a member of the polyomavirus family of small non-enveloped DNA tumor viruses, which have small, circular, double-stranded DNA genomes [46]. JCV is one of the first two human polyomaviruses to be discovered in 1971 [5], the other being polyomavirus BK [47], which can cause BKV-associated nephropathy in kidney transplant recipients who receive highly immunosuppressive drugs and is a leading cause of allograft failure [46]. JCV was first isolated from PML brain by Padgett et al [5] and was the first direct evidence for a neurotropic virus associated with PML. JCV is now the proven causative agent of PML [1–3]. Recently, 7 more human polyomaviruses were isolated that are not as closely related to each other as is the case for JCV and BKV. This has prompted the subdivision of the family Polyomaviridae into three genera: Orthopolyomavirus (including JCV, BKV and SV40), Wukipolyomavirus (the new human viruses) and Avipolyomavirus, which infect birds [48].
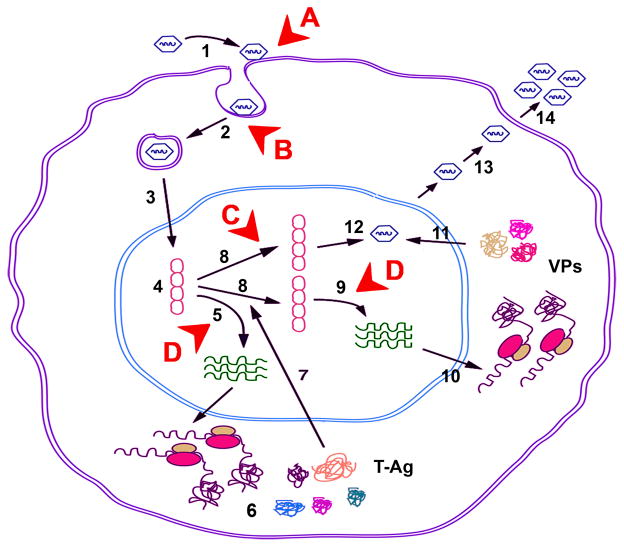
The JCV genome is divided into two protein-coding regions (early and late) transcribed in opposite directions starting from a common non-coding control region (NCCR). The NCCR is a bidirectional regulatory element containing promoter/enhancer elements for viral early and late genes and also the origin of viral DNA replication [46,49]. In cell culture, growth of JCV is largely restricted to primary human fetal glial cells. After infection and translocation of viral DNA to the nucleus, expression of JCV early mRNA occurs and this requires both on tissue-specific factors found only in glial cells and general transcription factors with ubiquitous tissue distribution [49,50]. During early phase, T-antigen accumulates and directs initiation of viral DNA replication and late transcription. Replicated DNA is packaged by viral capsid proteins to give mature virions, which are released from the cell [46,49,50]. In principle, any of the events that are part of the viral life cycle could provide a molecular target for therapeutic intervention. The events of the viral life cycle are illustrated in Figure 2 together with annotations of the targets where therapeutic drug intervention has been attempted.
Figure 2. Life cycle of JCV and therapeutic targets.
The steps in the life cycle of JCV are indicated by numbers in black as follow: 1 - adsorption of virus to cell surface receptors; 2 -entry by clathrin-dependent endocytosis; 3 - transport to the nucleus; 4 - uncoating; 5 -transcription of the early coding region; 6 - translation of early mRNAs to produce the early regulatory proteins, large T-antigen, small t-antigen and the alternatively spliced T′-antigens: T′135, T′136 and T′165; 7 - Nuclear localization of large T-antigen; 8 - replication of viral genomes; 9 - transcription of the viral late genes; 10 - translation of viral late transcript to produce agnoprotein and the capsid proteins (VP1, VP2 and VP3); 11 - nuclear localization of capsids; 12 - assembly of viral progeny in the nucleus; 13 - release of virions by an unknown mechanism; 14 - released virions. Targets for drug intervention are indicated by letters in red as follows: A - virus/receptor interaction; B - viral entry; C - viral replication; D - viral transcription.
The life cycle of JCV in the human body is considerably more complex and incompletely understood. Since antibodies to JCV can be detected in most people starting in childhood, it is generally held infection occurs early in life resulting in a primary viremia whereafter virus enters a “latent” state [2,49]. The events involved in primary infection, including the mode of viral transmission, route of entry and conversion to a latent state remain unknown since the primary infection is subclinical [2]. In most people, nothing more happens and the virus remains latent. However, in a few rare cases of individuals undergoing severe immunosuppression, JCV emerges from latency (reactivation) and multiplies in glial cells of CNS to cause PML [2]. There has been much debate on the nature of latency and its site(s) within the body. We have discussed this debate in detail in a recent review [2]. In summary, there are two main hypotheses.
Firstly, JCV latency and reactivation chiefly involves immune cells [3], especially B-lymphocytes in bone marrow, which act as a source of latent virus and allow virus to circulate around the body and enter brain. For example, natalizumab, which is associated with PML, may mobilize JCV-infected bone marrow cells leading to PML [51]. However, assessment of JCV DNA in blood and urine is not useful for predicting PML risk [44]. Further, JCV DNA was not detectable in PBMCs or magnetically separated CD34+ hematopoietic progenitor cells from the blood of 67 natalizumab-treated patients with MS and six PML patients, which does not support this hypothesis [52].
Secondly, JCV DNA may be present in brain prior to PML and that event(s) related to immunosuppression or HIV-1/AIDS initiates viral replication, i.e., brain is site of JCV latency and reactivation. As reviewed recently [2], our laboratory and four others published finding JCV DNA in normal brain. Since this review, two other laboratories have made similar reports [53,54].
While no clear consensus has emerged about the nature and site of JCV latency, these two hypotheses are not mutually exclusive [2]. Nevertheless, it is likely that transcriptional regulatory events that activate JCV early gene expression in glial cells are important in the JCV life cycle regardless of the time frame of their occurrence in relation to viral entry. Since this is crucial in the pathway from inactive viral DNA and the initiation of transcriptional activation and subsequently replication, this represents a fundamental interface of viral reactivation and commencement of PML [2,49,50]. Thus, it may be an important therapeutic target.
As indicated above in the section on the epidemiology of PML, it is a very rare disease. However, serological studies indicate that exposure to the virus is very common in the human population. Surveys in many countries found that antibody prevalence to JCV in adults can range from 66–92% indicating that exposure to the virus has a widespread geographic distribution [55]. Seroconversion begins in childhood and acquisition of antibody against JCV continues into middle age. We have recently reviewed seroepidemiological studies of JCV distribution within populations [2].
