Abstract
Recent epidemiologic studies clearly showed that early intensive glucose control has a legacy effect for preventing diabetic macrovascular complications. However, the cellular and molecular processes by which high glucose leads to macrovascular complications are poorly understood. Vascular smooth muscle cell (VSMC) dysfunction due to high glucose is a characteristic of diabetic vascular complications. Activation of nuclear factor-κB (NF-κB) may play a key role in the regulation of inflammation and proliferation of VSMCs. We examined whether VSMC proliferation and plasminogen activator inhibitor-1 (PAI-1) expression induced by high glucose were mediated by NF-κB activation. Also, we determined whether selective inhibition of NF-κB would inhibit proliferation and PAI-1 expression in VSMCs. VSMCs of the aorta of male SD rats were treated with various concentrations of glucose (5.6, 11.1, 16.7, and 22.2 mM) with or without an inhibitor of NF-κB or expression of a recombinant adenovirus vector encoding an IκB-α mutant (Ad-IκBαM). VSMC proliferation was examined using an MTT assay. PAI-1 expression was assayed by real-time PCR and PAI-1 protein in the media was measured by ELISA. NF-κB activation was determined by immunohistochemical staining, NF-κB reporter assay, and immunoblotting. We found that glucose stimulated VSMC proliferation and PAI-1 expression in a dose-dependent manner up to 22.2 mM. High glucose (22.2 mM) alone induced an increase in NF-κB activity. Treatment with inhibitors of NF-κB such as MG132, PDTC or expression of Ad-IκB-αM in VSMCs prevented VSMC proliferation and PAI-1 expression induced by high glucose. In conclusion, inhibition of NF-κB activity prevented high glucose-induced VSMC proliferation and PAI-1 expression.
Keywords: cell proliferation; glucose metabolism disorders; muscle, smooth, vascular; NF-κB; plasminogen activator inhibitor 1
Introduction
Cardiovascular complications are a primary cause of high morbidity and mortality in diabetes mellitus (UK Prospective Diabetes Study [UKPDS] Group, 1998). Recently, the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study and UKPDS post-trial study clearly showed that early intensive glucose control has a legacy effect for preventing diabetic macrovascular complications (DCCT/EDIC Study Research Group, 2005; Holman et al., 2008). However, the cellular and molecular processes by which high glucose leads to macrovascular complications are poorly understood, even though several mechanisms of diabetic vascular complication were suggested (Jeong and King, 2011).
The proliferation of vascular smooth muscle cells (VSMC) is one of the main features of atherosclerosis that are induced by high glucose conditions (Feener and King, 1997). Besides vascular cells, many kinds of inflammatory cells, such as monocytes and macrophages, along with cytokines are involved in atherosclerosis, which leads to the concept that atherosclerosis is an inflammatory disease (Ross, 1999). Nuclear factor κB (NF-κB) regulates transcription of various genes involved in immune and inflammatory responses, growth, and adhesion (Lenardo and Baltimore, 1989). Since the activation of NF-κB was shown to be involved in the human atherosclerotic artery (Brand et al., 1996) or VSMC proliferation sites in the balloon injured artery (Breuss et al., 2002), NF-κB was found to have an important role in atherosclerosis (Landry et al., 1997). Hyperglycemia (Monaco and Paleolog, 2004; Erl et al., 1999) as well as inflammatory cytokines (IL-1 and TNF-α) (Brand et al., 1997) can activate NF-κB through oxidative stress (Hattori et al., 2000).
Plasminogen activator inhibitor-1 (PAI-1) inhibits fibrinolysis and proteolysis as a physiologic inhibitor of tissue type plasminogen activator or urokinase-like plasminogen activator. Increases of PAI-1 have been observed in cases of thrombosis and fibrosis, obesity, diabetes, and insulin resistance (Meigs et al., 2000; Ma et al., 2004). Increases in PAI-1 mRNA expression were reported in the atherosclerotic artery of type 2 diabetic patients (Pandolfi et al., 2001) and in VSMCs cultured under high glucose conditions (Suzuki et al., 2002) or angiotensin-II (Lee et al., 2010). However, the signaling pathway involved in high glucose-induced PAI-1 expression and proliferation of VSMCs is not clear even though MAPK or PKC activation is partially involved (Suzuki et al., 2002).
Therefore, it is critical to determine whether the inhibition of proliferation and coagulation in the vasculature can serve as a novel therapeutic strategy to prevent vascular complications of diabetes. We studied the role of NF-κB activation in the proliferation of VSMCs or increases of PAI-1 expression due to high glucose. We also examined whether the inhibition of NF-κB by AdIκB-αM, an adenoviral vector encoding an IκB-α mutant protein as an IκBα super-repressor, or inhibitors of NF-κB such as MG132 and PDTC can prevent proliferation or increases in PAI-1 expression induced by high glucose in VSMCs.
Results
High glucose induces proliferation of VSMCs
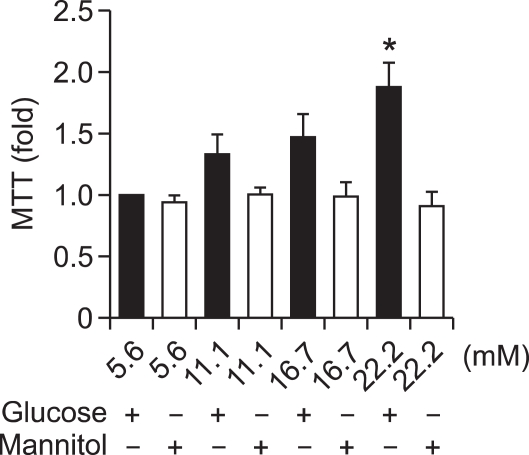
Incubation of VSMCs with various concentrations of glucose (5.6, 11.1, 16.7, and 22.2 mM) resulted in an increase of VSMC proliferation in a dose-dependent manner after 48 h of treatment. This increase was not seen in the osmotic control cells treated with mannitol to maintain the same osmolarity as cell grown in a high concentration of glucose (22.2 mM). We found that 22.2 mM glucose significantly increased VSMC proliferation (Figure 1) compared to control cells exposed to 5.6 mM glucose (P < 0.05).
