Abstract
Advanced prostate cancer (PCa) is the second-leading cause of cancer-related deaths among American men. The androgen receptor (AR) is vital for PCa progression, even in the face of castrate levels of serum testosterone following androgen ablation therapy, a mainstay therapy for advanced PCa. Downregulation of superoxide dismutase 2 (SOD2), a major intracellular antioxidant enzyme, occurs progressively during PCa progression to advanced states, and is known to promote AR activity in PCa. Therefore, this study investigated the effects of SOD mimetics on AR expression and function in AR-dependent LNCaP, CWR22Rv1, and LAPC-4AD PCa cells. Treatment with Tempol, a SOD mimetic, not only lowered cellular superoxide levels, but also concomitantly attenuated AR transcriptional activity and AR target gene expression in a dose- and time-dependent manner, in the presence and absence of dihydrotestosterone, the major endogenous AR agonist. Tempol's inhibition of AR was mediated, in large part, by its ability to decrease AR protein via increased degradation, in the absence of any inhibitory effects on other nuclear receptors. Tempol's inhibitory effects on AR were also reproducible with other SOD mimetics, MnTBAP and MnTMPyP. Importantly, Tempol's effects on AR function were accompanied by significant in vitro and in vivo reduction in castration-resistant PCa survival and growth. Collectively, this study has demonstrated for the first time that SOD mimetics, by virtue of their ability to suppress AR function, may be beneficial in treating the currently incurable castration-resistant PCa in which SOD2 expression is highly suppressed.
Keywords: androgen receptor, SOD mimetics, castration-resistant prostate cancer
Introduction
The androgen receptor (AR), a transcription factor that mediates the biological effects of androgens, testosterone and dihydrotestosterone (DHT), is vital for the development and progression of prostate cancer (PCa). After an initial response to androgen ablation therapy, which suppresses AR signaling, the majority of advanced tumors eventually transition to the currently incurable androgen-independent or castration-resistant prostate cancer (CRPC) (1, 2). Importantly, CRPC continues to be highly dependent on the persistent expression and function of AR to survive and progress (3, 4). Studies reporting significant inhibition of in vitro and in vivo growth of CRPC following disruption of AR expression and/or function (5, 6) have generated much interest in the AR as a key therapeutic target, and have intensified efforts to uncover potent AR inhibitors.
Elevated levels of cellular reactive oxygen species (ROS) significantly contribute to the initiation and progression of cancer (7, 8), and the degree of ROS generation correlates with the aggressive phenotype of PCa (8). Cellular ROS levels are normally kept in check by a very efficient cellular detoxifying system, which includes the mitochondrial antioxidant enzyme, superoxide dismutase 2 (SOD2), which catalyzes the conversion of superoxide (O2-) to hydrogen peroxide (9). The expression of SOD2 or manganese SOD is commonly downregulated in cancer cells, and restoration of SOD2 activity via SOD2 overexpression significantly inhibits in vitro and in vivo tumor growth, including PCa growth (10-12). SOD2 levels progressively decline during the transition from prostatic intraepithelial neoplasia (PIN) to androgen-dependent PCa (AD PCa) to CRPC (13-15). Strikingly, SOD2 levels in CRPC are just 11% of that found in AD PCa (15), supporting the notion that there may be selection for decreased SOD2 expression in advanced PCa. SOD2 downregulation increases AR transcriptional activity, and this effect is reversed with the antioxidant, N-acetylcysteine (16). These findings raise the possibility that therapies aimed at specifically augmenting SOD2 activity might offer an effective and feasible means of treating CRPC, by directly targeting the key player, the AR.
Use of SOD mimetics to augment the cell's natural antioxidant defenses has been beneficial in animal models of a number of neoplastic and non-neoplastic diseases in which oxidative stress is implicated in disease progression (17, 18). As oxidative stress is an integral component of and contributor to cancer progression (7, 8), use of SOD mimetics not only lowers tumor incidence (19-21), but also markedly inhibits in vitro and in vivo tumor growth (22-26). Although the effects of SOD mimetics on a variety of cancers have been investigated, their effects on AR function and PCa growth was hitherto unknown. Here, we show for the first time that SOD mimetics are effective in suppressing AR activity, and in vitro and in vivo CRPC growth.
Materials and Methods
Reagents, plasmid constructs, luciferase reporter gene assay, cell cycle analysis, immunoprecipitation and qRT-PCR analysis. See SI Materials and Methods
Tumor cell lines and culture
LNCaP, CWR22Rv1 and PC-3 PCa cells (ATCC, Manassas, VA) were maintained in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells are authenticated by ATCC. LAPC-4AD PCa cells (provided by Dr. Charles Sawyers, Memorial Sloan-Kettering Cancer Center, NY) were maintained in modified IMEM medium (Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin.
Western blot analysis
After the indicated treatments, whole cell lysates were prepared and subjected to Western blot analysis as described previously (27). After incubation with primary antibody, the blots were probed with an IRDye-labeled secondary antibody (LI-COR Biosciences, Lincoln, NE). Scanning of the blots and densitometric analysis of protein bands was done using the LI-COR Odyssey IR Imaging System. The intensity of a target protein band in each sample was normalized to that of β-actin in the same sample, and expressed as a fold-change, with expression in the control set at 1.
Analysis of cellular superoxide levels
This assay is based on the principle that hydroethidine is oxidized by O2- and converted to fluorescent hydroxyethidium (28). After the indicated treatments, cells were washed with PBS and incubated with 15 μM hydroethidine in serum-free medium at 37°C for 2 h. Fluorescence was read at 510nmEx/580nmEm, and represented as relative fluorescence units (RFU).
Cell viability assay
Cell viability was determined using the CellTiter-Fluor™ Cell Viability Assay (Promega) as per the manufacturer's protocol. Cell viability was assessed by measuring live-cell-protease activity, which is determined by measuring fluorescence emitted by the fluorogenic, cell-permeant peptide substrate, GF-AFC. The intensity of fluorescence generated is directly proportional to cell viability, and was read at 400nmEx/505nmEm.
In vivo xenograft tumor growth
Animal procedures were in accordance with UT Southwestern Medical Center Institutional Animal Care and Use Committee-approved animal protocol. 10-12 weeks-old male, NOD-SCID mice were injected subcutaneously in the flank region with 107 LAPC-4AD cells suspended in matrigel. Tumor volume was calculated by the formula: length × width2/2 (29). Once tumor volume reached 275 +/- 75 mm3, the mice were castrated. Following castration, once tumors exhibited the 1st measurable increase in growth, mice were randomized into 2 groups: 1) Control diet (n=6) and 2) Tempol diet (n=4). Tempol treatment was in the form of bacon-flavored mouse chow mixed with powdered Tempol at a concentration of 58 mM (10 mg/g) (Bioserv). Control animals received bacon-flavored mouse chow minus Tempol. The animals were individually caged, and food intake monitored at regular intervals. Individual relative tumor volume (RTV) at any given time (x) was calculated as follows: Vx/V1, where Vx is the tumor volume at time x, and V1 is the tumor volume at the start of treatment. Mean RTV and standard deviation for Control and Tempol groups was calculated at the end of each week of treatment, and statistical significance assessed by Unpaired t test. Animals were euthanized 9 weeks after commencement of control or Tempol diet, and tumors excised and processed for Western blotting.
Statistical Analyses
Data were tested for statistical significance by one-way ANOVA followed by Tukey post hoc test or by Unpaired t test where appropriate (as indicated in the figure legends) using the Graph-Pad Instat software. Data were considered statistically significant only if p<0.05.