The high prevalence of JCV infection and yet the rarity of PML raises the possibility that rare variants or mutants of the virus are involved. In this regard, two regions of the JCV genome are of importance: (i) the NCCR, which as a bidirectional regulatory element controls both viral transcription and replication; (ii) the VP1 genotyping region, which is on the surface of the virions, binds to host cell surface receptors, confers the viral hemagglutination activity and is a target for host cell-mediated immune responses [46].
With regard to the NCCR, two genetic forms of the virus are recognized: the archetype and PML-type [2]. The archetypical form of the virus is found in kidney, urine and sewage. It is typified by the CY strain of JCV and may represent the transmissible form of the virus [56]. The PML-type form of JCV, also called prototypical, is always associated with PML and is typified by the Mad-1 strain of JCV [57]. Relative to archetype NCCR, prototypical Mad-1 JCV is characterized by two deletions and a duplication. It is thought that these deletion and duplication events yield higher rates viral DNA replication and infectivity in glial cells [58]. Indeed, Gosert et al [59] compared the activity of PML-type JCV NCCRs found in the CSF of eight HIV/AIDS patients to archetype NCCR using a bidirectional reporter vector and found that they confer increased early gene expression and higher replication rates compared to archetype. Thus the prototypical configuration of the JCV NCCR is likely important in the pathogenesis of PML. We have recently reviewed in detail the relationship between archetype and PML-type JCV [2].
The other polymorphic part of the JCV genome is the VP1 genotyping region, which was first recognized by serology and restriction fragment length polymorphisms and then defined by sequencing of different isolates and strains by sequencing with at least 8 subtypes having now been described [60]. Several reports indicate the importance of these polymorphisms in the pathogenesis of PML. Agostini et al [61] reported that Type 2B is more frequently found in PML brain than expected based on its prevalence in urine samples from a control normal population. Cloning and sequencing of whole JCV genomes from 3 PML patients revealed the occurrence of amino acid substitutions in VP1, many of which were located in the surface loops [62]. Further analysis of CSF from 20 PML patients revealed VP1 outer loop mutations to be widespread, the mutations at most polymorphic locations showing a trend toward a change to specific amino acids and VP1 loop mutations being associated with the progression of PML [63]. Differences in the type of VP1 loop substitution have also been reported between fast- and slow- progressing PML patients [64]. Recently, an extensive analysis of VP1 sequences provided evidence for the adaptive evolution of certain amino acids in JCV from PML patients but not in normal urine. These amino acids were located within the sialic acid binding site predicted by 3-D modeling [65]. Indeed, it has been shown using virus-like particles that VP1 mutations associated with PML can alter sialic acid binding, hemagglutination activity and receptor specificity [66]. Thus changes in the VP1 coding sequence are likely important in the pathogenesis of PML. Investigating the effects of these mutations is important not only for the understanding of PML but also in the design of therapeutic agents that block the association of JC virions with host cell surface receptors as described in the next section.
TREATMENT OF PML
A number of treatment options have been employed for PML, largely without success. These have targeted various points in viral life cycle such as entry and replication (Table 1). It was reported interaction between JCV and the serotonin 2A receptor (5-HT2AR) is necessary for viral entry. 5HT2AR antagonists inhibited JCV infection and monoclonal antibodies to 5HT2AR blocked infection of glial cells by JCV [67]. Nukuzuma et al [68] also reported the 5HT2AR antagonists ketanserin and ritanserin inhibited JCV infection and propagation. However, Chapagain et al [69] found risperidone, an antipsychotic that tightly binds 5HT2AR, had no effect on JCV infection of primary glial cells. A clinical report [70] on non-HIV-related PML showed beneficial effect the clinical and MRI picture. Mirtazapine, which is approved for treatment of major depression, is a selective serotonin reuptake inhibitor. There are several case reports on HIV [71] and non-HIV [72–74] PML demonstrating good tolerability and efficacy of this treatment, with neurological and MRI improvement. In most of these patients, however, other therapies were initiated (i.e., cART, cidofovir, cytarabine) in combination with mirtazapine, making assessment of mirtazapine efficacy difficult. Chlorpromazine inhibits clathrin-dependent endocytosis, and this is required for JCV infection [75]. Baum et al [76] found clozapine and chlorpromazine can inhibit JCV infection of glial cells in culture and low-dose combinations of both acted synergistically. The only clinical report describing a non-HIV PML case treated with chlorpromazine and cidofovir did not show any significant beneficial effect [77].
TABLE 1.
Summary of agents tested for activity against JCV and PMLa
| AGENT | TYPE | TARGET | LABORATORY | CLINIC |
|---|---|---|---|---|
| AraC (Cytarabine, Cytosine arabinoside) | Nucleoside Analog | Viral DNA | +b | −c |
| Cidofovir | Nucleoside Analog | Viral DNA | −b | −d |
| Topotecan/Camptothecin | Topoisomerase Inhibitor | Viral DNA | +e | +/−f |
| Antipsychotics | 5HT2A Agonists | Viral Entry | +/−g | +/−h |
| IL-2 | Immunostimulator | T-cells | N/A | (+)i |
| Interferon α | Antiviral | Replication | +j | −k |
| Interferon β | Antiviral | Replication | +j | −l |
| CMX001m | Derivatized Nucleoside Analog | Viral DNA | +n | (+)o |
| Mefloquine | Unknown | Unknown | +p | In Progressq |
| HAART | Anti-HIV-1 | HIV-1 | N/A | +r |
| LSTcs | Pentasaccharide | Viral Entry | +t | ND |
| C/EBPβ LIP | Transcription Factor | Viral Transcription | +u | ND |
| SF2/ASF Factor | Transcription | Viral Transcription | +v | ND |
| siRNA | RNAi | Viral mRNAs | +w | ND |
[79].
AIDS Clinical Trials Group 243 Team reported no benefit for HIV/PML patients on HAART [99]. Levy et al [124] have suggested that convection-enhanced intraparenchymal delivery (CEID) of cytosine arabinoside is effective based on a case report. In a retrospective study [100], no significant effect was reported. Aksamit [97] reported some success in treating non-AIDS PML.
De Luca et al [80] reported that cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated PML. Epker et al [84] reported ineffectiveness for both HIV+ and non-HIV PML. Yagi et al [91] reported a case study of successful treatment of PML developed in incomplete Heerfordt syndrome.
[101]
Topotecan is poorly tolerated but Royal et al [125] reported some success with a small group of patients.
[109]
[114]
1-O-hexadecyloxypropyl cidofovir
One case report [126].