Figure 1.
High glucose-induced proliferation of vascular smooth muscle cells (VSMCs). VSMCs were incubated in various concentrations of glucose (5.6, 11.1, 16.7, and 22.2 mM) for 48 h. Mannitol was used as an osmotic control. Cell proliferation was measured by an MTT assay. A high concentration of glucose (22.2 mM) significantly increased VSMC proliferation compared to control cells exposed to 5.6 mM glucose (*P < 0.05). Same concentrations of mannitol did not increase proliferation of the VSMCs.
High glucose activates the NF-κB signaling pathway
Immunohistochemical staining
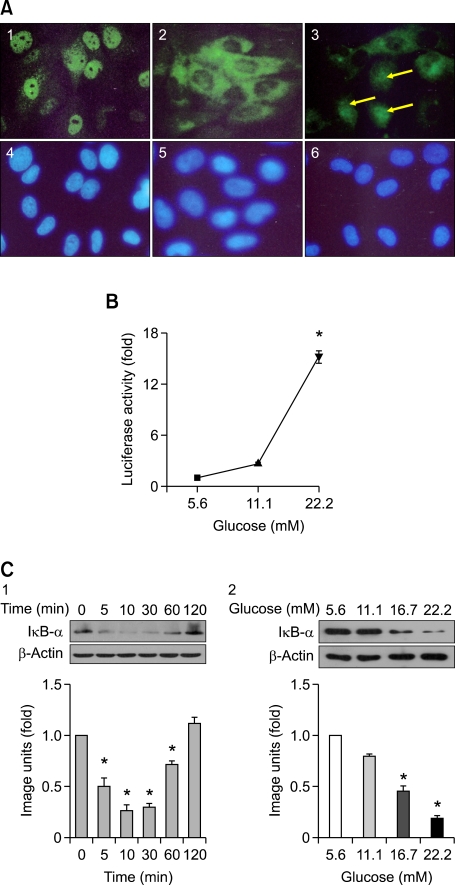
VSMCs in NG (5.6 mM) retained NF-κB p65 in the cytoplasm (green fluorescence) with little staining observed in the nuclei (Figure 2A-2). VSMC cultured in HG (22.2 mM) showed in a significant increase of NF-κB p65 nuclear translocation (Figure 2A-3) similar to the positive control (TNF-α, Figure 2A-1) which contained intense green fluorescence localized in the cell nuclei. Nuclei were stained blue by Hoechest staining.
Figure 2.
NF-κB activation by a high glucose concentration. (A) The effect of high glucose on the translocation of NF-κB. VSMCs were treated with 10 ng/ml TNF-α as a positive control (A-1), normal glucose (5.6 mM, A-2), and high glucose (22.2 mM, A-3). The cells were fixed and subjected to immunohistochemical staining to examine translocation of NF-kB. Nuclei were stained blue by Hoechest staining (A-4, 5, 6). VSMCs cultured in high glucose (22.2 mM) resulted in a significant increase of NF-κB p65 nuclear translocation (A-3, arrows). (B) The effect of glucose on luciferase activity. VSMCs were transfected with pNFκB-Luc and treated with 5.6, 11.1, and 22.2 mM glucose. High glucose (22.2 mM) significantly induced an increase of luciferase activity in the VSMCs (*P < 0.001). (C) The effect of glucose on the expression of IκB-α. VSMCs were cultured in high glucose (22.2 mM) for various time periods. Whole cell lysates were subjected to Western immunoblotting to analyze the protein levels of IκB-α. Densitometry quantification values (Image J) were measured and shown by fold over the control for each time point. Degradation of IκB-α started at 5 min, was maintained until 60 min, and returned to the basal level 120 min after high glucose (22.2 mM) treatment (C-1). Also, quiescent VSMCs were treated with various concentrations of glucose for 30 min. The representative immunoblot shows that high glucose (16.7 and 22.2 mM) significantly induced degradation of IκB-α (C-2) (*P < 0.05).
Luciferase activity
The NF-κB activity in cells treated for 48 h with 11.1 and 22.2 mM glucose was 2.8- and 15.7-fold, respectively, greater than that in cells treated with NG (5.6 mM). HG (22.2 mM) significantly increased NF-κB activity (P < 0.001; Figure 2B).
Immunoblotting (Western blot)
When VSMCs were cultured in HG (22.2 mM) for various time periods, degradation of IkB-α started at 5 min, maintained until 60 min, and returned to the basal level 120 min after HG treatment (Figure 2C-1). Also, after treatment with various concentrations (5.6, 11.1, 16.7, and 22.2 mM) of glucose for 30 min, the high glucose concentrations (16.7 and 22.2 mM) significantly induced degradation of IkB-α compared with that of 5.6 mM glucose (Figure 2C-2).