Results
Tempol-mediated decline in cellular O2- levels is accompanied by a significant reduction in AR transcriptional activity
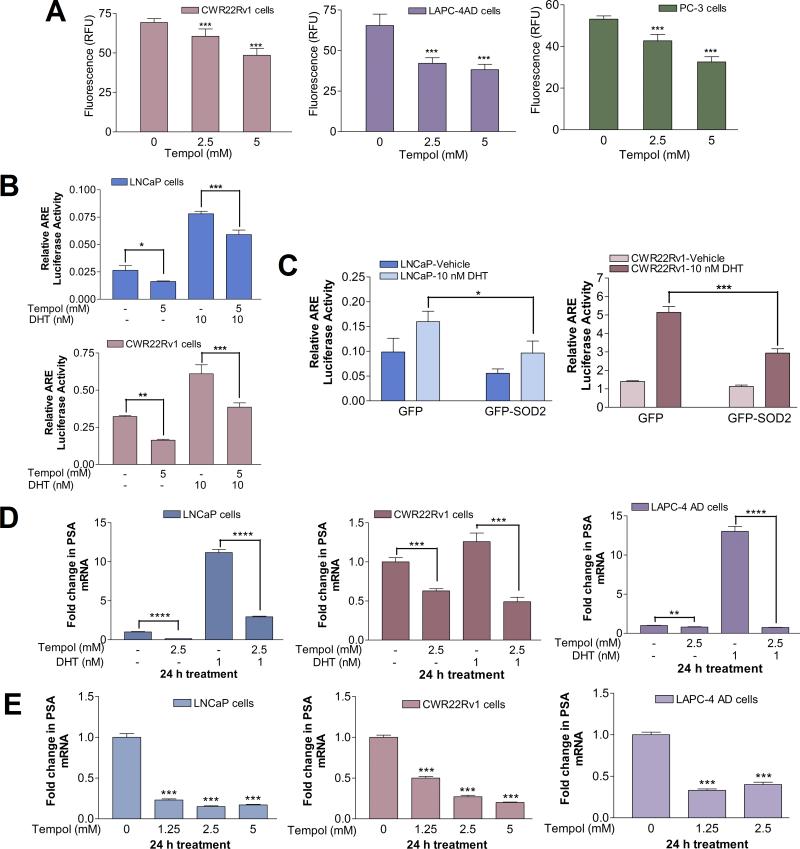
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) (Diagram S1) is a cell-membrane permeable, water-soluble compound belonging to the nitroxide class of SOD mimetics (30). Tempol dose-dependently attenuated O2- levels in AR-dependent CWR22Rv1 and LAPC-4AD cells, as well as in AR-negative PC-3 PCa cells (Fig.1A).
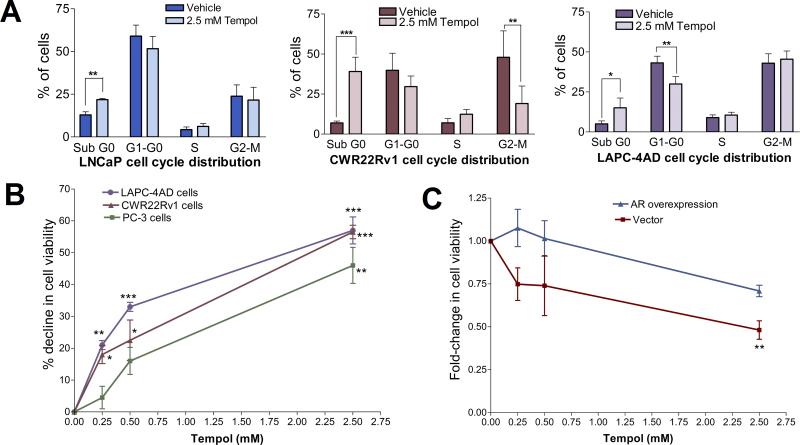
Fig. 1. Tempol-mediated decline in cellular O2- levels is accompanied by a significant reduction in AR transcriptional activity.
(A) Tempol decreases cellular O2- levels. Cells were treated with 0-5 mM Tempol in phenol-red-free (PR-free), serum-free medium for 24 h, followed by O2- analysis with hydroethidine. Data are mean ± S.D. Statistical significance assessed by One-way ANOVA is versus untreated cells. (B) Tempol inhibits androgen response element (ARE)-driven luciferase reporter activity, both in the presence and absence of DHT. Cells were transiently co-transfected with plasmids pGL3-TK-3xARE-FLuc and pGL4.75-RLuc, followed by treatment with 5 mM Tempol +/- 10 nM DHT for 24 h. Relative luciferase activity was determined and represented as mean ± S.D. Statistical significance was assessed by Unpaired t test. (C) Tempol's effect on ARE-driven luciferase reporter activity is closely mimicked by SOD2 overexpression. Cells were transiently co-transfected with GFP-SOD2 or GFP plasmids, along with pGL3-TK-3xARE-FLuc and pGL4.75-RLuc constructs, and subsequently treated for 24 h with vehicle or 10 nM DHT. Relative luciferase activity was determined and represented as mean ± S.D. Statistical significance was assessed by Unpaired t test. (D) Tempol decreases PSA mRNA, both in the presence and absence of DHT. Cells were treated for 24 h with 1 nM DHT +/- 2.5 mM Tempol in PR-free medium containing 5% charcoal-stripped (C/S) FBS. PSA mRNA was assessed by qPCR and expressed as fold change +/- S.D. (ΔΔCt method), with mRNA levels in the vehicle-treated control set at 1. Statistical significance was assessed by Unpaired t test. (E) Tempol elicits a dose-dependent decline in PSA mRNA. Cells were treated for 24 h with increasing doses of Tempol in serum-free medium, followed by qPCR analyses of PSA mRNA, which was expressed as fold change +/- S.D., with mRNA levels in the untreated control set at 1. Statistical significance assessed by One-way ANOVA is versus the control. For Figs. 1A-1E, * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, and **** indicates p<0.0001.
The AR-dependent PCa cell lines used in this study express AR variants that represent the spectrum found in CRPC: LNCaP cells harbor AR with a mutation in the ligand-binding domain (LBD), CWR22Rv1 cells express constitutively active, LBD-lacking, truncated AR and full-length AR with a mutated LBD, and LAPC-4AD cells predominantly express wild-type AR (31, 32). Tempol significantly decreased androgen response element (ARE)-driven luciferase reporter activity, in the presence and absence of DHT, the major AR agonist (Fig. 1B), suggesting that AR transcriptional activity is attenuated by Tempol. To determine how closely Tempol's inhibition of AR transcriptional activity mimics the effect of SOD2, we assessed ARE-driven luciferase reporter activity after SOD2 overexpression. Inhibition of ARE-driven luciferase reporter activity after SOD2 overexpression (Fig. 1C) closely paralleled that following Tempol treatment, both in the presence and absence of DHT (Fig. 1B).
The Tempol-mediated decrease in AR transcriptional activity was reflected in a significant decline in mRNA levels of AR target genes, PSA and FKBP5. Inhibitory effects of Tempol on AR target gene expression were evident in the presence and absence of DHT (Figs. 1D, S1), as well as in a dose- (Figs. 1E, S2) and time-dependent manner (Fig. S3). Dose-response studies revealed that Tempol decreased PSA mRNA levels by 83, 80 and 60% in LNCaP, CWR22Rv1 and LAPC-4AD cells, respectively, relative to that after vehicle treatment (Fig. 1E). A similar trend was seen with FKBP5 mRNA levels (Fig. S2). 2.5 mM Tempol elicited a significant, time-dependent decline in PSA and FKBP5 mRNA levels in LNCaP, CWR22Rv1 and LAPC-4AD cells at each time point under study, relative to that in the vehicle-treated cells at the same time points (Fig. S3).
These data suggest that Tempol is effective in suppressing AR transcriptional activity in PCa cells, and this suppression is closely tied to its ability to lower cellular O2- levels.
