Abstract
In an oxygen-depleted environment, endothelial cells initiate an adaptive pattern of synthesis, which may enable them to survive hypoxic crises. Using high-resolution two-dimensional gel electrophoresis in conjunction with mass spectroscopy, we obtained a 24 differential display of proteins in the pancreatic endothelial cell line, MS-1, at four time points following induction of hypoxia. The induction of Wee1 under hypoxia was confirmed both at the mRNA and protein levels. The phosphorylation of cell division cycle 2, which is downstream of Wee1, was also increased after hypoxic exposure. In addition, pre-exposure to hypoxia attenuated a decrease in hydrogen peroxide-induced cell number. The induction of bax (a pro-apoptotic protein) and reduction of bcl (an anti-apoptotic protein) after hypoxia stimulus were also attenuated by hypoxic pre-exposure. Moreover, hydrogen peroxide-induced morphologic damage did not appear in the wild-type Wee1-expressing cells. Taken together, our results suggest that Wee1 may have important role in hypoxia-induced pathophysiological situations in endothelial cells.
Keywords: cell hypoxia; endothelial cells; hydrogen peroxide; hypoxia inducible factor 1, α subunit; Wee1 protein, human
Introduction
Endothelial cells play an integral role in the maintenance of vascular homeostasis. As the cell type directly in contact with the blood, endothelial cells must adapt to changing conditions within the blood vessels and synthesize regulators of leukocyte and vascular smooth muscle cell behavior. Medical conditions, such as atherosclerosis, varicose veins, pulmonary hypertension, and tumor angiogenesis, may induce vascular hypoxia (Eyries et al., 2008; Han et al., 2008; Kubo et al., 2008; Osada-Oka et al., 2008a; Rankin et al., 2008), and the molecular aspects of hypoxia have been studied in order to explain the pathology underlying these diseases; however, many questions remain.
Hypoxic conditions may profoundly affect endothelial cells through a cascade of reactions involving neutrophils (Sumagin et al., 2006, 2008; Butler et al., 2008; Carpenter and Alexander, 2008; Hu et al., 2008) and smooth muscle cells (Michiels et al., 2000; Aley et al., 2008; Jernigan et al., 2008; Mayr et al., 2008; Osada-Oka et al., 2008a, 2008b; Sheshgiri et al., 2008). Hypoxia regulates the expression of many genes, and the list of hypoxia-related genes is growing (Yoshida et al., 2006; Eckardt et al., 2007; Said et al., 2007; Wang et al., 2007; Chen et al., 2008; Furuta et al., 2008; Yang et al., 2008). These molecular changes appear to favor endothelial cell survival of hypoxic crises. Many genes involved in tumor biology are regulated by levels of oxygen. In some human tumor types, hypoxia inducible factor-1α (HIF-1α) is overexpressed compared with corresponding normal tissues (Zhong et al., 1999; Furlan et al., 2007; Fan et al., 2008; Hamaguchi et al., 2008; Liang et al., 2008; Masamune et al., 2008; Sayed et al., 2008; Simiantonaki et al., 2008).
Wee1 encodes a nuclear protein, a tyrosine kinase belonging to the Ser/Thr family, which catalyzes the inhibitory tyrosine phosphorylation of cell division cycle 2 (cdc2)/cyclin B kinases (McGowan and Russell, 1995; Okamoto and Sagata, 2007). By shielding the nucleus from activated cytoplasm cdc2 kinase, Wee1 may coordinate the transition between DNA replication and mitosis (Yamada et al., 2004; De Schutter et al., 2007; Perry and Kornbluth, 2007). Phosphorylation of Wee1 is dependent upon mitotic cell cycle dependent kinase 1 (cdk1) activity (Stumpff et al., 2004; Harvey et al., 2005). The molecular mechanisms that regulate Wee1 are poorly understood; hence, a more detailed knowledge of these mechanisms will be an important step toward understanding what determines whether a cell undergoes mitosis.