High glucose stimulates PAI-1 mRNA expression and PAI-1 protein secretion into media
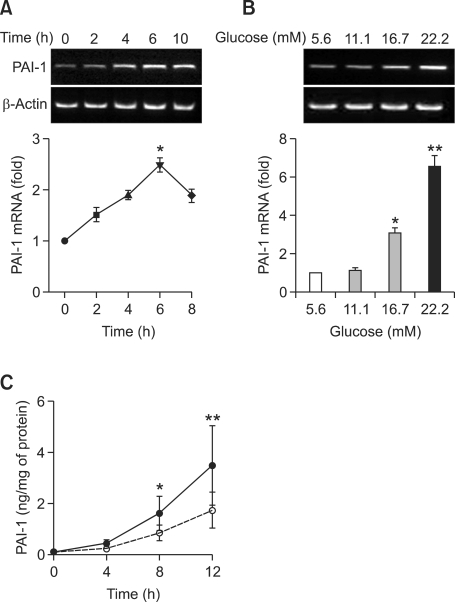
We examined the effects of high glucose on PAI-1 mRNA expression in VSMCs with semi-quantitative RT-PCR and real-time PCR. PAI-1 mRNA was observed at low levels in untreated control VSMCs. After HG (22.2 mM) treatment, PAI-1 mRNA increased at 2 h, peaked at 6 h, and remained constant at 10 h (Figure 3A).
Figure 3.
Effects of high glucose on PAI-1 mRNA expression (A, B) and PAI-1 protein secretion (C). (A) Time-dependent effects of high glucose on PAI-1 mRNA expression. Quiescent cells were stimulated with high glucose (22.2 mM) for the indicated times. PAI-1 mRNA expression was determined by semi-quantitative RT-PCR. PAI-1 mRNA expression started to increase at 2 h, peaked at 6 h, and then decreased after high glucose treatment. (B) Dose-dependent effects of high glucose on PAI-1 mRNA expression. Quiescent cells were stimulated with the indicated concentrations of glucose for 6 h and were subjected to RT-PCR. High concentrations of glucose (16.7 and 22.2 Mm) significantly stimulated PAI-1 mRNA expression. (**P < 0.01 versus 5.6 mM glucose, *P < 0.05 versus 5.6 mM glucose). Data are shown as mean × SD from at least four experiments. (C) Time-dependent effects of high glucose on secretion of PAI-1 protein in the media. Synchronized quiescent VSMC cells were treated with DMEM Low Glucose without Penol Red containing 5.6 (open circle) and 22.2 (closed circle) mM glucose for 4, 8, 12 h. PAI-1 concentrations of the culture medium were determined using a rat PAI-1 ELISA kit and were expressed as nanograms of active PAI-1 released from VSMC/mg of cell protein. (*P < 0.05 versus 5.6 mM glucose, **P < 0.01 versus 5.6 mM glucose).
To evaluate the effects of various concentrations of glucose on PAI-1 gene expression, VSMCs were treated with 5.6, 11.1, 16.7, and 22.2 mM glucose for 6 h. Figure 3B shows that PAI-1 mRNA expression was increased in a glucose concentration-dependent manner. High concentrations of glucose (16.7 and 22.2 mM) significantly stimulated PAI-1 mRNA expression compared to 5.6 mM glucose treatment (P < 0.05, 16.7 mM versus 5.6 mM glucose; P < 0.01, 22.2 mM versus 5.6 mM glucose).
The level of PAI-1 protein in the media was measured by ELISA. Figure 3C shows that the 22.2 mM HG stimulated the secretion of PAI-1 protein into media compared with 5.6 mM NG at 8 h and 12 h after treatment (*P < 0.05, 22.2 mM versus 5.6 mM glucose; **P < 0.01, 22.2 mM versus 5.6 mM glucose in Figure 3C).
NF-κB inhibition decreases high glucose-induced proliferation in VSMCs
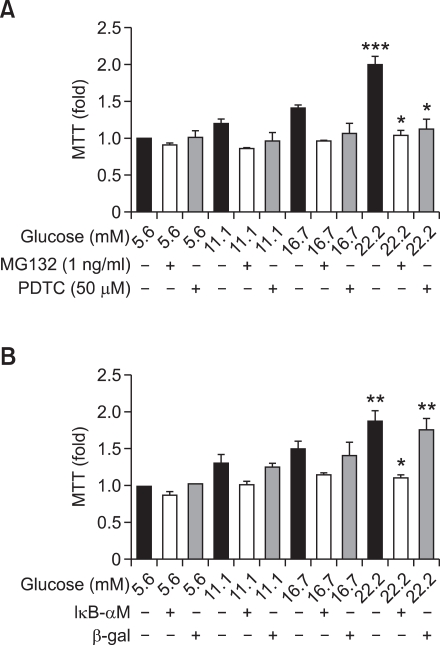
Addition of NF-κB inhibitors PDTC (50 µM) and MG132 (1 ng/ml) also led to a significant decrease in VSMC proliferation compared to that of cells treated with HG (22.2 mM). Two kinds of NF-κB inhibitors prevented high glucose-induced VSMC proliferation (P < 0.05 versus HG-treated cells; Figure 4A). Additionally, transfection of AdIκB-αM significantly inhibited high glucose-induced VSMC proliferation (P < 0.05 versus cells treated with 22.2 mM glucose or 22.2 mM glucose with Adβ-gal; Figure 4B).
Figure 4.
NF-κB inhibition decreases high glucose-induced proliferation in VSMC. VSMCs were treated with different glucose concentrations (5.6, 11.1, 16.7, and 22.2 mM) for 48 h in the presence or absence of NF-κB inhibitors: MG132 (A) or PDTC (A) or transfection of AdIκB-αM (B). (A) Cells were pretreated with MG132 (1 ng/ml) or PDTC (50 µM) for 2 h and then treated with the indicated concentrations of glucose for 48 h. Cell proliferation was measured by an MTT assay. High glucose (22.2 mM) significantly stimulated proliferation of VSMC (***P < 0.001 versus 5.6 mM glucose). The two NF-κB inhibitors prevented HG-induced VSMC proliferation (*P < 0.05 versus treatment with 22.2 mM glucose alone). (B) AdIκB-αM as a IκBα-super-repressor was transfected into VSMC for 4 h. VSMCs were treated with indicated concentrations of glucose for 48 h. High glucose (22.2 mM) significantly stimulated proliferation of VSMC (**P < 0.01 versus 5.6 mM glucose). AdIκB-αM significantly inhibited high glucose-induced VSMC proliferation (*P < 0.05 versus VSMCs treated with 22.2 mM glucose or Adβ-gal transfected cells treated with 22.2 mM glucose with).