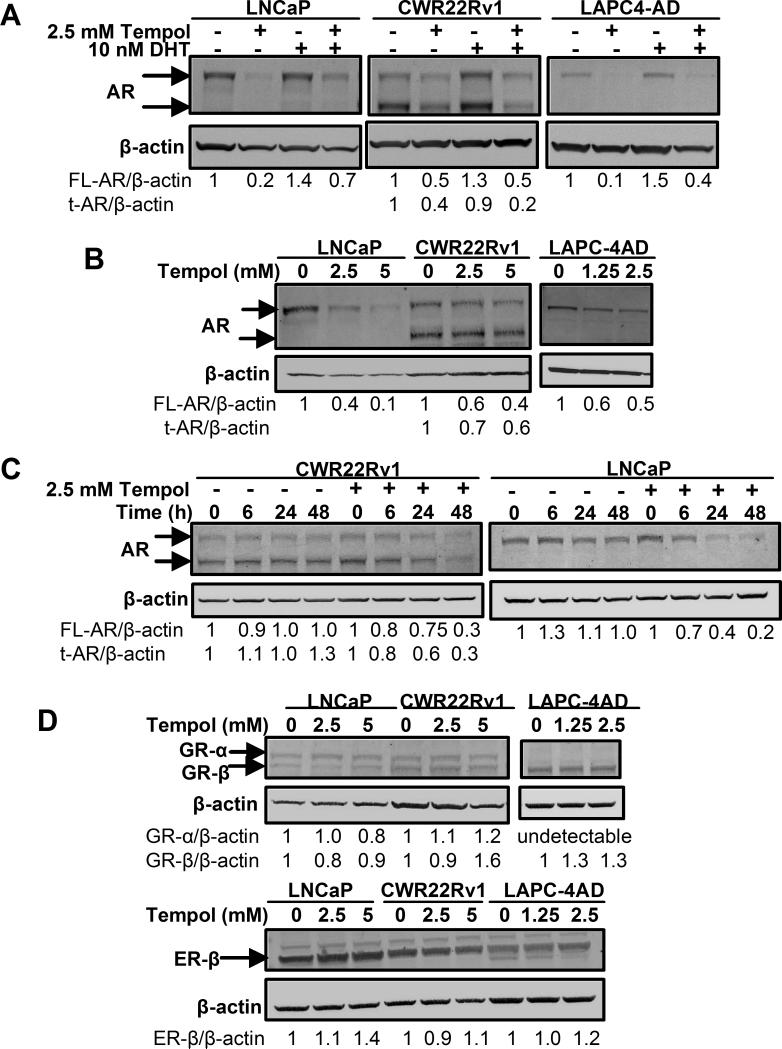
Tempol decreases AR protein, without significantly altering the expression of other nuclear receptors
To determine if the Tempol-mediated inhibition of AR transcription was due to a decrease in AR protein, we next assessed changes in AR protein following treatment with Tempol. Although DHT is known to increase AR protein (33), Tempol markedly decreased AR protein, both in the absence and presence of DHT (Fig. 2A). Remarkably, Tempol not only significantly reduced protein levels of full-length AR (high molecular weight AR band in LNCaP, CWR22Rv1 and LAPC-4AD cells), but also decreased levels of truncated AR (lower molecular weight AR band in CWR22Rv1 cells) (Fig. 2A). For all AR Western blot analyses, we used an antibody targeting the N-terminal domain of AR; hence, it can detect full-length AR and truncated ARs that lack the C-terminal LBD (31, 34).
Fig.2. Tempol decreases AR protein, without significantly altering the expression of other nuclear receptors.
(A) Tempol decreases AR protein, both in the presence and absence of DHT. Cells were grown in PR-free medium containing 5% C/S FBS for 2 d prior to 24-h treatment with 10 nM DHT +/- 2.5 mM Tempol. Cell lysates were prepared and AR protein analyzed by Western blotting. (B) Tempol elicits a dose-dependent decline in AR protein. Cells were treated for 24 h with increasing doses of Tempol in serum-free medium, followed by cell lysate preparation and AR protein analysis by Western blotting. (C) Tempol elicits a time-dependent decline in AR protein. Cells were treated with vehicle or 2.5 mM Tempol for 0-48 h in complete medium, followed by cell lysate preparation and AR protein analysis by Western blotting. AR expression was normalized to that of β-actin and expressed as fold-change, relative to that at the 0 h time point for Vehicle or 2.5 mM Tempol, respectively. (D) Tempol does not decrease protein levels of other nuclear receptors. Cells were treated for 24 h with increasing doses of Tempol in serum-free medium. Cell lysates were prepared and protein levels of nuclear receptors GR-α, GR-β and ER-β were assessed by Western blot analyses. For all Western blot analyses, nuclear receptor expression in each sample was normalized to that of the loading control, β-actin.
A consistent dose-dependent decline in full-length AR (LNCaP, CWR22Rv1 and LAPC-4AD cells) and truncated AR (CWR22Rv1 cells) was observed following treatment with Tempol (Fig. 2B). 2.5 mM Tempol elicited a significant time-dependent decline in both full-length and truncated AR over the course of 48 h, relative to that following vehicle treatment (Figs. 2C, S4). In contrast to its ability to dose-dependently decrease AR protein across all PCa cell lines under study, Tempol did not have similar suppressive effects on other nuclear receptors, such as glucocorticoid receptors-α and -β (GR-α and GR-β) or estrogen receptor-β (ER-β) (Fig. 2D), indicating that Tempol selectively decreases AR protein in PCa cells.
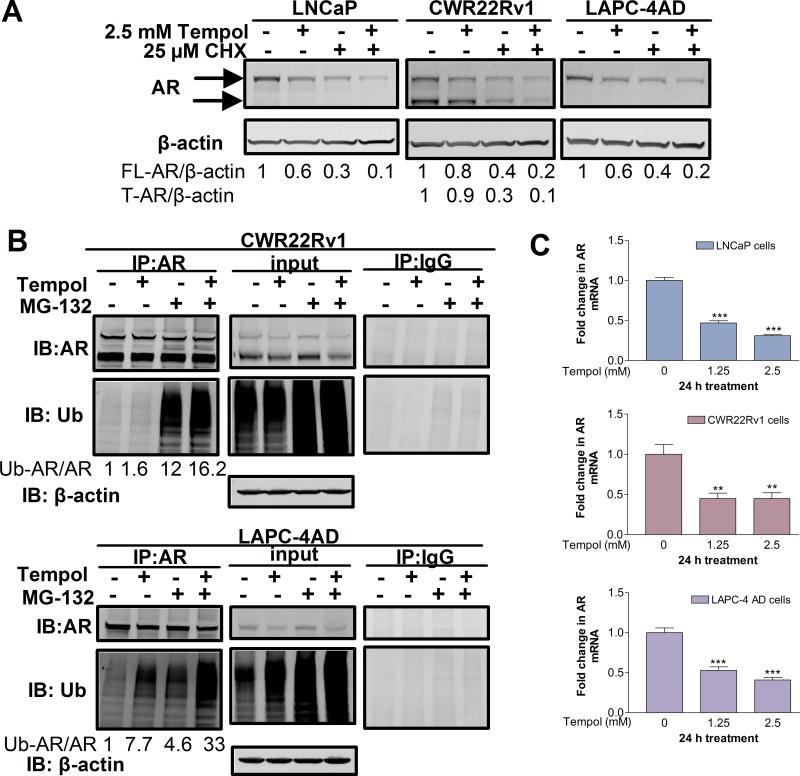
Tempol accelerates degradation of AR protein and decreases AR mRNA levels
To dissect the mechanism by which Tempol decreases AR protein, we assessed if Tempol promotes AR protein degradation. To this end, PCa cells were treated with the protein synthesis inhibitor, cycloheximide (CHX), in the presence or absence of Tempol (Fig. 3A). We reasoned that once new protein synthesis is inhibited with CHX, levels of residual AR protein would reflect the rate of AR degradation; therefore, more rapid degradation of AR would result in lower residual AR protein levels. Concomitant treatment with CHX and Tempol decreased AR protein levels more rapidly than did treatment with either CHX or Tempol alone (Fig. 3A), suggesting that Tempol enhances AR protein degradation.
Fig.3. Tempol accelerates degradation of AR protein and reduces AR mRNA levels.
(A) Tempol promotes AR protein degradation. Cells were treated with 25 μM CHX +/- 2.5 mM Tempol for 18 h in complete medium. CHX-treated samples were pre-treated with CHX for 6 h. AR expression was analyzed by Western blotting and expressed as fold-change, relative to vehicle control. (B) Tempol promotes ubiquitination of AR. Cells were treated with 5 mM Tempol +/- 2.5 μM MG-132 for 8 h, and AR was immunoprecipitated from the cell lysates. Samples immunoprecipitated with an isotype-matched control antibody were run in parallel. The immunoprecipitates and input lysates were subjected to Western blot analyses with antibodies specific for AR and ubiquitin (Ub). β-actin served as a loading control for the input. The intensity of ubiquitin in each immunoprecipitation sample was normalized to that of AR in the same sample and expressed as a fold-change, with the ubiquitinated AR/AR ratio in the untreated control set at 1. (C) Tempol dose-dependently decreases AR mRNA. Cells were treated for 24 h with increasing doses of Tempol in serum-free medium, followed by qPCR analyses of AR mRNA. AR mRNA levels were expressed as fold change +/- S.D. (ΔΔCt method), with mRNA levels in the untreated control set at 1. ** indicates p<0.01, and *** indicates p<0.001 versus the control (One-way ANOVA).