Results
Hypoxia induces Wee1 expression
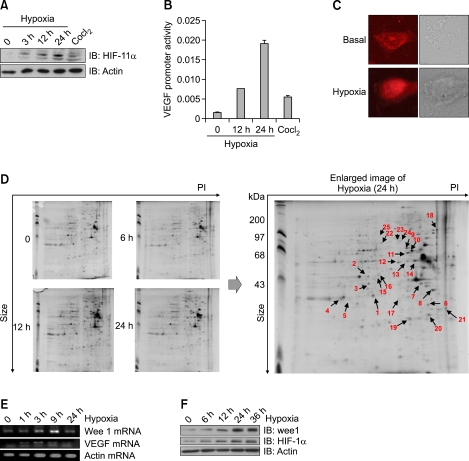
The expression of HIF-1α serves as a cellular marker for hypoxic conditions. In order to examine hypoxic systems, we investigated the expression of HIF-1α after exposure to hypoxia (Figure 1A). Hypoxia also induced a time-dependent activation of the vascular endothelial growth factor (VEGF) promoter downstream of HIF-1α (Figure 1B), suggesting that our hypoxic system was working appropriately. HIF-1α is known to translocate to the nucleus in hypoxic conditions. To confirm this in another way, we investigated the subcellular location of HIF-1α. We found that the majority of HIF-1α was located in the nucleus in hypoxic conditions (Figure 1C). These findings confirm the effectiveness of hypoxia in our experimental system. Two-dimensional protein expression maps were generated for the triplicate samples of MS-1 cells before and at measured intervals after induction of hypoxia (Figure 1D). Gel images were checked manually to minimize gel-to-gel variations. Following the shift to hypoxia, 44 spots indicated the differential expression. Spots that were 1.5-fold up- or down-regulated by hypoxia were defined as differentially expressed. Twenty-four proteins were identified and categorized (Table 1). Identified proteins were categorized as being involved with glycolysis, chaperone mediaion, or cell mobility. These proteins were already known to be involved with hypoxia, suggesting the relevance of our data. To validate the MS results, the level of Wee1 mRNA was examined (Figure 1E). The level of VEGF mRNA was also examined and used as a positive control for hypoxic conditions. Concurrently, the level of Wee1 protein in the hypoxic cells was measured and shown to increase (Figure 1F). The protein level of HIF-1α was used as a positive control for hypoxia. These findings support a specific role for Wee1 in hypoxia-mediated signaling events.
Figure 1.
Hypoxia induced Wee1 expression. (A) Induction of HIF-1α by hypoxia in MS-1 cells. Cells were exposed for the indicated times in a hypoxic chamber (0.1% O2) and then blotted with HIF-1α antibody. The lower panel demonstrates equivalent loading of total actin in the whole cell lysates. (B) The effect of hypoxia on the VEGF promoter. The promoter plasmid was transiently transfected and maintained for 24 h in MS-1 cells. After hypoxia (0.1% O2) for indicated times, the promoter activity was monitored. CoCl2 was treated and used as a chemical hypoxic inducer. (C) Effect of hypoxia on subcellular localization of HIF-1α. Before hypoxic treatment, MS-1 cells were preincubated for 30 min with hypoxia culture medium. The HIF-1α signal was then measured after staining with antibody. (D) Spectrometric analysis of hypoxic samples. Two-dimensional gels showing the silver-stained signal obtained from 200 µg of MS-1 cell before and at measured intervals after induction of hypoxia. The right image is the enlarged images of the 24 h spot in the 2D gels. The arrows indicate the identification of the protein by spot-picking, tryptic digestion, and MALDI-TOF. (E) Image of total RNA was prepared from MS-1 cells and amplified by RT-PCR using Wee1/VEGF-specific primers. Actin mRNA was used as a positive control. The PCR products were separated on 2% agarose gels and visualized with UV. (F) Effect of hypoxia on Wee1 expression. The MS-1 cells were exposed to hypoxia for the indicated times. Expression of Wee1 was then examined by blotting with antibodies to HIF-1α and Wee1. The lower panel demonstrates equivalent loading of total actin in the whole cell lysates.
Table 1.