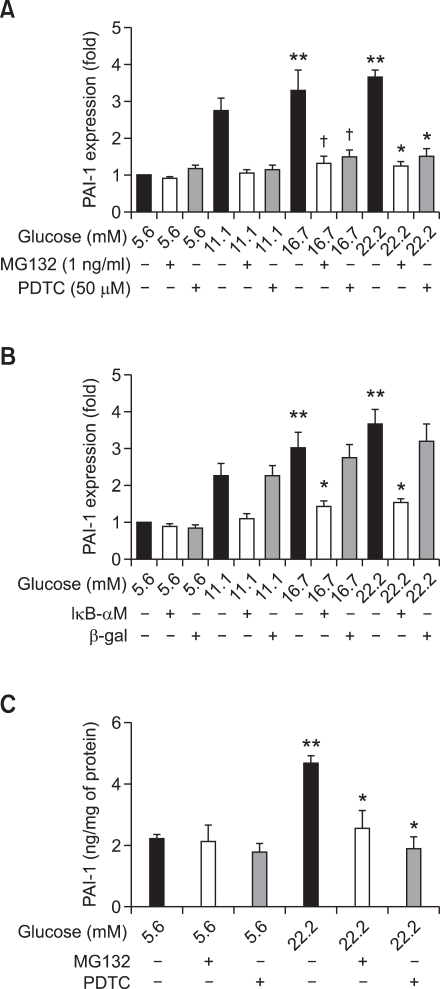
NF-κB inhibition prevents high glucose-induced PAI-1 mRNA expression and PAI-1 protein secretion in VSMC
High glucose significantly stimulated PAI-1 mRNA expression (P < 0.01 versus cells treated with 5.6 mM glucose). Pretreatment with 1 ng/ml MG132 or 50 µM PDTC (Figure 5A) prevented HG-induced PAI-1 mRNA expression (P < 0.05 versus cells treated with 22.2 mM glucose alone). AdIκB-αM expression (Figure 5B) also significantly inhibited HG-induced PAI-1 mRNA expression (P < 0.05 versus cells treated with 22.2 mM glucose or 22.2 mM glucose with β-gal). HG induced PAI-1 protein secretion was significantly reduced by 1 ng/ml MG132 or 50 µM PDTC (Figure 5C).
Figure 5.
NF-κB inhibition prevents high glucose-induced PAI-1 expression in VSMCs. VSMCs were treated with different glucose concentrations (5.6, 11.1, 16.7, and 22.2 mM) for 48 h in the presence or absence of NF-κB inhibitors, MG132 (A) or PDTC (A) or AdIκB-αM (B). (A) Cells were pretreated with 1 ng/ml MG132 or 50 µM PDTC for 2 h before being treated with the indicated concentrations of glucose for 6 h. After RNA isolation, PAI-1 mRNA expression was measured by real-time PCR. High glucose significantly stimulated PAI-1 mRNA expression (**P < 0.01 versus 5.6 mM glucose). The two NF-κB inhibitors prevented HG-induced PAI-1 mRNA expression (*P < 0.05 versus 22.2 mM glucose alone, †P < 0.05 versus 16.7 mM glucose alone). (B) High glucose significantly stimulated PAI-1 mRNA expression (**P < 0.01 versus 5.6 mM glucose). AdIκB-αM significantly inhibited high glucose-induced PAI-1 mRNA expression (*P < 0.05 versus glucose 22.2 mM or glucose 22.2 mM with β-gal). (C) HG induced PAI-1 protein secretion was inhibited by NF-κB inhibitors. Synchronized quiescent VSMC cells were treated with DMEM Low Glucose without Penol Red containing 5.6 and 22.2 mM glucose after pretreatment with or without NF-κB inhibitors, 1 ng/ml MG132 or 50 µM PDTC. The level of PAI-1 in the media was measured by ELISA. HG induced PAI-1 secretion was significantly reduced by NF-κB inhibitors (**P < 0.05 glucose 22.2 mM vs glucose 5.6 mM; *P < 0.05 glucose 22.2 mM vs glucose 22.2 mM with NF-κB inhibitors, 1 ng/ml MG132 or 50 µM PDTC).
Discussion
Our results demonstrated that high glucose stimulated proliferation and PAI-1 expression of rat VSMCs. Furthermore, inhibition of NF-κB prevented proliferation and PAI-1 expression induced by high glucose in VSMCs. We have reported for the first time that both proliferation and PAI-1 expression of VSMC are controlled by the NF-κB pathway.
Prolonged chronic hyperglycemia is one of the major causes of vascular complication in diabetes; this can be prevented by intensive early treatment as clearly shown in recent epidemiologic studies (DCCT/EDIC Study Research Group, 2005; Holman et al., 2008). Also, SHARE study showed that the degree of intima-media thickness of the carotid artery is relation of HbA1c (Gerstein et al., 2003). It suggested that the elevated HbA1c is a strong indicator of proliferation of VSMCs. We showed that proliferation of VSMCs was increased by glucose in a concentration-dependent manner. Furthermore, a high glucose concentration (22.2 mM) significantly stimulated the proliferation of VSMC.