Next, to explore if the enhanced degradation of AR by Tempol is due to increased ubiquitination, PCa cells were treated with the proteasomal inhibitor, MG-132, in the presence or absence of Tempol, and AR was immunoprecipitated and probed for ubiquitin (Figs. 3B, S5). Tempol increased AR ubiquitination, relative to that in the control. Also, the level of ubiquitinated AR after co-treatment with MG-132 and Tempol was significantly higher than that with either MG-132 or Tempol alone (Figs. 3B, S5). These findings suggest that Tempol promotes AR degradation via increased ubiquitination of AR.
A close assessment of AR protein levels in the MG-132 +/- Tempol input lysates revealed that despite the presence of MG-132, Tempol appreciably decreased AR protein relative to that in the vehicle control (Figs. 3B, S5). This observation pointed to the possibility that Tempol may also be decreasing AR synthesis. Indeed, Tempol dose-dependently decreased AR mRNA in LNCaP, CWR22Rv1 and LAPC-4AD cells (Fig. 3C). Collectively, we show that Tempol decreases AR via accelerated degradation of AR protein and a reduction in AR mRNA.
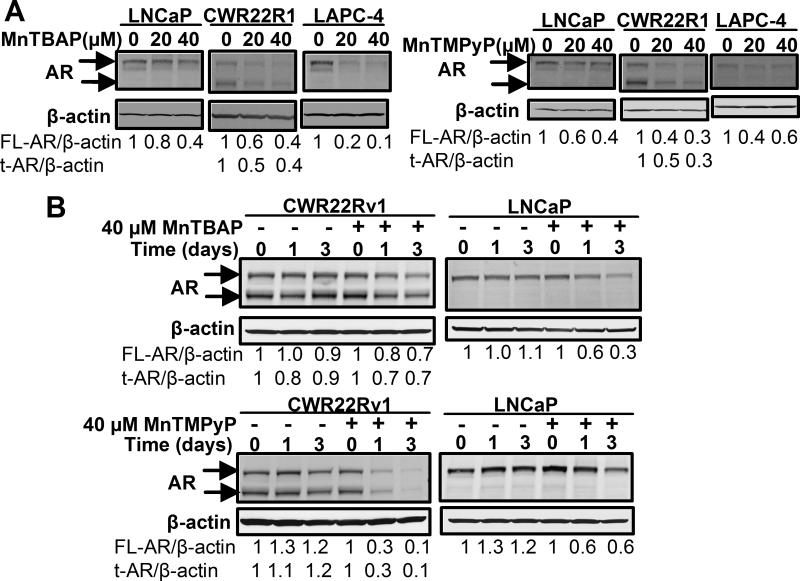
AR inhibition is also a property common to other SOD mimetics
To determine if the suppressive effects of Tempol on AR is a property also shared by other SOD mimetics, we tested the effects of MnTBAP and MnTMPyP, both of which belong to the class of SOD mimetics termed manganese (III) metalloporphyrins (Diagram S1). MnTBAP and MnTMPyP have significant anti-cancer activities against cancers of the colon, liver, esophagus and prostate (24-26). Both MnTBAP and MnTMPyP dose-dependently decreased full-length and truncated AR protein levels (Fig. 4A). 40 μM MnTBAP and MnTMPyP elicited a significant time-dependent decline in both full length and truncated AR protein in LNCaP and CWR22Rv1 cells (Fig. 4B). The above results closely paralleled those seen with Tempol (Figs. 2B, 2C), thereby indicating that the inhibitory effects of Tempol on AR are due to characteristics also shared by other SOD mimetics, and are not unique to Tempol alone.
Fig.4. AR inhibition is also a property common to other SOD mimetics.
(A) SOD mimetics, MnTBAP and MnTMPyP, elicit a dose-dependent decrease in AR protein. Cells were treated for 24 h with 0-40 μM MnTBAP or MnTMPyP in serum-free medium. AR protein was assessed by Western blotting. (B) MnTBAP and MnTMPyP decrease AR protein in a time-dependent manner. Cells were treated with vehicle or 40 μM MnTBAP or MnTMPyP, respectively, for 0-3 d in complete medium, and AR protein analyzed by Western blotting. The intensity of the AR protein band in each sample was normalized to that of β-actin in the same sample and expressed as a fold-change, relative to that at the 0 d time point for vehicle and 40 μM MnTBAP or MnTMPyP treatments, respectively.
Tempol decreases the viability of PCa cells
Studies have shown marked in vitro and in vivo declines in CRPC growth following inhibition of AR expression and/or function (5, 6). Since our data showed that Tempol significantly decreases AR protein levels and AR transcriptional activity in PCa cells (Figs. 1-3), we next investigated the effects of Tempol on PCa cell survival and proliferation. First, we determined the cell cycle phases that were altered by Tempol (Fig. 5A). The most consistent trend that emerged across PCa cell lines after treatment with 2.5 mM Tempol was a significant increase in the Sub G0 (dead cell) fraction, relative to that after vehicle treatment. Additionally, Tempol significantly decreased the G2-M fraction of CWR22Rv1 cells (Fig. 5A).
Fig.5. Tempol decreases PCa cell viability.
(A) Cell-cycle analysis of Tempol-treated cells reveals an increase in the dead cell fraction. Cells were treated with vehicle or 2.5 mM Tempol in PR-free medium containing 5% C/S FBS for 3 d, and cell-cycle distribution assessed by flow cytometry. Data are mean ± S.D., statistical significance was assessed by Unpaired t test. (B) Tempol elicits a dose-dependent decline in cell viability. Cells were treated with 0-2.5 mM Tempol in PR-free medium containing 5% C/S FBS for 2 d. Thereafter, cell viability was assessed and represented as % decline, relative to vehicle control. Data are mean ± S.D., and statistical significance was assessed by One-way ANOVA. (C) AR overexpression blunts Tempol-mediated decrease in PCa cell viability. LAPC-4AD cells were transfected with AR cDNA or empty vector, and treated for 1 d with 0-2.5 mM Tempol in PR-free medium containing 5% C/S FBS. Cell viability was assessed and expressed as fold-change relative to vehicle treatment. Data are mean ± S.D., and statistical significance was assessed by One-way ANOVA. For Figs. 5A-5C, * indicates p<0.05, ** indicates p<0.01, and *** indicates p<0.001 versus control.
To test if the Tempol-mediated suppression of AR leads to a selective decline in viability of AR-dependent over AR-negative cells, we assessed PCa cell viability after treatment with 0-2.5 mM Tempol (Fig. 5B). Tempol significantly decreased the viability of AR-expressing PCa cells at all doses. 0.25, 0.5 and 2.5 mM Tempol decreased CWR22Rv1 cell viability by 18, 23 and 57%, and LAPC-4AD cell viability by 21, 33 and 57%, respectively, relative to that after vehicle treatment. Although 0.25 and 0.5 mM Tempol had significant suppressive effects on viability of AR-positive PCa cells, it only elicited a 5 and 16% decrease in viability of AR-negative PC-3 cells, relative to vehicle treatment. However, 2.5 mM Tempol decreased PC-3 cell viability by 46% (Fig. 5B). Our data (Figs. 5A & 5B) indicate that Tempol significantly decreases PCa cell viability, with a more pronounced effect on viability of AR-dependent cells. Additionally, AR overexpression significantly blunted the ability of Tempol to decrease PCa cell viability, suggesting that the Tempol-mediated decline in viability of AR-dependent cells is predominantly via suppression of AR function (Fig. 5C).