[102]
[97]
NeuNAc-α2,6-Gal-β1,4-GlcNAc-β1,3-Gal-β1,4-Glc
[116]
[127]
[128]
Another class of possible therapeutic agents employed for PML inhibits viral replication. Cidofovir (CDV) is a nucleoside analogue commonly used for CMV- and HPV− related diseases. Due to its inhibitory action on DNA polymerase, its effect on JCV has been tested in vitro and in vivo with contradictory results. Andrei et al [78] demonstrated inhibition of mouse polyomavirus and SV40 replication in fibroblasts. However, a later study employing JCV and a human neuroglial cell line [79] showed no significant effect on replication. Since then, many case reports indicate efficacy of this drug on HIV− and non-HIV related PML. A recent study on 6 international cohorts of HIV+ patients affected by PML showed no significant impact of cidofovir on overall survival [80, confirming a previously reported pilot study [81] and other studies [82,83] and case reports [84]. Moreover, De Luca et al [80] reported an increased risk of neurological disability in patients treated with CDV. On the other hand, some studies suggest a role for CDV in prolonging survival in PML, although without significant effect on neurological outcome [85,86]. There are anecdotal reports on CDV efficacy in PML with different immune compromised states [87–93]. However, all clinical studies conducted so far are retrospective, observational and thus exposed to several biases, while the only pilot study was not randomized. To achieve a definite conclusion, a randomized clinical trial should be planned.
The low efficacy of CDV may reflect poor penetration and severe side effects (i.e., ocular and renal toxicity) limit use of this compound. Recently, a lipid derivative of CDV (CMX001) was found to reduce JCV replication up to 60% with no significant toxicity in a human cell line [93] suggesting it could be a promising candidate for the treatment of PML. Further evidence of the potential usefulness of this compound has recently been reported in a study that showed a significant inhibitory effect of CMX001 on JCV replication in primary human astrocytes, together with a very favorable toxicity profile [93,94].
Cytosine arabinoside (Ara-c) is another nucleoside analogue but was effective in decreasing JCV replication in cultured human neuroglial cells [79]. Different case reports show positive [72,95–97] and negative [98] results. The only randomized clinical trial on Ara-C administered either intravenously or intrathecally showed no efficacy in prolonging the survival [99]. An observational study on 13 HIV-PML patients showed poor prognosis but a non-significant trend toward longer survival was shown in patients treated with Ara-C [100]. Limitations of Ara-C could be short half-life, poor ability to cross the blood brain barrier (BBB) and bone marrow toxicity.
Other drugs that inhibit viral DNA replication act on DNA topoisomerase. Topotecan blocked JCV DNA replication with no effect on host transcription and translation [101]. Results of a phase II clinical trial on 11 patients with HIV-related PML showed response with prolonged survival in 27.2% (3/11) cases (Table 1).
Mefloquine is an anti-malarial agent effective in inhibiting JCV infection and replication in cell culture. The target of mefloquine is unknown but it may directly inhibit T-antigen [102]. Mefloquine has good penetration of the BBB. A randomized, clinical trial with mefloquine is currently under way (sponsored by Biogen Idec, NCT00746941). Some data on its efficacy come from two clinical reports on non-HIV PML [103,104] demonstrating neurological improvement and prolonged survival in both patients.
Since PML is associated with severe immunosuppression, other therapies aim to restore immune function. Recombinant IL-2 was anecdotally reported to give favorable outcomes in PML presenting with myelodysplastic syndromes [105] or after bone marrow transplantation [106,107]. The beneficial effect of IL-2 could be related to restoration of the immune system, as shown by an increase in the number of CD4+ cells [106,107].
T-cell therapy is another possible therapeutic option. A single case of PML was reported where the patient had received prolonged immunosuppression to treat severe graft versus host disease. The patient was infused with adoptive cytotoxic T lymphocytes from a donor after stimulation in vitro with 15-mer peptides derived from VP1 and large T viral proteins. Neurological improvement after cell therapy was reported [108].
In vitro,α- and β-interferons (IFN) were reported to inhibiting JCV replication and neutralizing anti-IFN antibody rescued the inhibitory effect of IFN [109]. Furthermore, β-IFN enhances expression of promyelocytic leukemia domains, which are nuclear bodies normally involved in gene expression and leads to inhibition of viral infection [110]. Supporting these experimental findings, an open-label non randomized study [111] reported longer survival and neurological improvement of PML, while other studies [112–114] failed to demonstrate any efficacy of IFN. These discrepancies may be due to systemic administration of IFN leading to poor CNS availability and hence IFN could be acting only as an immunomodulant rather than an antiviral.
In summary, all drugs tested generally show no significant impact on survival or neurological improvement (Table 2). The reasons are several, and include low ability to cross BBB and penetrate the CNS, variability of viral structure and complex virus/host interactions including the presence of more than one receptor for viral entry. The only regimen that seems to be effective is cART, which restores immune function rather than having a specific anti-JC viral activity.
TABLE 2.
Results of PML clinical trials.
| Drug | Pts | On drug | cART | Survival# | Neurological conditions# | Limits of the study | ||
|---|---|---|---|---|---|---|---|---|
| [81] | HIV-PML | CDV | 24 | 7 | 17 | = | = | Non randomized |
| [85] | HIV-PML | CDV | 46 | 22 | 46 | = (ns >) | = | Observational retrospective |
| [86] | HIV-PML | CDV | 43 | 16 | 43 | > | Initial, not maintained | Observational retrospective |
| [80] | HIV-PML | CDV | 370 | 185 | 370 | = | < | Observational retrospective |
| [82] | HIV-PML | CDV | 118 | 44 | 118 | Nr | = | Observational retrospective |
| [83] | HIV-PML | CDV | 33 | 14 | 17 | = | nr | Observational retrospective |
| [97] | Non HIV-PML | Ara-C | 19 | 19 | - | > in 7 pts | > in 7 pts | Open label non randomized |
| [99] | HIV-PML | Ara-C | 57 | 39 | 57 | = | nr | Open-label |
| [100] | HIV-PML | Ara-C | 13 | 8 | 7 AZT | => | = > | Observational retrospective |
| [125] | HIV-PML | Topotecan | 11 | 11 | 11 | Nr | = (>ns) | small sample size |
| [111] | HIV-PML | α-IFN | 77 | 21 | na | > | > | open label non randomized |
| [112] | HIV-PML | α-IFN | 4 | 4 | na | = | = | open label uncontrolled |
| [113] | HIV-PML | α-IFN | 97 | 36 | 32 | Nr | = | retrospective |
Nr: not reported; Ns: not significant; Na: not available
Survival and neurological conditions are reported as a comparison between patients receiving the medication and patients on cART alone.