Proliferation and migration of VSMCs are characteristic of progression of atherosclerotic lesion from a fatty streak to a more complex plaque (Glass and Witztum, 2001). A wide spectrum of inflammatory mediators is involved in atherosclerosis. NF-κB is one of the key factors of atherosclerosis detected in VSMCs, macrophages, and endothelial cells from human atherosclerotic lesions (Brand et al., 1996). Even though inflammatory cytokines such as TNF-α (Lee et al., 2011) or advanced glycation endproducts (Yoon et al., 2009) promoted the progression of atherosclerosis through activation of NF-κB pathway, that is not adequate evidences which explain about the direct effect of hyperglycemia itself in diabetic vasculopathy. Therefore, in this study, we observed that activation of NF-κB was stimulated by high concentrations of glucose as evidenced by translocation of the p65 subunit, increased luciferase activity, and degradation of IκB-α. Activation of the NF-κB pathway by high glucose concentrations has been shown in VSMCs from rat (Hattori et al., 2000), swine (Yerneni et al., 1999), and humans (Ruiz et al., 2006). However, the role of NF-κB activation by hyperglycemia in VSMCs has not been clearly shown. We demonstrated that both proliferation and PAI-1 expression of VSMC were mediated by NF-κB activation. Several studies showed that a link between high glucose induced NF-κB activation and cell proliferation was blocked by use of antioxidant or anti-inflammatory drugs such as α-lipoic acid (Kim et al., 2007), pentoxifylline, and c-peptide (Cifarelli et al., 2008). These NF-κB blockades are non-specific inhibitors and may influence other intracellular pathways. Thus, we overexpressed a mutant form of IκBα, AdIκB-αM. Other investigators showed that cytokine- or growth factor-induce VSMC proliferation is inhibited by liposomal delivery of an NF-κB inhibitory peptide, IκBα (Selzman et al., 1999). However, this study showed for the first time that high glucose-induced proliferation and PAI-1 expression was prevented by expression of AdIκB-αM, an IκBα super-repressor.
Even though progression of atherosclerotic lesions can lead to ischemic symptoms as a result of progressive narrowing of the vessel lumen, acute cardiovascular events usually results from plaque rupture and thrombosis. Decreased fibrinolysis is believed to accelerate arterial atherosclerosis by facilitating thrombosis. PAI-1 is one of the major regulators of fibrinolysis (Meigs et al., 2000). Levels of plasma PAI-1 are elevated under various thrombotic conditions as well as during diabetes and insulin resistance (Irigoyen et al., 1999). Even though platelets account for more than 90% of PAI-1 in blood, high glucose increases PAI-1 synthesis in arterial wall cells in culture by activating MAPK and PKC (Suzuki et al., 2002). We showed that PAI-1 gene expression was increased by glucose in a concentration-dependent manner. High glucose concentrations (16.7 and 22.2 mM) significantly increased the expression of PAI-1. Inhibition of NF-κB prevented PAI-1 expression. Recently, the association between PAI-1 and VSMC proliferation was shown by Chen et al. (2006). PAI-1 transgenic mice show increased VSMC proliferation through NF-κB and ERK. According to our data, inhibition of NF-κB prevented high glucose-induced proliferation and PAI-1 expression in VSMCs. We did not study about the direct effect of PAI-1 on the high glucose-induced proliferation of VSMC. We just demonstrated that increased PAI-1 expression and proliferation induced by high glucose were mediated through NF-κB activation. However, the direct role of PAI-1in the proliferation of VSMCs is very conflicted and complex. Several researchers showed the role PAI-1 as an inhibitor of vascular proliferation. Stefansson and Lawrence (1996) showed that PAI-1 binds vitronectin (VN) and blocks its interactions with u-PAR (urokinase-type plasminogen activator receptor) and αγβ3 integrin thus inhibiting migration and cell detachment. Wu et al. (2009) showed that active stable mutant recombinant PAI-1 inhibited VSMC proliferation which was lost in VN-deficient VSMCs. On the other hand, overexpression of PAI-1 increased proliferation of VSMC due to a direct inhibitory effect of PAI-1 on the activity of caspase-3. Meilhac et al. (2003) showed pericellular plasmin induced smooth muscle cell anoikis which is a kind of apoptosis induced by detachment of adherent cells from extracellular matrix. This plasminogen activator-induced proteolysis can result plaque rupture and aneurismal formation. It suggests that PAI-1 can enhance proliferation primarily by controlling proteolytic activity. Therefore the balance between PAI-1's inhibitory action on plasmin and interaction with vitronectin and other proteases may play a critical role in interfering with vascular proliferative responses. This PAI-1 paradox is speculated as several ways. Diebold et al. (2008) suggested dose dependent effect of PAI-1 and Konstantinides et al. (2002) argued the different effect of PAI-1 by various stage of atherosclerosis.
Even though we did not elucidate the direct role of PAI-1 in the VSMC proliferation, NF-κB activation directly increased VSMC proliferation through enhancing Bcl-2 expression. Antiapototic effect of NF-κB was reported that the activation of NF-κB directly stimulated antiapoptotic protein, Bcl-2 in macrophage (Lawrence et al., 2005). Our results mean that activation of NF-κB represents another mechanism of high glucose-induced PAI-1 expression in VSMC.
In conclusion, we have demonstrated that high glucose-induced proliferation and PAI-1 expression in VSMC was prevented by inhibition of NF-κB activation. Direct administration of selective inhibitory proteins such as IκBα or NF-κB inhibitors might be a clinically-accessible strategy for responding to vascular events in diabetic patients.
Methods
Animals
8-week old male Sprague-Dawley rats were used after adaptation for 7 days in an animal facility kept at 24-26℃ and 60% humidity with 12-h light/dark cycles.
Isolation and culture of VSMCs
VSMCs were isolated from the aorta of male SD rats using an enzyme solution mixed with collagenase type I and elastase type III (Yoo et al., 1997). Isolated VSMCs were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Gibco) containing normal glucose (NG; 5.6 mM), 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin in a CO2 incubator (5% CO2, 95% O2, 37℃). These VSMCs were shaped so that they had a 'hill-and-valley' appearance. At passages 5-12, the cells were used for this experiment.