List of summary of MS analysis
Cdc2 was phosphorylated under hypoxia
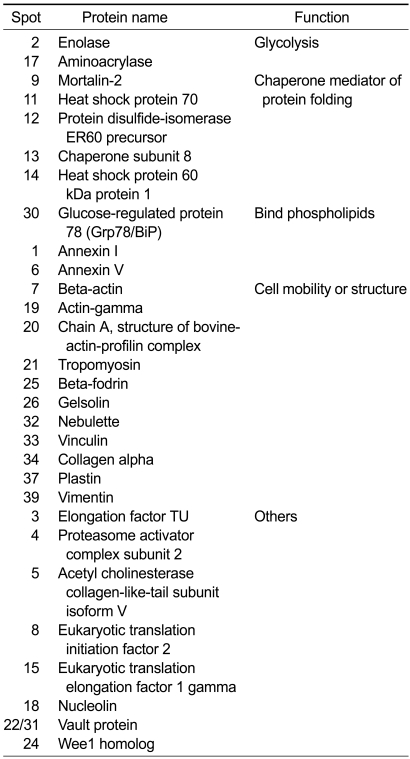
To trace the effects of Wee1 in hypoxia-mediated signaling, we measured the phosphorylation of cdc2, a Wee1 downstream molecule. Exposure of cells to hypoxia for 10 min induced phosphorylation of cdc2 (Tyr15; Figure 2A), confirming Wee1 activation in hypoxia-induced signaling. We then monitored cell numbers following exposure to hypoxia for 24 h, and found a reduction in cell proliferation (Figure 2B), which indicated that both Wee1 and cdc2 were involved in the hypoxia-induced signaling pathway.
Figure 2.
Cdc2 phosphorylation (Tyr15) in hypoxia. (A) Effect of hypoxia on cdc2 phosphorylation. To induce hypoxic conditions, MS-1 cells were incubated for the indicated times in a hypoxic chamber. The phosphorylation of cdc2 was analyzed with western blotting. The lower panel demonstrates equivalent loading of total cdc2 and actin in the whole cell lysates. (B) Number of MS-1 cells after hypoxia. Cells were exposed to hypoxia for the indicated times. The results are expressed as the mean ± SEM for three separate experiments.
Hypoxia attenuates the reduction in cell viability induced by hydrogen peroxide
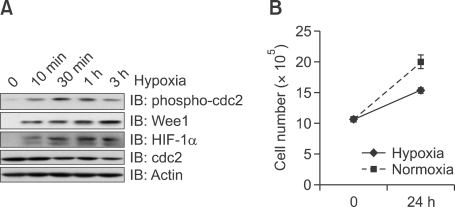
Pre-exposure of cells to hypoxia (24 h) attenuated the hydrogen peroxide-induced suppression of cell viability (Figure 3A). While 1 mM of hydrogen peroxide suppressed cell viability by approximately 80%, this suppression was attenuated to 50%-60% in cells pre-exposed to hypoxia. Bcl-2 is the member of the Bcl family of apoptosis regulator protein. It is thought to be involved in resistance to cancer treatment, so regarded as anti-apoptosis protein. Bax is a pro-apoptotic protein Bcl family protein. It promotes apoptosis by competing Bcl protein. This hypoxic pre-exposure also suppressed the induction of bax, and delayed the downregulation of bcl after hydrogen peroxide treatment. While bax was induced after 6 h of exposure to hydrogen peroxide in normoxic conditions, this increase appeared at 24 h in cells pre-exposed to hypoxia (Figure 3B). The expression of bcl began to decease at 6 h in normoxia. This pattern was delayed under conditions of pre-exposure hypoxia. Thus, Wee1 signaling due to hypoxia may be involved in protection from oxidative stress.
Figure 3.
Hypoxia attenuates the reduction in cell viability induced by hydrogen peroxide. (A) Viability of MS-1 cells after hydrogen peroxide exposure. Cells were exposed to hydrogen peroxide either with or without hypoxic preconditioning. The results are expressed as the percentage of absorbance (MTT) relative to the control. Each value represents the mean ± SEM for three separate experiments. Statistical analyses was conducted in comparison to control values; P < 0.001. (B) Effect of hypoxia on hydrogen peroxide-mediated apoptosis-related protein expression. Before hydrogen peroxide treatment, MS-1 cells were exposed to hypoxia for indicated times. Anti-bax and bcl expression were then examined after hydrogen peroxide treatment, and protein expression was examined by blotting with bax and bcl antibodies. The lower panel demonstrates equivalent loading of total actin in the whole cell lysates.