FUTURE PERSPECTIVES
So far, the only therapeutic approach with some efficacy is restoration of the immune function, i.e., cART in HIV patients and rapid interruption of the immunosuppressant drug in non-HIV PML, e.g., patients receiving natalizumab. To identify effective therapies, unanswered questions remain such as where JCV rearranges and reactivates, the site of latency virus and the nature of specific humoral and cellular components involved in immune system response. The first issue is crucial, since the correct diagnostic assessment in an early phase of the disease, with the identification of the specific viral strain involved in the pathogenetic process, would help to tailor therapeutic regimen. Although in most cases the prototype virus is found in CSF and brain of PML patients, there are reports of archetype in the CSF of long-survivors PML [115]. Also, there is no direct evidence that prototype virus derived from archetype sequence in the patient and thus, the possibility of a second later infection by another type of JCV cannot be excluded [2].
There are a variety reasons why compounds have failed to show efficacy. These might be related to the properties of the drug itself such as low CNS penetration, toxicity or other factors. Also important may be properties of the virus or virus-host interactions. As discussed above, different JCV genotypes may vary in their receptor specificity. Also factors related to the host such as pharmacodynamics cannot be excluded. To overcome these limitations and optimize the efficacy of the treatment, we believe that a combination of different therapies targeting both virus and host immune system should be administered early in disease. Treatments against virus should interfere with both entry and replication. These targets are likely to be important regardless of the site of viral latency. Block of viral entry might be achieved with antagonists of proposed JCV receptors, 5-HT2AR and N-linked glycoprotein with α(2–6)-linked sialic acids. Compounds interacting with 5-HT2AR showed some efficacy, but more studies are needed. Recently, features of the N-linked glycoprotein have delineated a linear sialylated pentasaccharide present on host glycoproteins and glycolipids as specifically binding VP1 pentamers and may mediate the viral entry. Experimental data show that mutations in this oligosaccharide prevent cell attachment and viral spread [116]. The simultaneous administration of therapies interfering with both JCV receptors might thus increase overall efficacy. As noted above, one recent report has suggested that the generation of mutations in the VP1 genotyping region during the development of PML can alter the receptor specificity of JCV [62]. If this is confirmed to be the case, this may necessitate modifications to the design strategies employed for therapeutic entry inhibitors.
Other therapeutic strategies may interfere with viral DNA replication and transcription, but their main limitations are toxicity and low CNS penetration. These problems may be solved with an adjustment of structure such as addition of a hydrophobic moiety [93,94]. A possible further target is represented by proteins involved in the regulation of gene expression and viral replication, such as T-antigen and agnoprotein. Experimental data show that siRNA against T-antigen and agnoprotein inhibit JCV replication in culture [117,118] and treatment with siRNA against agnoprotein inhibited virus in JCV-infected human cells inoculated into nude mice brains [119].
The other main therapeutic target is the immune system. The Swiss HIV Cohort Study reported that PML survivors showed significant increases in JCV-specific T-cells and IgG responses and that IgG responses in survivors positively correlated with CD4+ count and negatively with HIV load [15]. The humoral immune response, i.e., virus-specific IgG in the blood or intrathecal synthesis of IgG in the CSF, is unable to contain the progression of PML, while T-CD4+ and B cells might be implicated to some extent and T-CD8+ cells seem to be the main effectors of the cellular response, eliminating infected cells and preventing viral spread [120]. Thus, boosting the immune system is considered a valuable therapeutic approach although a rapid restoration of immune response can lead to a severe progression of symptoms due to immune reconstitution inflammatory syndrome (IRIS), which is predominantly related to an exaggerated reaction of T-CD8+ cells [121]. Therefore, the ideal approach should specifically target the population of cells activated by PML, rather than promote a broad-spectrum recruitment of the immune system. For example, it was recently reported that JCV-specific T-CD8+ cells expansion can be elicited by dendritic cells stimulation even during the early stage of the disease and may impact on survival and neurological improvement [122]. A better comprehension of immune response against JCV infection is needed to identify strategies with a high efficacy and low iatrogenic complications.
In conclusion, although elements of JCV pathogenesis remain to be elucidated, several possible therapeutic targets have been identified. A combined approach with multiple compounds both interfering with different phases of the viral cycle and boosting specific components of the immune system involved in the response to JCV infection should be used. The advantages of this combined treatment are several, including the possibility to administer lower dosages of each single therapy. This may reduce drug-related toxicity and the risk of side effects while increasing overall therapeutic efficacy. We would also like to highlight the importance of a correct diagnostic assessment of the viral strain involved in the pathogenesis of PML, and the urgent need to identify possible risk factors and sensitive biomarkers detectable in the early phase of the disease.
Acknowledgments
We wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for their continued support, insightful discussion, and sharing of reagents and ideas. We also wish to thank C. Papaleo for editorial assistance. This work was made possible by grants awarded by the NIH to MKW and KK.