MTT assay
VSMC proliferation was measured by an MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) assay to compare the effect of normal glucose (NG; 5.6 mM; D-glucose, Sigma, St. Louis, MO) and high glucose (HG; 11.1, 16.7, and 22.2 mM) on the cell proliferation.
VSMCs were cultured in 10% FBS-DMEM (Gibco) on 96-well plates (5 × 103 cells/well). When the VSMCs reached a 60% confluent state, the cells were rendered quiescent by incubation in DMEM (Gibco) containing NG and 0.1% FBS for 24 h. Next, the VSMCs were treated with different glucose concentrations (5.6, 11.1, 16.7, and 22.2 mM) for 48 h in the absence or presence of NF-κB inhibitors: 1 ng/ml MG132 (Calbiochem, La Jolla, CA) or 50 µM PDTC (Sigma) or AdIκB-αM (kindly provided by Dr. Myung-Shik Lee). 1 µM MG-132 or 50 µM PDTC was used to treat the cells 2 h before glucose treatment. After aspiration of supernatants, VSMCs were treated with indicated concentrations of glucose for 48 h. The cells of 96 wells were incubated with 100 µl of 0.5 mg/ml MTT at 37℃ for 4 h, washed with cold PBS, and lysed with 100 µl of DMSO (Sigma). After the insoluble crystals were completely dissolved, the optical density of each well was immediately measured at 570 nm using an automatic micro-plate reader (Molecular Devices, Sunnyvale, CA).
Adenoviral transfection of AdIκB-αM
AdIκB-αM, an adenoviral vector encoding an IκB-α mutant protein as an IκBα super-repressor, whose serine 32, 36 were replaced with alanines and control virus (Adβ-gal). Adenovirus was propagated in HEK 293 cells and concentrated by standard CsCl ultracentrifugation. VSMCs were infected with AdIκB-αM at a multiplicity of infection of 100 in a serum free media for 4 h. After incubation of 24 h, the cells were treated with indicated glucose before MTT assays.
NF-κB activity
Immunohistochemical staining
VSMCs were cultured in chamber slides (500 cells/chamber, Lab-Tek; Nalge Nunc International, IL) in 10% FBS-DMEM (Gibco) for 48 h and were made quiescent by incubation in DMEM (Gibco) containing NG and 0.2% BSA for 24 h. The cells were then treated with 5.6 and 22.2 mM glucose solutions for 6 h. TNF-α (10 µg/ml) was used as a positive control. After treatment, the cells were fixed in 4% paraformaldehyde, washed with PBS, and incubated with anti-RelA/p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4℃. The cells were then washed and incubated with biotinylated anti-mouse IgG, streptavidin-TRITC, and Hoechst33342. Stained cells were observed using a fluorescence microscope connected to a digital camera and interfaced with a computer, and the number of cells showing nuclear translocation of RelA/p65 was counted by a single observer blinded to the treatment groups.
NF-κB reporter assay
NF-κB reporter activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Fitchburg, WI). VSMC were co-transfected with 0.5 µg of an NF-κB-responsive reporter gene construct carrying two copies of κB sequences linked to the luciferase gene (pNFκB-Luc; Stratagene, La Jolla, CA) along with 0.01 µg of Renilla luciferase (Prl-TK; Promega) using Lipofectamine (Invitrogen, Grand Island, NY). At 24 h after transfection, cells were treated with NG or HG (22.2 mM) for an additional 5 h. Afterward, activities of the luciferase and Renilla luciferase were measured using the Dual-Luciferase Reporter Assay Kit (Promega). Results were presented as luciferase activity normalized to Renilla luciferase activity.
Western blotting
VSMCs were cultured in a 6-well plate (2 × 105 cells/well) and made quiescence in DMEM containing 0.1% FBS and NG, then treated with high glucose (HG: 22.2 mM) for various times (0, 10, 20, 30, 50, and 60 min). Following treatment, whole cell lysates were made; protein concentrations were determined and an equal amount of each sample was subjected to SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Following the transfer, the membranes were reversibly stained with a Ponceau S solution (Amresco, Ohio) to confirm sample loading and gel transfer equivalence. After blocking with 5% nonfat milk for 1 h, the membranes were incubated with an anti-IκB-α antibody (1:1,000, Santa Cruz, CA) for overnight at 4℃ and followed by incubation with an goat-anti-rabbit Ig G secondary antibody (1:2,000, Santa Cruz, CA) for 1 h. Antibody binding was detected by enhanced chemiluminescence (ECL, Pierce). X-ray film (Kodak) was used to visualize the bands. Densitometry quantification values (Image J, program from NIH) were measured and shown by fold over the control for each time point.
RNA isolation, semi-quantitative reverse transcription (RT)-polymerase chain reaction (PCR), and real-time RT-PCR of PAI-1 mRNA
Total RNA was isolated from VSMCs (2.5 × 105 cells/60 mm dish/2 ml DMEM with 0.1% FBS) that had been serum-starved overnight in 0.1% FBS and treated with HG (22.2 mM) for 0, 2, 4, 6, 8 h. The method was based on the phenol/guanidine isothiocyanate based on RNAzol B (Cinna/Biotecx, Houston, TX) after VSMCs were treated with indicated concentrations of glucose for 6 h with or without NF-κB inhibitors such as MG132 or AdIκB-αM transfected cells. Total RNA (1 µg) was reverse transcribed using MMLV reverse transcriptase (Gibco) with random hexamer priming. For semi-quantitative PCR, aliquots of cDNA were amplified in a 20 µl PCR mixture according to the protocol provided by the manufacturer (TaKaRa Bio, Kyoto, Japan). The PCR conditions for PAI-1 were as follows: 28 cycles at 95℃ for 45 s, 60℃ for 45 s, and 72℃ for 45 s. PCR products were separated by electrophoresis on 1.5% agarose gels containing ethidium bromide, and the bands were visualized under UV light. The primers were synthesized by Bioneer Co. Ltd (Seoul, South Korea).