Tempol decreases AR protein in vivo and suppresses growth of CRPC xenografts
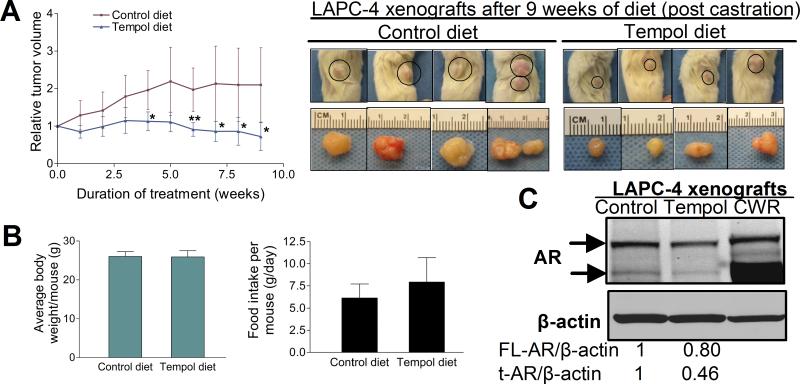
Since our in vitro studies revealed a significant Tempol-mediated decrease in AR protein levels and PCa cell viability, we next evaluated the effect of Tempol on CRPC growth in vivo. To this end, subcutaneous LAPC-4AD tumor-bearing mice were surgically castrated and placed on control diet or Tempol-containing diet for 9 weeks. This form of Tempol treatment has been shown to produce an average serum Tempol concentration of 90-100 μM, and to decrease ROS and oxidative damage in vivo, without selectively inducing cytotoxicity (21). The average relative tumor volume (RTV) and cellularity in the Tempol group was consistently and significantly lower than that in the control group (Fig. 6A; S7). After 9 weeks of treatment, 5 of the 6 mice in the control group had relatively large tumors, with individual RTVs of 3.34, 3.18, 1.93, 1.86 and 1.55; 1 control mouse had a regressing tumor with an individual RTV of 0.75. In contrast, all 4 mice in the Tempol group had comparably smaller tumors, with 3 being regressing tumors (individual RTVs of 0.39, 0.51, 0.66), and 1 slow-growing tumor (individual RTV of 1.35) (Fig. S6). After 9 weeks of treatment, the average RTV of tumors in the Tempol group was 0.72, versus 1.89 in the control group (Fig. 6A). We did not observe a statistically significant difference in food intake or body weight between mice in the control and Tempol groups (Fig. 6B, left and right panels), suggesting that differential food intake was probably not a factor influencing tumor growth in the Tempol group.
Fig.6. Tempol decreases AR protein in vivo and suppresses growth of castration-resistant prostate cancer xenografts.
(A, left panel) Tempol significantly suppresses growth of castration-resistant LAPC-4 PCa xenografts. Subcutaneous LAPC-4AD tumor-bearing mice were surgically castrated and placed on control diet (n=6) or Tempol diet (n=4) for 9 weeks. Mean relative tumor volume +/- S.D. for each group was calculated at the end of each week of treatment. * indicates p<0.05 and ** indicates p<0.01 versus the control for each week (Unpaired t test). (A, right panel) Representative photographs of subcutaneous LAPC-4AD tumor-bearing mice that were on control or Tempol diet for 9 weeks following surgical castration, and the corresponding harvested tumors are shown. (B, left panel) No significant difference in average body weight per mouse after 9 weeks of treatment with control or Tempol diet. Data are mean ± S.D. of body weight of 6 mice in the control group and 4 in the Tempol group. (B, right panel) No statistically significant difference in daily food intake between mice on control diet (n=6) or Tempol diet (n=4). Data are mean ± S.D. (C) Tempol decreases both full-length and truncated AR protein in castration-resistant LAPC-4 xenografts. Pooled lysates of LAPC-4 xenografts from castrated mice on control diet (n=4) or Tempol diet (n=4) were analyzed for AR protein by Western blotting. CWR22Rv1 (CWR) cell lysate was run in parallel.
We next assessed the extent to which 9 weeks of Tempol treatment modulated AR protein in the PCa xenografts (Fig. 6C). Tempol treatment elicited a 20% decrease in full-length AR. Interestingly, the LAPC-4AD xenografts also expressed a truncated AR variant which had the same mobility as the truncated AR in the CWR22Rv1 cell lysate, which was run in parallel. Notably, expression of the truncated AR variant was suppressed by more than 50% in the Tempol group, relative to that in the control group (Fig. 6C).
Discussion
Current androgen deprivation strategies to treat advanced, metastatic PCa have been unable to adequately suppress AR function, resulting in a vast majority of patients failing treatment and progressing to the lethal CRPC (1). Clearly, there is an urgent need for the discovery of AR inhibitors that will remain highly effective, notwithstanding the numerous current-therapy-evading AR molecular alterations that crop up both during and following the development of castration resistance. This study is the first to identify SOD mimetics as effective AR inhibitors in PCa cells, and also the first to document significant in vivo tumor growth suppressive effects with the use of a SOD mimetic in castrated, tumor-bearing mice.
Several reports (31, 34, 35) on the intriguing discovery of novel AR splice variants that lack the LBD in CRPC have necessitated a re-evaluation of strategies aimed at targeting the AR in advanced PCa. Targeting truncated AR variants is critical because: a) they are constitutively active (31, 34, 35); b) their expression is significantly increased during PCa progression, and they confer androgen-independent PCa growth (31, 34, 35); and c) their expression correlates with the risk of tumor recurrence after radical prostatectomy (31, 35). We show that SOD mimetics downregulate full-length and truncated AR protein levels, both in vitro and in vivo, without significantly altering levels of other nuclear receptors. In CWR22Rv1 cells which express high levels of truncated AR, all three SOD mimetics under study, i.e., Tempol, MnTBAP and MnTMPyP, significantly decreased both full-length and truncated AR forms in vitro. Truncated AR protein was not readily detectable in LAPC-4AD cells in vitro; however, the LAPC-4AD xenografts accumulated appreciable levels of truncated AR. This finding is in conformity with findings by Dehm et al which showed that xenograft-based models of CRPC were enriched with truncated AR splice variants (34). Notably, the accumulation of truncated AR in LAPC-4AD xenografts was significantly blunted in mice that were on Tempol-containing diet. Interestingly, Tempol had a more pronounced suppressive effect on truncated AR rather than full-length AR in vivo (Fig. 6C). This finding raises an interesting question about the relative contributions of full-length and truncated AR forms to tumor growth in vivo. Although our study has not specifically addressed this question, Guo et al have previously demonstrated that specific shRNA-mediated knockdown of truncated AR expression, without altering full-length AR, was sufficient to attenuate PCa growth in xenograft models (31). Furthermore, truncated AR regulates a unique set of genes which are not regulated by full-length AR (31). Together, these observations, as well as ours, imply that expression of the truncated AR is critical for regulating PCa growth.
Our data reveal a Tempol-mediated decline in viability of AR-positive, as well as AR-negative PCa cells. However, it is noteworthy that AR-positive PCa cells are more susceptible to the growth-inhibitory effects of Tempol and exhibit a greater decline in viability when treated with lower doses of Tempol than the AR-negative PC-3 cells at the corresponding dose (Fig. 5B). Further, AR overexpression blunts Tempol's suppressive effects on viability of AR-dependent PCa cells (Fig. 5C). Together, these observations suggest that (i) Tempol's effect on AR-dependent PCa cell viability is predominantly mediated via AR, and (ii) the AR-expressing PCa cells depend on the growth-promoting effects of AR signaling (4-6, 36), and hence, even a minor suppression of AR function causes them to become acutely sensitive. On the other hand, AR-negative PCa cells may have developed alternative AR-independent, but ROS-dependent means of survival and proliferation, and hence they require a higher dose of SOD mimetic to lower ROS levels to an extent that will sufficiently suppress those ROS-dependent cell survival pathways. This notion is in partial agreement with a finding by Venkataraman et al (10) which shows that SOD2 overexpression inhibits growth of AR-negative PC-3 cells, suggesting that reduced cellular ROS levels inhibit tumor growth, independent of the AR. In line with this view, we have shown a significant Tempol-mediated reduction in cellular O2- levels in AR-positive and AR-negative PCa cells (Fig. 1A). Collectively, our data suggest that Tempol may be exerting its anti-proliferative effects via AR-dependent, as well as AR-independent, but ROS-dependent mechanisms (37, 38). Safety and toxicity studies conducted by many research groups have confirmed that concentrations of Tempol needed to suppress tumor growth in vivo do not elicit signs of general or organ toxicity, and that Tempol is significantly more effective in inhibiting the growth of a range of neoplastic than non-neoplastic cell lines (20-23). The above findings, together with our demonstration of significant AR suppressive effects by Tempol, provide a mechanistic rationale for the use of Tempol and other SOD mimetics in PCa treatment.