Abbreviations
- PML
Progressive multifocal leukoencephalopathy
- JCV
Polyomavirus JC
- cART
Combination antiretroviral therapy
- WM
White matter
- 5-HT2AR
Serotonin 2A receptor
- NCCR
Non-coding control region
- CDV
Cidofovir
- HPV
Human papillomavirus
- Ara-c
Cytosine arabinoside
- BBB
Blood brain barrier
References
- 1.Berger JR. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf. 2010;33:969–883. doi: 10.2165/11537510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy – revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 4.Åström KE, Mancall EL, Richardson EP. Progressive multifocal encephalopathy: A hitherto unrecognized complication of chronic lymphocytic leukemia and lymphoma. Brain. 1958;81:99–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Padgett BL, Zu Rhein GM, Walker DL, Echroade R, Dessel B. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;i:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 6.Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6:667–679. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- 7.Focosi D, Marco T, Kast RE, Maggi F, Ceccherini-Nelli L, Petrini M. Progressive multifocal leukoencephalopathy: what’s new? Neuroscientist. 2010;16:308–323. doi: 10.1177/1073858409356594. [DOI] [PubMed] [Google Scholar]
- 8.Marshall LJ, Major EO. Molecular regulation of JC virus tropism: insights into potential therapeutic targets for progressive multifocal leukoencephalopathy. J Neuroimmune Pharmacol. 2010;5:404–417. doi: 10.1007/s11481-010-9203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warnke C, Menge T, Hartung HP, et al. Natalizumab and progressive multifocal leukoencephalopathy: what are the causal factors and can it be avoided? Arch Neurol. 2010;67:923–930. doi: 10.1001/archneurol.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JR, Kaszovitz B, Post MJ, Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med. 1987;107:78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie SM, Chang Y, Lemp G, et al. Progressive multifocal leukoencephalopathy in persons infected with human immunodeficiency virus, San Francisco, 1981–1989. Ann Neurol. 1991;30:597–604. doi: 10.1002/ana.410300413. [DOI] [PubMed] [Google Scholar]
- 13.Martínez AJ, Sell M, Mitrovics T, et al. The neuropathology and epidemiology of AIDS. A Berlin experience. A review of 200 cases. Pathol Res Pract. 1995;191:427–443. doi: 10.1016/S0344-0338(11)80730-2. [DOI] [PubMed] [Google Scholar]
- 14.d’Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 15.Khanna N, Elzi L, Mueller NJ, et al. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV Cohort Study. Clin Infect Dis. 2009;48:1459–1466. doi: 10.1086/598335. [DOI] [PubMed] [Google Scholar]
- 16.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 2009;60:3761–3765. doi: 10.1002/art.24966. [DOI] [PubMed] [Google Scholar]
- 17.Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9:625–636. doi: 10.1016/S1473-3099(09)70226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delbue S, Marchioni E, Sotgiu G, et al. Longitudinal study of two cases of progressive multifocal leukoencephalopathy with a clinical benign evolution. J Neurovirol. 2007;13:268–273. doi: 10.1080/13550280701291796. [DOI] [PubMed] [Google Scholar]
- 19.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 20.Koralnik IJ, Wüthrich C, Dang X, Rottnek M, Gurtman A, Simpson D, Morgello S. JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–580. doi: 10.1002/ana.20431. [DOI] [PubMed] [Google Scholar]
- 21.Viallard JF, Ellie E, Lazaro E, Lafon ME, Pellegrin JL. JC virus meningitis in a patient with systemic lupus erythematosus. Lupus. 2005;14:964–966. doi: 10.1191/0961203305lu2229cr. [DOI] [PubMed] [Google Scholar]
- 22.Blake K, Pillay D, Knowles W, Brown DW, Griffiths PD, Taylor B. JC virus associated meningoencephalitis in an immunocompetent girl. Arch Dis Child. 1992;67:956–957. doi: 10.1136/adc.67.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Bonington A. BKV-DNA and JCV-DNA in CSF of patients with suspected meningitis or encephalitis. Infection. 2003;31:374–378. doi: 10.1007/s15010-003-3078-5. [DOI] [PubMed] [Google Scholar]
- 24.Fontoura P, Vale J, Lima C, Scaravilli F, Guimarães J. Progressive myoclonic ataxia and JC virus encephalitis in an AIDS patient. J Neurol Neurosurg Psychiatry. 2003;72:653–656. doi: 10.1136/jnnp.72.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallantyre EC, Paine SM, Sharp CP, Lowe JS, Gran B. Atypical progressive multifocal leukoencephalopathy associated with an unusual JC polyomavirus mutation. Arch Neurol. 2009;66:1021–1024. doi: 10.1001/archneurol.2009.94. [DOI] [PubMed] [Google Scholar]
- 26.Du Pasquier RA, Corey S, Margolin DH, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61:775–782. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- 27.Del Valle L, White MK, Khalili K. Potential mechanisms of the human polyomavirus JC in neural oncogenesis. J Neuropathol Exp Neurol. 2008;67:729–740. doi: 10.1097/NEN.0b013e318180e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilleux MH, Steiner RE, Young IR. MR imaging in progressive multifocal leukoencephalopathy. AJNR Am J Neuroradiol. 1986;7:1033–1035. [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993;187:233–240. doi: 10.1148/radiology.187.1.8451420. [DOI] [PubMed] [Google Scholar]
- 30.Shah R, Bag AK, Chapman PR, Curé JK. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol. 2010;65:431–439. doi: 10.1016/j.crad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Giancola ML, Rizzi EB, Lorenzini P, et al. Progressive multifocal leukoencephalopathy in HIV-infected patients in the era of HAART: radiological features at diagnosis and follow-up and correlation with clinical variables. AIDS Res Hum Retroviruses. 2008;24:155–162. doi: 10.1089/aid.2006.0252. [DOI] [PubMed] [Google Scholar]
- 32.Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896–1906. [PMC free article] [PubMed] [Google Scholar]
- 33.Mark AS, Atlas SW. Progressive multifocal leukoencephalopathy in patients with AIDS: appearance on MR images. Radiology. 1989;173:517–520. doi: 10.1148/radiology.173.2.2798883. [DOI] [PubMed] [Google Scholar]
- 34.Bergui M, Bradac GB, Oguz KK, Boghi A, Geda C, Gatti G, Schiffer D. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology. 2004;46:22–25. doi: 10.1007/s00234-003-1115-9. [DOI] [PubMed] [Google Scholar]
- 35.Richardson EP. Progressive multifocal leukoencephalopathy. N Engl J Med. 1961;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- 36.Dousset V, Armand JP, Lacoste D, Mièze S, Letenneur L, Dartigues JF, Caill JM. Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. Groupe d’Epidémiologie Clinique du SIDA en Aquitaine. AJNR Am J Neuroradiol. 1997;18:895–901. [PMC free article] [PubMed] [Google Scholar]