For real-time RT-PCR, the resultant cDNA was amplified using a Roche Light Cycler (Roche Diagnostics Ltd., Lewes, UK). Real-time PCR analysis was carried out with SYBR Green I (Roche Diagnostics Ltd.) and primers for rat PAI-1 (5'-ATGAGATCAGTACTGCGGACGCCATCTTTG-3', 5'-ACAAGTCTGATGGTAGCACCATCTCCGTGC-3') or β-actin (5'-AATGCCTCCTGCACCACCAAC-3', 5'-AAGGCCATGCCAGTGAGCTTC-3'). PCR reactions with SYBR Green I and primers were performed in a 20 µl volume with 2 µl cDNA, 0.5 µM primers, and 4 mM MgCl2. Taq polymerase, PCR buffer, dNTPs and SYBR Green I dye were included in the Light Cycler-Fast Start DNA Master SYBR Green I mix (Roche Diagnostics Ltd.). Thermal cycling profile consisted of a pre-incubation step at 95℃ for 10 min followed by 40 or 50 cycles of a 95℃ denaturation step for 10 s, 59℃ annealing step for 5 s, and 72℃ extension step for 20 s. After each extension step, the temperature was raised to 88℃ to measure SYBR Green I fluorescence at a temperature 2℃ below the product Tm and above the Tm of the primer-dimers to prevent interference from non-specific primers-dimers. At the end of the PCR, a melting curve analysis was performed by gradually increasing the temperature from 65℃ to 95℃ (0.1℃/s) to confirm the amplification specificity of the PCR products. The level of expression of each mRNA and their estimated crossing points (Cp) for each sample were determined relative to the standard preparation using LightCycler computer software (v 3.5). The PCR standards for PAI-1 and β-actin consisted of a known number of PCR products and were made to a concentration of 108 copies/µl. PCR amplification was performed with a series of standards prepared by successive dilutions, and a linear standard curve was automatically generated. A standard curve was constructed for each PCR run. All samples to be compared were run in the same assay.
ELISA of PAI-1 protein
The levels of PAI-1 in the culture media were determined by ELISA kit (Molecular Innovations). VSMC cells were cultured in 10% FBS-DMEM Low Glucose without Penol Red (GIBCO) and cells were made quiescent by incubation in DMEM Low Glucose without Penol Red containing 0.1% FBS and NG for 24 h and were treated with 5.6 and 22.2 mM glucose solutions for 4, 8, 12 h. PAI-1 concentrations of the culture medium were determined using a rat PAI-1 ELISA kit and were expressed as nanograms of active PAI-1 released from VSMC/mg of cell protein. Also, synchronized quiescent VSMC cells were treated with DMEM Low Glucose without Penol Red containing 5.6 and 22.2 mM glucose for 12 h after pretreatment with or without NF-κB inhibitors, 1 ng/ml MG132 or 50 µM PDTC. The level of PAI-1 in the media was measured by ELISA. HG induced PAI-1 secretion was significantly reduced by NF-κB inhibitors (**P < 0.05 glucose 22.2 mM vs glucose 5.6 mM; *P < 0.05 glucose 22.2 mM vs glucose 22.2 mM with NF-κB inhibitors, 1 ng/ml MG132 or 50 µM PDTC).
Statistical analysis
Results were expressed as means ± SD. Statistical significance was assessed with a non-parametric Student's t-test or one-way analysis of variance (ANOVA) using Prism software (Graphpad, San Diego, CA). P < 0.05 was considered statistically significant. Tukey-Kramer post-tests were performed when appropriate.
Acknowledgements
This work was supported by a grant from Korean Diabetes Association in 2003 and Kyung Hee University in 2006 (KHU-20060484).
Abbreviations
- Ad-IκBαM
adenovirus vector encoding an IκB-alpha mutant
- PAI-1
plasminogen activator inhibitor-1
- VSMC
vascular smooth muscle cell
References
- 1.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand K, Page S, Walli AK, Baeuerle PA. Role of nuclear factor-κB in atherosclerosis. Exp Physiol. 1997;82:297–304. doi: 10.1113/expphysiol.1997.sp004025. [DOI] [PubMed] [Google Scholar]
- 3.Breuss JM, Cejna M, Bergmeister H, Kadl A, Baumgartl G, Steurer S, Xu Z, Koshelnick Y, Lipp J, De Martin R, Losert U, Lammer J, Binder BR. Activation of nuclear factor-kappa B significantly contributes to lumen loss in a rabbit iliac artery balloon angioplasty model. Circulation. 2002;105:633–638. doi: 10.1161/hc0502.102966. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 5.Cifarelli V, Luppi P, Tse HM, He J, Piganelli J, Trucco M. Human proinsulin C-peptide reduces high glucose-induced proliferation and NF-kappaB activation in vascular smooth muscle cells. Atherosclerosis. 2008;201:248–257. doi: 10.1016/j.atherosclerosis.2007.12.060. [DOI] [PubMed] [Google Scholar]
- 6.Diebold I, Kraicun D, Bonello S, Görlach A. The 'PAI-1 paradox' in vascular remodeling. Thromb Haemost. 2008;100:984–991. [PubMed] [Google Scholar]
- 7.Erl W, Hannson GK, de Martin R, Draude G, Wever KSC, Weber G. Nuclear factor-κB regulates induction of apoptosis and inhibitor of apoptosis proteon-1 expression in vascular smooth muscle cells. Circ Res. 1999;84:668–677. doi: 10.1161/01.res.84.6.668. [DOI] [PubMed] [Google Scholar]
- 8.Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350:SI9–SI13. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Anand S, Yi QL, Vuksan V, Lonn E, Teo K, Malmberg K, McQueen M, Yusuf S SHARE Investigators. The relationship between dysglycemia and atherosclerosis in South Asian, Chinese, and European individuals in Canada: a randomly sampled cross-sectional study. Diabetes Care. 2003;26:144–149. doi: 10.2337/diacare.26.1.144. [DOI] [PubMed] [Google Scholar]