Wee1 suppression of hydrogen peroxide-induced cellular injury
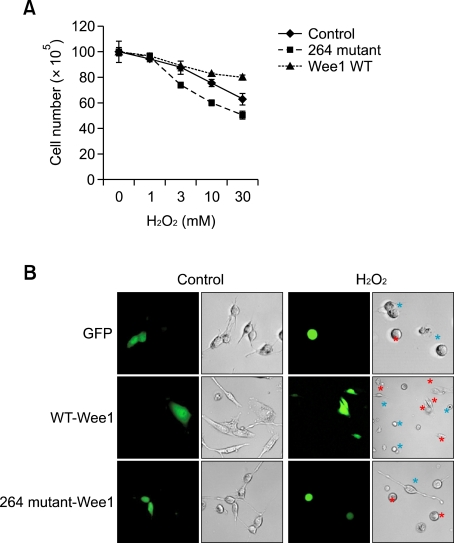
To confirm the role of Wee1 in hydrogen peroxide-mediated signaling, we compared the decrease in hydrogen peroxide-induced cell numbers between the wild-type Wee1 and mutant Wee1-expressing cells. After treatment with hydrogen peroxide for different times and at different doses, the number of cells was counted. The wild-type expressing cells, but not those with the mutant gene, resisted the anti-proliferative effects of hydrogen peroxide in a time- and dose-dependent manner (Figure 4A). In parallel experiments, we found that hydrogen peroxide did not alter the morphology of cells which expressed green fluorescent protein (GFP)-tagged wild-type Wee1, but severely shrank the morphology of cells which expressed GFP-tagged mutant Wee1 (Figure 4B). The morphology of cells which expressed both wild-type and mutant Wee1 did not change under control conditions. This result supports the hypothesis that the physiologic role of Wee1 is to protect cells in response to hydrogen peroxide.
Figure 4.
Wee1 expression prevents hydrogen peroxide-induced cellular injury. (A) Number of cells after Wee1 expression under hydrogen peroxide. Cells were transiently transfected with either the wild-type or mutant Wee1 and then maintained for 24 h, prior to hydrogen peroxide stimulation. Cells were exposured to hydrogen peroxide for 24 h with indicated doses. The results are expressed as the mean ± SEM for three separate experiments. (B) Morphologic changes of MS-1 cells after Wee1 expression. Cells were transiently transfected with either Green Fluorescent Protein-tagged wild type or mutant Wee1, prior to hydrogen peroxide exposure. Images were obtained with confocal microscopy. Green color indicates GFP-transfected cell, red color indicate non-transfected cell.
Discussion
The primary finding of this study is that Wee1 mediates some of the adaptive synthetic pattern which may enable cells to survive a hypoxic crisis. The role of Wee1 has previously been evaluated in control cell cycle arrest and DNA replication checkpoints (Watanabe et al., 1995). In response to DNA damage, Wee1 can inactivate the cdc2/cyclin B complexes in order to activate the G2 DNA damage checkpoints and inhibit the G2/M transition in the cell cycle in order to prevent cells from progressing through to mitosis prior to completion of repair. This subsequently results in an irreversible alteration to the genome that can have profound effects on cellular viability and coordinated cell proliferation and development (Igarashi et al., 1991; Raleigh and O'Connell, 2000; Stumpff et al., 2004). In the present study, we showed that phosphorylation of cdc2 was induced by hypoxic stimuli, suggesting that Wee1 may promote the cell cycle checkpoint pathway for hypoxic conditions in endothelial cells.
Hypoxia, as well as heavy metals, oxygen-free radicals, inflammation, and ischemia, may also induce heat shock proteins (Hsps), or chaperone proteins. As molecular chaperones, the Hsps stabilize intracellular proteins to facilitate their biosynthesis and folding. Induction of Hsp70 by hypoxia, for example, may increase resistance of the heart to ischemic injury (Zhong et al., 2000). Hsp90 is one of the most abundant and ubiquitous molecular chaperones. In the fission yeast, Wee1 was identified as a Hsp90 substrate (Aligue et al., 1994). These facts suggest that Wee1 may have important role in hypoxia through regulating molecular chaperone, such as Hsp90.
In conclusion, we have demonstrated that hypoxia induces Wee1, and that this protein protects endothelial cells against hypoxic damage. Further studies will be focused on elucidating the role of Wee1 in hypoxia-related pathologic conditions, such as in tissue ischemia caused by atherosclerosis.
Methods
Reagents
Antibodies to HIF-1α, Wee1, bax, bcl, actin, and cdc2 were purchased from Cell Signaling Technology Inc. (New England Biolabs, Beverly, MA). Anti-phospho-cdc2 (Tyr15) was obtained from Stressgen Bioreagents Corporation (Victoria, B.C., Canada). Horseradish peroxidase-conjugated secondary antibodies were obtained from the Kirkegaard and Perry Lab (Gaithersburg, MD). Cobalt-chloride was purchased from Sigma-Aldrich (St. Louis, MO) and hydrogen peroxide was obtained from Calbiochem (San Diego, CA).