In summary, we have discovered SOD mimetics to be a promising, novel class of AR inhibitors with the ability to suppress CRPC growth. Given that SOD2 expression progressively declines in the spectrum from benign prostatic epithelium to CRPC (13-15), and that ROS and AR play a critical role in every stage of PCa development (4, 8, 10, 16, 39, 40), this new class of AR inhibitors could be beneficial in PCa treatment, with maximal beneficial effects seen in CRPC, in which SOD2 expression is decreased the most. Our demonstration of significant suppression of both full-length and truncated ARs with SOD mimetics, could also open up the prospect for the use of SOD mimetics in PCas that are not amenable to treatment with therapeutic modalities that inhibit the synthesis of androgens and those that competitively block access to the AR LBD.
Supplementary Material
Supporting Information
SI Materials and Methods
Reagents
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) was purchased from Sigma-Aldrich (St. Louis, MO), MnTBAP and MnTMPyP were from Alexis Biochemicals (San Diego, CA), and hydroethidine was from Invitrogen (Carlsbad, CA). Anti-AR, anti-ubiquitin, anti-GR and anti-ER-β antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-β-actin antibody was from Sigma-Aldrich.
Plasmid constructs and luciferase reporter gene assay
Plasmid constructs pGL3-TK-3xARE-FLuc and the bi-cistronic GFP-SOD2 expression vector, pLZRS-eGFP-SOD2, were obtained courtesy of Dr. Elaine Hurt (NCI, Bethesda, MD). pIRES2-eGFP control plasmid for the SOD2 overexpression experiment was from BD Biosciences. pGL3-TK-3xARE-FLuc contains 3 copies of the androgen response element (ARE) linked to the thymidine kinase basal promoter and firefly luciferase gene. Plasmid pGL4.75-RLuc was used as a transfection efficiency control (Promega, Madison, WI), and it contains the CMV promoter linked to the renilla luciferase gene. AR cDNA construct for the AR overexpression experiment was obtained from Origene Technologies (Rockville, MD), and the empty vector was pCS2.
For the luciferase assay, cells were seeded in 48-well plates, and a day later, they were transiently transfected with the luciferase constructs, pGL3-TK-3xARE-FLuc and pGL4.75-RLuc using Lipofectamine 2000 transfection reagent (Invitrogen). Where indicated, GFP or GFP-SOD2 plasmid constructs were co-transfected with the luciferase constructs. 24 h post-transfection, the cells were treated as indicated in phenol red-free (PR-free), serum-free medium for 24 h, followed by cell lysate preparation and dual-luciferase reporter assay as per the Dual-Luciferase Reporter System protocol (Promega). Briefly, the firefly luciferase activity from pGL3-TK-3xARE-FLuc and renilla luciferase activity from pGL4.75-RLuc were sequentially measured from each sample using a single-sample luminometer. The activity of pGL3-TK-3xARE-FLuc was normalized to the activity of the pGL4.75-RLuc internal control, and represented as relative luciferase activity.
Cell cycle analysis
After the indicated treatments, cells were harvested, washed with PBS and fixed in 70% ethanol overnight at -20°C. The fixed cells were rehydrated by washing with PBS and resuspended in 1 ml of propidium iodide (PI) staining solution (20 μg/ml PI and 20 μg/ml RNase A in PBS) for 1 h at RT. Samples were then analyzed by flow cytometry using a Beckman Coulter FC500 flow cytometer.
Immunoprecipitation
After the indicated treatments, whole cell lysates were prepared and immunoprecipitation was performed as described previously (1). AR was immunoprecipitated from the cell lysates with AR antibody conjugated to protein G-agarose beads. The samples were boiled in SDS sample buffer, and proteins were electrophoretically separated on 4–20% gradient gels and transferred to PVDF membranes for Western blot analysis. Blots were incubated sequentially with primary antibodies, rabbit anti-AR and mouse anti-ubiquitin, followed by incubation with the relevant IR dye-labeled secondary antibodies (LI-COR Biosciences, Lincoln, NE). Blots were scanned and densitometric analysis of protein bands done using the LI-COR Odyssey IR Imaging System Software.
qRT-PCR analysis
After the indicated treatments, total cellular RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer's instructions. Total RNA (1 μg) isolated from cells was reverse transcribed to cDNA using oligo-dT and random primers. qPCR analysis of the cDNA was done in triplicate using Absolute qPCR SYBR Low Rox Mix (Fisher, Waltham, MA) and the following specific primers: House-keeping gene, large ribosomal protein P0 (RPLP0) Forward primer: 5'-CGAGGGCACCTGGAAAAC-3 ’ , Reverse Primer: 5'-CACATTCCCCCGGATATGA-3'; Androgen Recept or Forward primer: 5 ’-CAGCTGCTCCGCTGACCTTA-3’, Reverse Primer: 5’-TCCTTGGAGGAAGTGGGAGC-3’
The primer sequences for PSA and FKBP5 have been previously published (2). The qPCR reactions were run using a 7500 Real-Time PCR machine (Applied Biosystems, Foster City, CA). Each experiment was repeated at least twice. The PSA, FKBP5 and AR mRNA levels in each sample were normalized to that of the house-keeping gene (RPLP0) and expressed as fold change +/- SD of the fold change (Comparative Ct or ΔΔCt method) relative to the mRNA levels in the vehicle-treated sample. The mRNA level in the vehicle-treated control was set at 1.
Diagram S1. Structures of (A) Tempol (4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl), (B) MnTMPyP.pentachloride (Manganese (III) tetrakis (1-methyl-4-pyridyl)porphyrin. 5Cl-), (C) MnTBAP chloride (Manganese (III) tetrakis (4-benzoic acid)porphyrin chloride).
Fig. S1. Tempol decreases FKBP5 mRNA levels, both in the presence and absence of DHT. Cells were cultured for 2 days in PR-free medium containing 5% charcoal-stripped (C/S) FBS prior to 24-h treatment with 1 nM DHT +/- 2.5 mM Tempol with the same medium. FKBP5 mRNA was assessed by qPCR analyses and expressed as fold change +/- SD of the fold change (ΔΔCt method), with mRNA levels in the vehicle-treated control set at 1. Statistical significance assessed by Unpaired t test is versus the indicated treatments; * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, and **** indicates p<0.0001.
Fig. S2. Tempol elicits a dose-dependent decline in FKBP5 mRNA levels. Cells were treated for 24 h with increasing doses of Tempol in serum-free medium, followed by qPCR analyses of FKBP5 mRNA which was expressed as fold change +/- SD of the fold change, with mRNA levels in the untreated control set at 1. Statistical significance assessed by One-way ANOVA is versus the control; * indicates p<0.05, ** indicates p<0.01 and *** indicates p<0.001.
Fig. S3. Tempol elicits a time-dependent decline in PSA and FKBP5 mRNA levels. PCa cells were treated with vehicle or 2.5 mM Tempol for 6, 24 and 48 h in complete medium, followed by qPCR analyses. PSA and FKBP5 mRNA at each time point was expressed as fold change +/- SD of the fold change relative to the mRNA level of the vehicle-treated control at the same time point, which was set at 1. Statistical significance assessed by Unpaired t test is versus indicated treatments; * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, and **** indicates p<0.0001.
Fig. S4.Tempol elicits a time-dependent decline in AR protein. LAPC-4AD cells were treated with vehicle or 2.5 mM Tempol for 0-48 h in complete medium, followed by cell lysate preparation and AR protein analysis by Western blotting. AR expression was normalized to that of β-actin in the same sample and expressed as fold-change, relative to that at the 0 h time point for Vehicle or 2.5 mM Tempol, respectively, which was set at 1.