- 37.Hurley RA, Ernst T, Khalili K, Del Valle L, Simone IL, Taber KH. Identification of HIV-associated progressive multifocal leukoencephalopathy: magnetic resonance imaging and spectroscopy. J Neuropsychiatry Clin Neurosci. 2003;15:1–6. doi: 10.1176/jnp.15.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JH, Bang OY, Kim HS. Progressive Multifocal Leukoencephalopathy in AIDS: Proton MR Spectroscopy Patterns of Asynchronous Lesions Confirmed by Serial Diffusion-Weighted Imaging and Apparent Diffusion Coefficient Mapping. J Clin Neurol. 2007;3:200–203. doi: 10.3988/jcn.2007.3.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253–260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 40.Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43:4175–4177. doi: 10.1128/JCM.43.8.4175-4177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43:4175–4177. doi: 10.1128/JCM.43.8.4175-4177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landry ML, Eid T, Bannykh S, Major E. False negative PCR despite high levels of JC virus DNA in spinal fluid: Implications for diagnostic testing. J Clin Virol. 2008;43:247–249. doi: 10.1016/j.jcv.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorelik L, Goelz S, Sandrock AW. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:2487–2488. doi: 10.1056/NEJMc0909622. [DOI] [PubMed] [Google Scholar]
- 44.Rudick RA, O’Connor PW, Polman CH, et al. Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol. 2010;68:304–310. doi: 10.1002/ana.22107. [DOI] [PubMed] [Google Scholar]
- 45.Dubois V, Lafon ME, Ragnaud JM, et al. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. AIDS. 1996;10:353–358. doi: 10.1097/00002030-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott, Williams & Wilkins; Philadelphia: 2007. pp. 2263–2298. [Google Scholar]
- 47.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K) isolated from urine after renal transplantation. Lancet. 1971;19:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 48.Johne R, Buck CB, Allander T, Atwood WJ, Garcea RL, Imperiale MJ, Major EO, Ramqvist T, Norkin LC. Taxonomical developments in the family Polyomaviridae. Arch Virol. 2011 doi: 10.1007/s00705-011-1008-x. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalili K, Safak M, Del Valle L, White MK. JC virus molecular biology and the human demyelinating disease, progressive multifocal leukoencephalopathy. In: Shoshkes Reiss C, editor. Neurotropic virus infections. Vol. 10. Cambridge University Press; Cambridge, UK: 2008. pp. 190–211. [Google Scholar]
- 50.White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ransohoff RM. Natalizumab and PML. Nat Neurosci. 2005;8:1275. doi: 10.1038/nn1005-1275. [DOI] [PubMed] [Google Scholar]
- 52.Warnke C, Smolianov V, Dehmel T, et al. CD34+ progenitor cells mobilized by natalizumab are not a relevant reservoir for JC virus. Mult Scler. 2011;17:151–156. doi: 10.1177/1352458510385834. [DOI] [PubMed] [Google Scholar]
- 53.Lam WY, Leung BW, Chu IM, Chan AC, Ng HK, Chan PK. Survey for the presence of BK, JC, KI, WU and Merkel cell polyomaviruses in human brain tissues. J Clin Virol. 2010;48:11–14. doi: 10.1016/j.jcv.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Tan CS, Ellis LC, Wüthrich C, et al. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol. 2010;84:9200–9209. doi: 10.1128/JVI.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker DL, Padgett BL. The epidemiology of human polyomaviruses. Prog Clin Biol Res. 1983;105:99–106. [PubMed] [Google Scholar]
- 56.Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniel AM, Swenson JJ, Mayreddy RP, Khalili K, Frisque RJ. Sequences within the early and late promoters of archetype JC virus restrict viral DNA replication and infectivity. Virology. 1996;216:90–101. doi: 10.1006/viro.1996.0037. [DOI] [PubMed] [Google Scholar]
- 59.Gosert R, Kardas P, Major EO, Hirsch HH. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J Virol. 2010;84:10448–10456. doi: 10.1128/JVI.00614-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cubitt CL, Cui X, Agostini HT, et al. Predicted amino acid sequences for 100 JCV strains. J Neurovirol. 2001;7:339–344. doi: 10.1080/13550280152537201. [DOI] [PubMed] [Google Scholar]
- 61.Agostini HT, Ryschkewitsch CF, Singer EJ, Baumhefner RW, Stoner GL. JC virus type 2B is found more frequently in brain tissue of progressive multifocal leukoencephalopathy patients than in urine from controls. J Hum Virol. 1998;1:200–206. [PubMed] [Google Scholar]
- 62.Zheng HY, Takasaka T, Noda K, et al. New sequence polymorphisms in the outer loops of the JC polyomavirus major capsid protein (VP1) possibly associated with progressive multifocal leukoencephalopathy. J Gen Virol. 2005;86:2035–2045. doi: 10.1099/vir.0.80863-0. [DOI] [PubMed] [Google Scholar]
- 63.Zheng HY, Ikegaya H, Takasaka T, et al. Characterization of the VP1 loop mutations widespread among JC polyomavirus isolates associated with progressive multifocal leukoencephalopathy. Biochem Biophys Res Commun. 2005;333:996–1002. doi: 10.1016/j.bbrc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Delbue S, Branchetti E, Bertolacci S, et al. JC virus VP1 loop-specific polymorphisms are associated with favorable prognosis for progressive multifocal leukoencephalopathy. J Neurovirol. 2009;15:51–56. doi: 10.1080/13550280802425467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sunyaev SR, Lugovskoy A, Simon K, Gorelik L. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML) PLoS Genet. 2009;5:e1000368. doi: 10.1371/journal.pgen.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorelik L, Reid C, Testa M, et al. Progressive Multifocal Leukoencephalopathy (PML) Development Is Associated With Mutations in JC Virus Capsid Protein VP1 That Change Its Receptor Specificity. J Infect Dis. 2011;204:103–114. doi: 10.1093/infdis/jir198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 68.Nukuzuma S, Nakamichi K, Nukuzuma C, Takegami T. Inhibitory effect of serotonin antagonists on JC virus propagation in a carrier culture of human neuroblastoma cells. Microbiol Immunol. 2009;53:496–501. doi: 10.1111/j.1348-0421.2009.00156.x. [DOI] [PubMed] [Google Scholar]
- 69.Chapagain ML, Sumibcay L, Gurjav U, Kaufusi PH, Kast RE, Nerurkar VR. Serotonin receptor 2A blocker (risperidone) has no effect on human polyomavirus JC infection of primary human fetal glial cells. J Neurovirol. 2008;14:448–454. doi: 10.1080/13550280802235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Focosi D, Kast RE, Maggi F, Ceccherini-Nelli L, Petrini M. Risperidone-induced reduction in JC viruria as a surrogate marker for efficacy against progressive multifocal leukoencephalopathy and hemorrhagic cystitis. J Clin Virol. 2007;39:63–64. doi: 10.1016/j.jcv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Cettomai D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. 2009;66:255–258. doi: 10.1001/archneurol.2008.557. [DOI] [PubMed] [Google Scholar]