Cell cultures
Mouse pancreatic endothelial (MS-1) cells were cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM; GIBCO™, Auckland, NZ) containing 0.584 g/L of L-glutamate and 4.5 g/L of glucose, and F-12 medium containing 0.146 g/L of L-glutamate, 1.8 g/L of glucose, 100 g/ml of gentamicin, 2.5 g/L of sodium carbonate, and 10% heat-inactivated fetal bovine serum.
Immunoblot analysis
The cells were lysed in 100 µl of lysis buffer (50 mM Tris-Cl [pH 7.4], 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM ethylenediaminetetraacetic acid [EDTA], and 150 mM NaCl). The samples were then briefly sonicated, heated for 5 min at 95℃, and centrifuged for 3 min. Supernatant proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 8%) gels, and then transferred to polyvinylidene difluoride membranes. Blots were incubated with primary antibodies, and then washed 6 times in Tris-buffered saline/0.1% Tween-20 prior to a 1-h probe with horseradish peroxidase-conjugated secondary antibodies. Blots were developed using ECL (Amersham Biosciences, Buckinghamshire, UK).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Levels of VEGF, Wee1, and actin expression were determined with RT-PCR, as described below. The synthetic oligonucleotide primer sequences for VEGF, Wee1, and actin were as follows: VEGF (forward), 5'-TGTTCGTGTCC TTCATCCTG-3', VEGF (reverse), 5'-GAAGGAGATGGCA AGGAGTG-3'; Wee1 (forward), 5'-GATGTGCGACAGACT CCTCA-3', Wee1 (reverse), 5'-CAAAGCGTTCTGCTCATC AA-3'; actin (forward), 5'-CGATGCTGGCGCTGAGTAC-3', and actin (reverse), 5'-CGTTCAGCTCAGGGATGACC-3'. Amplifications using the above-listed primers were performed in a 25-µl reaction for 30 amplification cycles in a Gene Amp PCR system 2400 (Perkin Elmer, Wellesley, MA) with 30 s at 94℃, 30 s at 62℃, and 30 s at 72℃.
Two-dimensional (2D)-PAGE
Cells were lysed in protein extraction buffer (7 M urea, 2 M thiourea, 4% [3-(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, 100 mM dithiothreitol, and 1% pharmalyte [pH 3-10]), and lysates were centrifuged at 356,000 × g for 10 min in a Beckman Optima TL Tabletop Ultracentrifuge (Beckman Instruments Inc., Palo Alto, CA). The protein concentration was determined using the Bio-Rad protein assay reagent. Immobilized pH gradient strips (pH 3-10, linear, 18 cm) were rehydrated overnight in 400 µl of lysate that contained 250 µg of protein. Isoelectric focusing on a Multiphor II system (Amersham Biosciences, Buckinghamshire, UK) and SDS-PAGE on 10% gels using the Investigator System (Genomic Solutions, Chelmsford, MA) were performed.
In-gel trypsin digestion of protein and mass spectrometry
After gels were destained in 10% acetic acid, protein spots were excised and macerated with a scalpel. Portions (typically, 1/20) of the complete trypsin digest were co-crystallized in a matrix of a-cyano-4-hydroxycinnamic acid and analyzed by using a PerSeptive BioSystems matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometer. Peptides were eluted isocratically for 10 min, followed by a linear gradient (0.95%/min) to a final mobile phase composition of 63% acetonitrile/0.082% trifluoroacetic acid in H2O. Eluting peptides were detected with an ABI 785A UV detector. Protein identities were derived using the MS spectra with the MS-Tag program.
Plasmid transfection and luciferase assays
Cells were seeded in 6-well tissue culture plates 24 h before transfection. Sub-confluent cells were transfected with VEGF promoter-luciferase using the FuGEN6 transfection reagent (Roche Diagnostics, Mannheim, Germany). After 24 h, the cells were treated with hypoxia (0.1% O2), as appropriate. The luciferase assay was done using the luciferase assay kit (Promega, Madison, WI).
Immunofluorescence and confocal microscopy
Cells grown on glass coverslips were fixed in 4% phosphate-buffered paraformaldehyde for 15 min. Cells were permeabilized in methanol at -20℃ for 4 min. After permeabilization, cells were incubated with primary antibody to Wee1 for 2 h at room temperature, washed 3 times in phosphate-buffered saline, and then incubated with secondary antibodies conjugated with 546-Alexa (red) from Molecular Probes (Invitrogen, Carlsbad, CA). Confocal scanning analysis was performed using a confocal microscope (Zeiss LSM 510, Oberkochen, Germany).