Fig. S5. Tempol promotes ubiquitination of AR. LNCaP cells were treated with 5 mM Tempol +/- 2.5 μM MG-132 for 8 h, and AR was immunoprecipitated from the cell lysates. Samples immunoprecipitated with an isotype-matched control antibody were run in parallel. The resulting immunoprecipitates, as well as input lysates, were subjected to Western blot analyses with antibodies specific for AR and ubiquitin (Ub). β-actin served as a loading control for the input. The intensity of ubiquitin in each immunoprecipitation sample was normalized to that of AR in the same sample and expressed as a fold-change, with the ubiquitinated AR/AR ratio in the untreated control set at 1.
Fig.S6. Tempol suppresses and regresses growth of castration-resistant prostate cancer xenografts. Subcutaneous LAPC-4AD tumor-bearing mice were surgically castrated and randomized to either control diet group (n=6) or tempol diet group (n=4). Response of the tumors to treatment was expressed as relative tumor volume (RTV). RTV at any given time (x) was calculated as the ratio of tumor volume in mm3 at time (x)/tumor volume in mm3 at the start of treatment. Pre-treatment RTV, i.e. RTV at week 0 of either control or Tempol treatment is therefore 1. Individual RTV of each tumor in the Control and Tempol groups at the end of the 9th week of treatment is represented on a waterfall plot.
Fig. S7. Hematoxylin and Eosin staining of Control and Tempol-treated LAPC-4 prostate cancer xenografts. Tempol treatment markedly decreases tumor cellularity.
References
1. Thomas, R., and Kim, M. H. (2009) Prostate 69, 263-275
2. Mostaghel, E. A., Page, S. T., Lin, D. W., Fazli, L., Coleman, I. M., True, L. D., Knudsen, B., Hess, D. L., Nelson, C. C., Matsumoto, A. M., Bremner, W. J., Gleave, M. E., and Nelson, P. S. (2007) Cancer Res 67, 5033-5041
Acknowledgements
We thank Drs. Jer-Tsong Hsieh, Daxing Xie, Amy Sieve, Katja Schuster, Georgia Konstantinidou and Andrea Rabellino for technical input, Dr. Charles Sawyers for the LAPC-4AD cells, and Dr. Elaine Hurt for pGL3-TK-3xARE-FLuc and eGFP-SOD2 plasmid constructs. This work was supported by a US Army Medical Research and Materiel Command Postdoctoral Prostate Cancer Grant # PC094309 to R.T., a Physician Research Training Grant # PC080193, a Prostate Cancer Foundation Young Investigator Award and a Howard Hughes Medical Institute Physician-Scientist Early Career Award to N.S.
Footnotes
Conflicts of interest: None
References
- 1.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. Jama. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 4.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 5.Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–15. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 6.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- 7.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–81. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 8.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–85. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 9.Gelain DP, Dalmolin RJ, Belau VL, Moreira JC, Klamt F, Castro MA. A systematic review of human antioxidant genes. Front Biosci. 2009;14:4457–63. doi: 10.2741/3541. [DOI] [PubMed] [Google Scholar]
- 10.Venkataraman S, Jiang X, Weydert C, Zhang Y, Zhang HJ, Goswami PC, et al. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene. 2005;24:77–89. doi: 10.1038/sj.onc.1208145. [DOI] [PubMed] [Google Scholar]
- 11.Weydert C, Roling B, Liu J, Hinkhouse MM, Ritchie JM, Oberley LW, et al. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2:361–9. [PubMed] [Google Scholar]
- 12.Liu R, Oberley TD, Oberley LW. Transfection and expression of MnSOD cDNA decreases tumor malignancy of human oral squamous carcinoma SCC-25 cells. Hum Gene Ther. 1997;8:585–95. doi: 10.1089/hum.1997.8.5-585. [DOI] [PubMed] [Google Scholar]
- 13.Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000;89:123–34. [PubMed] [Google Scholar]
- 14.Baker AM, Oberley LW, Cohen MB. Expression of antioxidant enzymes in human prostatic adenocarcinoma. Prostate. 1997;32:229–33. doi: 10.1002/(sici)1097-0045(19970901)32:4<229::aid-pros1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Best CJ, Gillespie JW, Yi Y, Chandramouli GV, Perlmutter MA, Gathright Y, et al. Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res. 2005;11:6823–34. doi: 10.1158/1078-0432.CCR-05-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifi N, Hurt EM, Thomas SB, Farrar WL. Effects of manganese superoxide dismutase silencing on androgen receptor function and gene regulation: implications for castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6073–80. doi: 10.1158/1078-0432.CCR-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, et al. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–60. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31:S76–84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- 19.Zhang QS, Eaton L, Snyder ER, Houghtaling S, Mitchell JB, Finegold M, et al. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Res. 2008;68:1601–8. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, et al. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 21.Schubert R, Erker L, Barlow C, Yakushiji H, Larson D, Russo A, et al. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13:1793–802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 22.Gariboldi MB, Lucchi S, Caserini C, Supino R, Oliva C, Monti E. Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic Biol Med. 1998;24:913–23. doi: 10.1016/s0891-5849(97)00372-9. [DOI] [PubMed] [Google Scholar]
- 23.Gariboldi MB, Ravizza R, Petterino C, Castagnaro M, Finocchiaro G, Monti E. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol--a potential new therapeutic agent for gliomas. Eur J Cancer. 2003;39:829–37. doi: 10.1016/s0959-8049(02)00742-6. [DOI] [PubMed] [Google Scholar]
- 24.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–56. [PubMed] [Google Scholar]
- 25.Martin RC, Liu Q, Wo JM, Ray MB, Li Y. Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res. 2007;13:5176–82. doi: 10.1158/1078-0432.CCR-07-1152. [DOI] [PubMed] [Google Scholar]
- 26.Tian J, Peehl DM, Knox SJ. Metalloporphyrin synergizes with ascorbic acid to inhibit cancer cell growth through fenton chemistry. Cancer Biother Radiopharm. 2010;25:439–48. doi: 10.1089/cbr.2009.0756. [DOI] [PubMed] [Google Scholar]
- 27.Thomas R, Kim MH. Targeting the hypoxia inducible factor pathway with mitochondrial uncouplers. Mol Cell Biochem. 2007;296:35–44. doi: 10.1007/s11010-006-9295-3. [DOI] [PubMed] [Google Scholar]
- 28.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeflich KP, Herter S, Tien J, Wong L, Berry L, Chan J, et al. Antitumor efficacy of the novel RAF inhibitor GDC-0879 is predicted by BRAFV600E mutational status and sustained extracellular signal-regulated kinase/mitogen-activated protein kinase pathway suppression. Cancer Res. 2009;69:3042–51. doi: 10.1158/0008-5472.CAN-08-3563. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–69. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–25. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 33.Wang LG, Liu XM, Kreis W, Budman DR. Phosphorylation/dephosphorylation of androgen receptor as a determinant of androgen agonistic or antagonistic activity. Biochem Biophys Res Commun. 1999;259:21–8. doi: 10.1006/bbrc.1999.0655. [DOI] [PubMed] [Google Scholar]
- 34.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–9. [PubMed] [Google Scholar]
- 37.Chen Q, Olashaw N, Wu J. Participation of reactive oxygen species in the lysophosphatidic acid-stimulated mitogen-activated protein kinase kinase activation pathway. J Biol Chem. 1995;270:28499–502. doi: 10.1074/jbc.270.48.28499. [DOI] [PubMed] [Google Scholar]
- 38.Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–65. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 40.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
SI Materials and Methods
Reagents
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) was purchased from Sigma-Aldrich (St. Louis, MO), MnTBAP and MnTMPyP were from Alexis Biochemicals (San Diego, CA), and hydroethidine was from Invitrogen (Carlsbad, CA). Anti-AR, anti-ubiquitin, anti-GR and anti-ER-β antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-β-actin antibody was from Sigma-Aldrich.