- 72.Vulliemoz S, Lurati-Ruiz F, Borruat FX, et al. Favourable outcome of progressive multifocal leucoencephalopathy in two patients with dermatomyositis. J Neurol Neurosurg Psychiatry. 2006;77:1079–1082. doi: 10.1136/jnnp.2006.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Owczarczyk K, Hilker R, Brunn A, Hallek M, Rubbert A. Progressive multifocal leucoencephalopathy in a patient with sarcoidosis--successful treatment with cidofovir and mirtazapine. Rheumatology (Oxford) 2007;46:888–890. doi: 10.1093/rheumatology/kem049. [DOI] [PubMed] [Google Scholar]
- 74.Verma S, Cikurel K, Koralnik IJ, et al. Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis. 2007;196:709–711. doi: 10.1086/520514. [DOI] [PubMed] [Google Scholar]
- 75.Pho MT, Ashok A, Atwood WJ. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74:2288–2292. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baum S, Ashok A, Gee G, Dimitrova S, Querbes W, Jordan J, Atwood WJ. Early events in the life cycle of JC virus as potential therapeutic targets for the treatment of progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9 (Suppl 1):32–37. doi: 10.1080/13550280390195342. [DOI] [PubMed] [Google Scholar]
- 77.Pöhlmann C, Hochauf K, Röllig C, et al. Chlorpromazine combined with cidofovir for treatment of a patient suffering from progressive multifocal leukoencephalopathy. Intervirology. 2007;50:412–417. doi: 10.1159/000112916. [DOI] [PubMed] [Google Scholar]
- 78.Andrei G, Snoeck R, Vandeputte M, De Clercq E. Activities of various compounds against murine and primate polyomaviruses. Antimicrob Agents Chemother. 1997;41:587–593. doi: 10.1128/aac.41.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou J, Major EO. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol. 1998;4:451–456. doi: 10.3109/13550289809114545. [DOI] [PubMed] [Google Scholar]
- 80.De Luca A, Ammassari A, Pezzotti P, et al. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS. 2008;22:1759–1767. doi: 10.1097/QAD.0b013e32830a5043. [DOI] [PubMed] [Google Scholar]
- 81.Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS. 2002;16:1791–1797. doi: 10.1097/00002030-200209060-00012. [DOI] [PubMed] [Google Scholar]
- 82.Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2003;36:1047–1052. doi: 10.1086/374048. [DOI] [PubMed] [Google Scholar]
- 83.Kraemer C, Evers S, Nolting T, Arendt G, Husstedt IW. Cidofovir in combination with HAART and survival in AIDS-associated progressive multifocal leukoencephalopathy. J Neurol. 2008;255:526–531. doi: 10.1007/s00415-008-0731-z. [DOI] [PubMed] [Google Scholar]
- 84.Epker JL, van Biezen P, van Daele PL, van Gelder T, Vossen A, van Saase JL. Progressive multifocal leukoencephalopathy, a review and an extended report of five patients with different immune compromised states. Eur J Intern Med. 2009;20:261–267. doi: 10.1016/j.ejim.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 85.Gasnault J, Kousignian P, Kahraman M, Rahoiljaon J, Matheron S, Delfraissy JF, Taoufik Y. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J Neurovirol. 2001;7:375–381. doi: 10.1080/13550280152537274. [DOI] [PubMed] [Google Scholar]
- 86.De Luca A, Giancola ML, Ammassari A, et al. Potent anti-retroviral therapy with or without cidofovir for AIDS-associated progressive multifocal leukoencephalopathy: extended follow-up of an observational study. J Neurovirol. 2001;7:364–368. doi: 10.1080/13550280152537256. [DOI] [PubMed] [Google Scholar]
- 87.Portilla J, Boix V, Román F, Reus S, Merino E. Progressive multifocal leukoencephalopathy treated with cidofovir in HIV-infected patients receiving highly active anti-retroviral therapy. J Infect. 2000;41:182–184. doi: 10.1053/jinf.2000.0704. [DOI] [PubMed] [Google Scholar]
- 88.De Luca A, Fantoni M, Tartaglione T, Antinori A. Response to cidofovir after failure of antiretroviral therapy alone in AIDS-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52:891–892. doi: 10.1212/wnl.52.4.891. [DOI] [PubMed] [Google Scholar]
- 89.De Luca A, Fantoni M, Tartaglione T, Antinori A. Response to cidofovir after failure of antiretroviral therapy alone in AIDS-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52:891–892. doi: 10.1212/wnl.52.4.891. [DOI] [PubMed] [Google Scholar]
- 90.Razonable RR, Aksamit AJ, Wright AJ, Wilson JW. Cidofovir treatment of progressive multifocal leukoencephalopathy in a patient receiving highly active antiretroviral therapy. Mayo Clin Proc. 2001;76:1171–1175. doi: 10.4065/76.11.1171. [DOI] [PubMed] [Google Scholar]
- 91.Yagi T, Hattori H, Ohira M, et al. Progressive multifocal leukoencephalopathy developed in incomplete Heerfordt syndrome, a rare manifestation of sarcoidosis, without steroid therapy responding to cidofovir. Clin Neurol Neurosurg. 2010;112:153–156. doi: 10.1016/j.clineuro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Reilmann R, Imai T, Ringelstein EB, Gaubitz M, Niederstadt TU, Paulus W, Husstedt IW. Remission of progressive multifocal leucoencephalopathy in SLE after treatment with cidofovir: a 4 year follow up. J Neurol Neurosurg Psychiatry. 2005;76:1304–1305. doi: 10.1136/jnnp.2004.057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang ZG, Cohen J, Marshall LJ, Major EO. Hexadecyloxypropyl-cidofovir (CMX001) suppresses JC virus replication in human fetal brain SVG cell cultures. Antimicrob Agents Chemother. 2010;54:4723–4732. doi: 10.1128/AAC.00837-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gosert R, Rinaldo CH, Wernli M, Major EO, Hirsch HH. CMX001 (1-O-hexadecyloxypropyl-cidofovir) inhibits polyomavirus JC replication in human brain progenitor-derived astrocytes. Antimicrob Agents Chemother. 2011;55:2129–2136. doi: 10.1128/AAC.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Portegies P, Algra PR, Hollak CE, Prins JM, Reiss P, Valk J, Lange JM. Response to cytarabine in progressive multifocal leucoencephalopathy in AIDS. Lancet. 1991;337:680–681. doi: 10.1016/0140-6736(91)92504-u. [DOI] [PubMed] [Google Scholar]
- 96.Nicoli F, Chave B, Peragut JC, Gastaut JL. Efficacy of cytarabine in progressive multifocal leucoencephalopathy in AIDS. Lancet. 1992;339:306. doi: 10.1016/0140-6736(92)91376-j. [DOI] [PubMed] [Google Scholar]
- 97.Aksamit AJ. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol. 2001;7:386–390. doi: 10.1080/13550280152537292. [DOI] [PubMed] [Google Scholar]
- 98.Guarino M, D’Alessandro R, Rinaldi R, et al. Progressive multifocal leucoencephalopathy in AIDS: treatment with cytosine arabinoside. AIDS. 1995;9:819–820. [PubMed] [Google Scholar]