- 10.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 11.Hattori Y, Hattori S, Sato N, Kasai K. High glucose-induced nuclear factor κB activation in vascular smooth muscle cells. Cardiovasc Res. 2000;46:188–197. doi: 10.1016/s0008-6363(99)00425-3. [DOI] [PubMed] [Google Scholar]
- 12.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 13.Irigoyen JP, Muñoz-Cánoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci. 1999;56:104–132. doi: 10.1007/PL00000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong IK, King GL. New perspectives on diabetic vascular complications: the loss of endogenous protective factors induced by hyperglycemia. Diabetes Metab J. 2011;35:8–11. doi: 10.4093/dmj.2011.35.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp Mol Med. 2007;39:106–113. doi: 10.1038/emm.2007.12. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinides S, Schäfer K, Loskutoff DJ. Do PAI-1 and vitronectin promote or inhibit neointima formation? The exact role of the fibrinolytic system in vascular remodeling remains uncertain. Arterioscler Thromb Vasc Biol. 2002;22:1943–1945. doi: 10.1161/01.atv.0000047462.65341.22. [DOI] [PubMed] [Google Scholar]
- 17.Landry DB, Couper LL, Bryant SR, Lindner V. Activation of the NF-kappa B and I kappa B system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am J Pathol. 1997;151:1085–1095. [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 19.Lenardo MJ, Baltimore D. NF-κB: a pleiotropic mediator of inducible and tissue specific gene control. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee CW, Lin CC, Lee IT, Lee HC, Yang CM. Activation and induction of cytosolic phospholipase A2 by TNF-α mediated through Nox2, MAPKs, NF-κB, and p300 in human tracheal smooth muscle cells. J Cell Physiol. 2011;226:2103–2114. doi: 10.1002/jcp.22537. [DOI] [PubMed] [Google Scholar]
- 21.Lee KM, Seo HY, Kim MK, Min AK, Ryu SY, Kim YN, Park YJ, Choi HS, Lee KU, Park WJ, Park KG, Lee IK. Orphan nuclear receptor small heterodimer partner inhibits angiotensin II-stimulated PAI-1 expression in vascular smooth muscle cells. Exp Mol Med. 2010;42:21–29. doi: 10.3858/emm.2010.42.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughan DE, Fogo AB. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 23.Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, Lipinska I, D'Agostino RB, Wilson PW. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283:221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 24.Meilhac O, Ho-Tin-Noé B, Houard X, Philippe M, Michel JB, Anglés-Cano E. Pericellular plasmin induces smooth muscle cell anoikis. FASEB J. 2003;17:1301–1303. doi: 10.1096/fj.02-0687fje. [DOI] [PubMed] [Google Scholar]
- 25.Monaco C, Paleolog E. Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res. 2004;61:671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfi A, Cetrullo D, Polishuck R, Alberta MM, Calafiore A, Pellegrini G, Vitacolonna E, Capani F, Consoli A. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler Thromb Vasc Biol. 2001;21:1378–1382. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz E, Gordillo-Moscoso A, Padilla E, Redondo S, Rodriguez E, Reguillo F, Briones AM, van Breemen C, Okon E, Tejerina T. Human vascular smooth muscle cells from diabetic patients are resistant to induced apoptosis due to high Bcl-2 expression. Diabetes. 2006;55:1243–1251. doi: 10.2337/db05-0949. [DOI] [PubMed] [Google Scholar]
- 29.Selzman CH, Shames BD, McIntyre RC, Jr, Banerjee A, Harken AH. The NFkappaB inhibitory peptide, IkappaBalpha, prevents human vascular smooth muscle proliferation. Ann Thorac Surg. 1999;67:1227–1231. doi: 10.1016/s0003-4975(99)00252-0. [DOI] [PubMed] [Google Scholar]
- 30.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature. 1996;383:441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Akimoto K, Hattori Y. Glucose upregulates plasminogen activator inhibitor-1 gene expression in vascular smooth muscle cells. Life Sci. 2002;72:59–66. doi: 10.1016/s0024-3205(02)02182-3. [DOI] [PubMed] [Google Scholar]
- 32.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 34.Wu J, Peng L, McMahon GA, Lawrence DA, Fay WP. Recombinant plasminogen activator inhibitor-1 inhibits intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2009;29:1565–1570. doi: 10.1161/ATVBAHA.109.189514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 36.Yoo HJ, Kozaki K, Akishita M, Watanabe M, Eto M, Nagano K, Sudo N, Hashimoto M, Kim S, Yoshizumi M, Toba K, Ouchi Y. Augmented Ca2+ influx is involved in the mechanism of enhanced proliferation of cultured vascular smooth muscle cells from spontaneously diabetic Goto-Kakizaki rats. Atherosclerosis. 1997;131:167–175. doi: 10.1016/s0021-9150(97)06098-x. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SJ, Yoon YW, Lee BK, Kwon HM, Hwang KC, Kim M, Chang W, Hong BK, Lee YH, Park SJ, Min PK, Rim SJ. Potential role of HMG CoA reductase inhibitor on oxidative stress induced by advanced glycation endproducts in vascular smooth muscle cells of diabetic vasculopathy. Exp Mol Med. 2009;41:802–811. doi: 10.3858/emm.2009.41.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]