Plasmid constructs and luciferase reporter gene assay
Plasmid constructs pGL3-TK-3xARE-FLuc and the bi-cistronic GFP-SOD2 expression vector, pLZRS-eGFP-SOD2, were obtained courtesy of Dr. Elaine Hurt (NCI, Bethesda, MD). pIRES2-eGFP control plasmid for the SOD2 overexpression experiment was from BD Biosciences. pGL3-TK-3xARE-FLuc contains 3 copies of the androgen response element (ARE) linked to the thymidine kinase basal promoter and firefly luciferase gene. Plasmid pGL4.75-RLuc was used as a transfection efficiency control (Promega, Madison, WI), and it contains the CMV promoter linked to the renilla luciferase gene. AR cDNA construct for the AR overexpression experiment was obtained from Origene Technologies (Rockville, MD), and the empty vector was pCS2.
For the luciferase assay, cells were seeded in 48-well plates, and a day later, they were transiently transfected with the luciferase constructs, pGL3-TK-3xARE-FLuc and pGL4.75-RLuc using Lipofectamine 2000 transfection reagent (Invitrogen). Where indicated, GFP or GFP-SOD2 plasmid constructs were co-transfected with the luciferase constructs. 24 h post-transfection, the cells were treated as indicated in phenol red-free (PR-free), serum-free medium for 24 h, followed by cell lysate preparation and dual-luciferase reporter assay as per the Dual-Luciferase Reporter System protocol (Promega). Briefly, the firefly luciferase activity from pGL3-TK-3xARE-FLuc and renilla luciferase activity from pGL4.75-RLuc were sequentially measured from each sample using a single-sample luminometer. The activity of pGL3-TK-3xARE-FLuc was normalized to the activity of the pGL4.75-RLuc internal control, and represented as relative luciferase activity.
Cell cycle analysis
After the indicated treatments, cells were harvested, washed with PBS and fixed in 70% ethanol overnight at -20°C. The fixed cells were rehydrated by washing with PBS and resuspended in 1 ml of propidium iodide (PI) staining solution (20 μg/ml PI and 20 μg/ml RNase A in PBS) for 1 h at RT. Samples were then analyzed by flow cytometry using a Beckman Coulter FC500 flow cytometer.
Immunoprecipitation
After the indicated treatments, whole cell lysates were prepared and immunoprecipitation was performed as described previously (1). AR was immunoprecipitated from the cell lysates with AR antibody conjugated to protein G-agarose beads. The samples were boiled in SDS sample buffer, and proteins were electrophoretically separated on 4–20% gradient gels and transferred to PVDF membranes for Western blot analysis. Blots were incubated sequentially with primary antibodies, rabbit anti-AR and mouse anti-ubiquitin, followed by incubation with the relevant IR dye-labeled secondary antibodies (LI-COR Biosciences, Lincoln, NE). Blots were scanned and densitometric analysis of protein bands done using the LI-COR Odyssey IR Imaging System Software.
qRT-PCR analysis
After the indicated treatments, total cellular RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer's instructions. Total RNA (1 μg) isolated from cells was reverse transcribed to cDNA using oligo-dT and random primers. qPCR analysis of the cDNA was done in triplicate using Absolute qPCR SYBR Low Rox Mix (Fisher, Waltham, MA) and the following specific primers: House-keeping gene, large ribosomal protein P0 (RPLP0) Forward primer: 5'-CGAGGGCACCTGGAAAAC-3 ’ , Reverse Primer: 5'-CACATTCCCCCGGATATGA-3'; Androgen Recept or Forward primer: 5 ’-CAGCTGCTCCGCTGACCTTA-3’, Reverse Primer: 5’-TCCTTGGAGGAAGTGGGAGC-3’
The primer sequences for PSA and FKBP5 have been previously published (2). The qPCR reactions were run using a 7500 Real-Time PCR machine (Applied Biosystems, Foster City, CA). Each experiment was repeated at least twice. The PSA, FKBP5 and AR mRNA levels in each sample were normalized to that of the house-keeping gene (RPLP0) and expressed as fold change +/- SD of the fold change (Comparative Ct or ΔΔCt method) relative to the mRNA levels in the vehicle-treated sample. The mRNA level in the vehicle-treated control was set at 1.
Diagram S1. Structures of (A) Tempol (4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl), (B) MnTMPyP.pentachloride (Manganese (III) tetrakis (1-methyl-4-pyridyl)porphyrin. 5Cl-), (C) MnTBAP chloride (Manganese (III) tetrakis (4-benzoic acid)porphyrin chloride).
Fig. S1. Tempol decreases FKBP5 mRNA levels, both in the presence and absence of DHT. Cells were cultured for 2 days in PR-free medium containing 5% charcoal-stripped (C/S) FBS prior to 24-h treatment with 1 nM DHT +/- 2.5 mM Tempol with the same medium. FKBP5 mRNA was assessed by qPCR analyses and expressed as fold change +/- SD of the fold change (ΔΔCt method), with mRNA levels in the vehicle-treated control set at 1. Statistical significance assessed by Unpaired t test is versus the indicated treatments; * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, and **** indicates p<0.0001.
Fig. S2. Tempol elicits a dose-dependent decline in FKBP5 mRNA levels. Cells were treated for 24 h with increasing doses of Tempol in serum-free medium, followed by qPCR analyses of FKBP5 mRNA which was expressed as fold change +/- SD of the fold change, with mRNA levels in the untreated control set at 1. Statistical significance assessed by One-way ANOVA is versus the control; * indicates p<0.05, ** indicates p<0.01 and *** indicates p<0.001.
Fig. S3. Tempol elicits a time-dependent decline in PSA and FKBP5 mRNA levels. PCa cells were treated with vehicle or 2.5 mM Tempol for 6, 24 and 48 h in complete medium, followed by qPCR analyses. PSA and FKBP5 mRNA at each time point was expressed as fold change +/- SD of the fold change relative to the mRNA level of the vehicle-treated control at the same time point, which was set at 1. Statistical significance assessed by Unpaired t test is versus indicated treatments; * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, and **** indicates p<0.0001.
Fig. S4.Tempol elicits a time-dependent decline in AR protein. LAPC-4AD cells were treated with vehicle or 2.5 mM Tempol for 0-48 h in complete medium, followed by cell lysate preparation and AR protein analysis by Western blotting. AR expression was normalized to that of β-actin in the same sample and expressed as fold-change, relative to that at the 0 h time point for Vehicle or 2.5 mM Tempol, respectively, which was set at 1.
Fig. S5. Tempol promotes ubiquitination of AR. LNCaP cells were treated with 5 mM Tempol +/- 2.5 μM MG-132 for 8 h, and AR was immunoprecipitated from the cell lysates. Samples immunoprecipitated with an isotype-matched control antibody were run in parallel. The resulting immunoprecipitates, as well as input lysates, were subjected to Western blot analyses with antibodies specific for AR and ubiquitin (Ub). β-actin served as a loading control for the input. The intensity of ubiquitin in each immunoprecipitation sample was normalized to that of AR in the same sample and expressed as a fold-change, with the ubiquitinated AR/AR ratio in the untreated control set at 1.
Fig.S6. Tempol suppresses and regresses growth of castration-resistant prostate cancer xenografts. Subcutaneous LAPC-4AD tumor-bearing mice were surgically castrated and randomized to either control diet group (n=6) or tempol diet group (n=4). Response of the tumors to treatment was expressed as relative tumor volume (RTV). RTV at any given time (x) was calculated as the ratio of tumor volume in mm3 at time (x)/tumor volume in mm3 at the start of treatment. Pre-treatment RTV, i.e. RTV at week 0 of either control or Tempol treatment is therefore 1. Individual RTV of each tumor in the Control and Tempol groups at the end of the 9th week of treatment is represented on a waterfall plot.
Fig. S7. Hematoxylin and Eosin staining of Control and Tempol-treated LAPC-4 prostate cancer xenografts. Tempol treatment markedly decreases tumor cellularity.
References
1. Thomas, R., and Kim, M. H. (2009) Prostate 69, 263-275
2. Mostaghel, E. A., Page, S. T., Lin, D. W., Fazli, L., Coleman, I. M., True, L. D., Knudsen, B., Hess, D. L., Nelson, C. C., Matsumoto, A. M., Bremner, W. J., Gleave, M. E., and Nelson, P. S. (2007) Cancer Res 67, 5033-5041