Hypoxia system
Cultured cells were transferred into a hypoxic chamber (model 1025; Forma Scientific, Marietta, OH) for the indicated times at 0.1% O2.
Data analysis
Statistical analyses were conducted using SigmaStat (SPSS Inc., Chicago, IL). A P-value < 0.05 was considered statistically significant.
Abbreviations
- cdc2
cell division cycle 2
- cdk1
cycle dependent kinase 1
- HIF-1α
hypoxia inducible factor-1α
- Hsp
heat shock protein
References
- 1.Aley PK, Wilkinson JA, Bauer CC, Boyle JP, Porter KE, Peers C. Hypoxic remodelling of Ca (2+) signalling in proliferating human arterial smooth muscle. Mol Cell Biochem. 2008;318:101–108. doi: 10.1007/s11010-008-9861-y. [DOI] [PubMed] [Google Scholar]
- 2.Aligue R, Akhavan-Niak H, Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler LM, Khan S, Ed Rainger G, Nash GB. Effects of endothelial basement membrane on neutrophil adhesion and migration. Cell Immunol. 2008;251:56–61. doi: 10.1016/j.cellimm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter AC, Alexander JS. Endothelial PKC delta activation attenuates neutrophil transendothelial migration. Inflamm Res. 2008;57:216–229. doi: 10.1007/s00011-007-7031-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen SC, Wang BW, Wang DL, Shyu KG. Hypoxia induces discoidin domain receptor-2 expression via the p38 pathway in vascular smooth muscle cells to increase their migration. Biochem Biophys Res Commun. 2008;374:662–667. doi: 10.1016/j.bbrc.2008.07.092. [DOI] [PubMed] [Google Scholar]
- 6.De Schutter K, Joubes J, Cools T, Verkest A, Corellou F, Babiychuk E, Van Der Schueren E, Beeckman T, Kushnir S, Inze D, De Veylder L. Arabidopsis Wee1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell. 2007;19:211–225. doi: 10.1105/tpc.106.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckardt KU, Bernhardt W, Willam C, Wiesener M. Hypoxia-inducible transcription factors and their role in renal disease. Semin Nephrol. 2007;27:363–372. doi: 10.1016/j.semnephrol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103:432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 9.Fan LF, Dong WG, Jiang CQ, Qian Q, Yu QF. Role of Hypoxia-inducible factor-1 alpha and Survivin in colorectal carcinoma progression. Int J Colorectal Dis. 2008;23:1057–1064. doi: 10.1007/s00384-008-0511-3. [DOI] [PubMed] [Google Scholar]
- 10.Furlan D, Sahnane N, Carnevali I, Cerutti R, Uccella S, Bertolini V, Chiaravalli AM, Capella C. Up-regulation and stabilization of HIF-1alpha in colorectal carcinomas. Surg Oncol. 2007;16(Suppl 1):S25–S27. doi: 10.1016/j.suronc.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi T, Iizuka N, Tsunedomi R, Hamamoto Y, Miyamoto T, Iida M, Tokuhisa Y, Sakamoto K, Takashima M, Tamesa T, Oka M. Glycolysis module activated by hypoxia-inducible factor 1alpha is related to the aggressive phenotype of hepatocellular carcinoma. Int J Oncol. 2008;33:725–731. [PubMed] [Google Scholar]
- 13.Han YL, He DL, Luo Y, Cheng HP, Zhu GF. Over-expression of hypoxia-inducible factor 1 alpha increases angiogenesis of LNCaP cells in vivo. Zhonghua Nan Ke Xue. 2008;14:439–444. (Chinese) [PubMed] [Google Scholar]
- 14.Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–420. doi: 10.1016/j.cell.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1(+)-like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- 17.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo T, Sugita T, Shimose S, Matsuo T, Arihiro K, Ochi M. Expression of hypoxia-inducible factor-1alpha and its relationship to tumour angiogenesis and cell proliferation in cartilage tumours. J Bone Joint Surg Br. 2008;90:364–370. doi: 10.1302/0301-620X.90B3.19806. [DOI] [PubMed] [Google Scholar]
- 19.Liang X, Yang D, Hu J, Hao X, Gao J, Mao Z. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28:1659–1666. [PubMed] [Google Scholar]
- 20.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–G717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- 21.Mayr M, Zampetaki A, Sidibe A, Mayr U, Yin X, De Souza AI, Chung YL, Madhu B, Quax PH, Hu Y, Griffiths JR, Xu Q. Proteomic and metabolomic analysis of smooth muscle cells derived from the arterial media and adventitial progenitors of apolipoprotein E-deficient mice. Circ Res. 2008;102:1046–1056. doi: 10.1161/CIRCRESAHA.108.174623. [DOI] [PubMed] [Google Scholar]