- 99.Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338:1345–1351. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- 100.Moreno S, Miralles P, Díaz MD, et al. Cytarabine therapy for progressive multifocal leukoencephalopathy in patients with AIDS. Clin Infect Dis. 1996;23:1066–1068. doi: 10.1093/clinids/23.5.1066. [DOI] [PubMed] [Google Scholar]
- 101.Kerr DA, Chang CF, Gordon J, Bjornsti MA, Khalili K. Inhibition of human neurotropic virus (JCV) DNA replication in glial cells by camptothecin. Virology. 1993;196:612–618. doi: 10.1006/viro.1993.1517. [DOI] [PubMed] [Google Scholar]
- 102.Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–1849. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gofton TE, Al-Khotani A, O’Farrell B, Ang LC, McLachlan RS. Mefloquine in the treatment of progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2011;82:452–455. doi: 10.1136/jnnp.2009.190652. [DOI] [PubMed] [Google Scholar]
- 104.Kishida S, Tanaka K. Mefloquine treatment in a patient suffering from progressive multifocal leukoencephalopathy after umbilical cord blood transplant. Intern Med. 2010;49:2509–2513. doi: 10.2169/internalmedicine.49.3227. [DOI] [PubMed] [Google Scholar]
- 105.Kunschner L, Scott TF. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL-2. Neurology. 2005;65:1510. doi: 10.1212/01.wnl.0000183064.10227.b5. [DOI] [PubMed] [Google Scholar]
- 106.Buckanovich RJ, Liu G, Stricker C, et al. Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin’s lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy. Ann Hematol. 2002;81:410–413. doi: 10.1007/s00277-002-0481-4. [DOI] [PubMed] [Google Scholar]
- 107.Przepiorka D, Jaeckle KA, Birdwell RR, Fuller GN, Kumar AJ, Huh YO, McCutcheon I. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 1997;20:983–987. doi: 10.1038/sj.bmt.1701010. [DOI] [PubMed] [Google Scholar]
- 108.Balduzzi A, Lucchini G, Hirsch HH, et al. Polyomavirus JC-targeted T-cell therapy for progressive multiple leukoencephalopathy in a hematopoietic cell transplantation recipient. Bone Marrow Transplant. 2010;46:987–992. doi: 10.1038/bmt.2010.221. [DOI] [PubMed] [Google Scholar]
- 109.Co JK, Verma S, Gurjav U, Sumibcay L, Nerurkar VR. Interferon- alpha and - beta restrict polyomavirus JC replication in primary human fetal glial cells: implications for progressive multifocal leukoencephalopathy therapy. J Infect Dis. 2007;196:712–718. doi: 10.1086/520518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gasparovic ML, Maginnis MS, O’Hara BA, Dugan AS, Atwood WJ. Modulation of PML protein expression regulates JCV infection. Virology. 2009;390:279–288. doi: 10.1016/j.virol.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang SS, Skolasky RL, Dal Pan GJ, Royal W, 3rd, McArthur JC. Survival prolongation in HIV-associated progressive multifocal leukoencephalopathy treated with alpha-interferon: an observational study. J Neurovirol. 1998;4:324–332. doi: 10.3109/13550289809114533. [DOI] [PubMed] [Google Scholar]
- 112.Counihan T, Venna N, Craven D, Sabin TD. Alpha Interferon in AIDS-Related Progressive Multifocal Leukoencephalopathy. J NeuroAIDS. 1996;1:79–88. doi: 10.1300/j128v01n04_08. [DOI] [PubMed] [Google Scholar]
- 113.Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol. 2001;7:353–357. doi: 10.1080/13550280152537238. [DOI] [PubMed] [Google Scholar]
- 114.Nath A, Venkataramana A, Reich DS, Cortese I, Major EO. Progression of progressive multifocal leukoencephalopathy despite treatment with beta-interferon. Neurology. 2006;66:149–150. doi: 10.1212/01.wnl.0000191322.93310.a1. [DOI] [PubMed] [Google Scholar]
- 115.Ferrante P, Delbue S, Pagani E, et al. Analysis of JC virus genotype distribution and transcriptional control region rearrangements in human immunodeficiency virus-positive progressive multifocal leukoencephalopathy patients with and without highly active antiretroviral treatment. J Neurovirol. 2003;9 (Suppl 1):42–46. doi: 10.1080/13550280390195405. [DOI] [PubMed] [Google Scholar]
- 116.Neu U, Maginnis MS, Palma AS, et al. Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe. 2010;8:309–319. doi: 10.1016/j.chom.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orba Y, Sawa H, Iwata H, Tanaka S, Nagashima K. Inhibition of virus production in JC virus-infected cells by postinfection RNA interference. J Virol. 2004;78:7270–7273. doi: 10.1128/JVI.78.13.7270-7273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radhakrishnan S, Gordon J, Del Valle L, Cui J, Khalili K. Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection. J Virol. 2004;78:7264–7269. doi: 10.1128/JVI.78.13.7264-7269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matoba T, Orba Y, Suzuki T, et al. An siRNA against JC virus (JCV) agnoprotein inhibits JCV infection in JCV-producing cells inoculated in nude mice. Neuropathol. 2008;28:286–294. doi: 10.1111/j.1440-1789.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 120.Matoba T, Orba Y, Suzuki T, et al. An siRNA against JC virus (JCV) agnoprotein inhibits JCV infection in JCV-producing cells inoculated in nude mice. Neuropathol. 2008;28:286–294. doi: 10.1111/j.1440-1789.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 121.Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol. 2005;109:449–455. doi: 10.1007/s00401-005-0983-y. [DOI] [PubMed] [Google Scholar]
- 122.Marzocchetti A, Lima M, Tompkins T, Kavanagh DG, Gandhi RT, O’Neill DW, Bhardwaj N, Koralnik IJ. Efficient in vitro expansion of JC virus-specific CD8(+) T-cell responses by JCV peptide-stimulated dendritic cells from patients with progressive multifocal leukoencephalopathy. Virology. 2009;383:173–177. doi: 10.1016/j.virol.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hernández B, Dronda F, Moreno S. Treatment options for AIDS patients with progressive multifocal leukoencephalopathy. Expert Opin Pharmacother. 2009;10:403–416. doi: 10.1517/14656560802707994. [DOI] [PubMed] [Google Scholar]
- 124.Levy RM, Major E, Ali MJ, Cohen B, Groothius D. Convection-enhanced intraparenchymal delivery (CEID) of cytosine arabinoside (AraC) for the treatment of HIV-related progressive multifocal leukoencephalopathy (PML) J Neurovirol. 2001;7:382–385. doi: 10.1080/13550280152537283. [DOI] [PubMed] [Google Scholar]
- 125.Royal W, Dupont B, McGuire D, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9:411–419. doi: 10.1080/13550280390201740. [DOI] [PubMed] [Google Scholar]
- 126.Patel A, Patel J, Ikwuagwu J. Treatment of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. Antimicrob Chemother. 2010;65:2489–2492. doi: 10.1093/jac/dkq389. [DOI] [PubMed] [Google Scholar]
- 127.Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPbeta. Virus Res. 2010;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sariyer IK, Khalili K. Regulation of human neurotropic JC virus replication by alternative splicing factor SF2/ASF in Glial Cells. Plos One. 2011;6:e14630. doi: 10.1371/journal.pone.0014630. [DOI] [PMC free article] [PubMed] [Google Scholar]