- 22.McGowan CH, Russell P. Cell cycle regulation of human Wee1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michiels C, Arnould T, Remacle J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim Biophys Acta. 2000;1497:1–10. doi: 10.1016/s0167-4889(00)00041-0. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto K, Sagata N. Mechanism for inactivation of the mitotic inhibitory kinase Wee1 at M phase. Proc Natl Acad Sci USA. 2007;104:3753–3758. doi: 10.1073/pnas.0607357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osada-Oka M, Ikeda T, Akiba S, Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J Cell Biochem. 2008a;104:1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- 26.Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb. 2008b;15:26–33. doi: 10.5551/jat.e533. [DOI] [PubMed] [Google Scholar]
- 27.Perry JA, Kornbluth S. Cdc25 and Wee1: analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raleigh JM, O'Connell MJ. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J Cell Sci. 2000;113:1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- 29.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Said HM, Hagemann C, Staab A, Stojic J, Kuhnel S, Vince GH, Flentje M, Roosen K, Vordermark D. Expression patterns of the hypoxia-related genes osteopontin, CA9, erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in vivo. Radiother Oncol. 2007;83:398–405. doi: 10.1016/j.radonc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Sayed KA, Khanfar MA, Shallal HM, Muralidharan A, Awate B, Youssef DT, Liu Y, Zhou YD, Nagle DG, Shah G. Latrunculin A and its C-17-O-carbamates inhibit prostate tumor cell invasion and HIF-1 activation in breast tumor cells. J Nat Prod. 2008;71:396–402. doi: 10.1021/np070587w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheshgiri R, Rao V, Tumiati LC, Xiao R, Prodger JL, Badiwala M, Librach C, Delgado DH. Progesterone induces human leukocyte antigen-g expression in vascular endothelial and smooth muscle cells. Circulation. 2008;118:S58–S64. doi: 10.1161/CIRCULATIONAHA.107.757781. [DOI] [PubMed] [Google Scholar]
- 33.Simiantonaki N, Taxeidis M, Jayasinghe C, Kurzik-Dumke U, Kirkpatrick CJ. Hypoxia-inducible factor 1 alpha expression increases during colorectal carcinogenesis and tumor progression. BMC Cancer. 2008;8:320. doi: 10.1186/1471-2407-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stumpff J, Duncan T, Homola E, Campbell SD, Su TT. Drosophila Wee1 kinase regulates Cdk1 and mitotic entry during embryogenesis. Curr Biol. 2004;14:2143–2148. doi: 10.1016/j.cub.2004.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumagin R, Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 2006;291:H2116–H2125. doi: 10.1152/ajpheart.00248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CF, Yuan CZ, Wang SH, Zhang H, Hu XX, Zhang L, Wu C, Li N. Differential gene expression of aldolase C (ALDOC) and hypoxic adaptation in chickens. Anim Genet. 2007;38:203–210. doi: 10.1111/j.1365-2052.2007.01605.x. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N, Broome M, Hunter T. Regulation of the human Wee1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada A, Duffy B, Perry JA, Kornbluth S. DNA replication checkpoint control of Wee1 stability by vertebrate Hsl7. J Cell Biol. 2004;167:841–849. doi: 10.1083/jcb.200406048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida D, Kim K, Noha M, Teramoto A. Anti-apoptotic action by hypoxia inducible factor 1-alpha in human pituitary adenoma cell line, HP-75 in hypoxic condition. J Neurooncol. 2006;78:217–225. doi: 10.1007/s11060-005-9017-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 43.Zhong N, Zhang Y, Fang QZ, Zhou ZN. Intermittent hypoxia exposure-induced heat-shock protein 70 expression increases resistance of rat heart to ischemic injury. Acta Pharmacol Sin. 2000;21:467–472. [PubMed] [Google Scholar]