Abstract
During NREM sleep and under certain types of anaesthesia, the mammalian brain exhibits a distinctive slow (<1 Hz) rhythm. At the cellular level, this rhythm correlates with so-called UP and DOWN membrane potential states. In the neocortex, these UP and DOWN states correspond to periods of intense network activity and widespread neuronal silence, respectively, whereas in thalamocortical (TC) neurons, UP/DOWN states take on a more stereotypical oscillatory form, with UP states commencing with a low-threshold Ca2+ potential (LTCP). Whilst these properties are now well recognised for neurons in cats and rats, whether or not they are also shared by neurons in the mouse is not fully known. To address this issue, we obtained intracellular recordings from neocortical and TC neurons during the slow (<1 Hz) rhythm in anaesthetised mice. We show that UP/DOWN states in this species are broadly similar to those observed in cats and rats, with UP states in neocortical neurons being characterised by a combination of action potential output and intense synaptic activity, whereas UP states in TC neurons always commence with an LTCP. In some neocortical and TC neurons, we observed ‘spikelets’ during UP states, supporting the possible presence of electrical coupling. Lastly, we show that, upon tonic depolarisation, UP/DOWN states in TC neurons are replaced by rhythmic high-threshold bursting at ~5 Hz, as predicted by in vitro studies. Thus, UP/DOWN state generation appears to be an elemental and conserved process in mammals that underlies the slow (<1 Hz) rhythm in several species, including humans.
Keywords: EEG, Oscillations, Sleep, Neocortex, Thalamocortical, Electroencephalogram, T-type calcium channel, Thalamus, Neocortical neurons
Introduction
During NREM sleep and under certain types of anaesthesia, the EEG of both humans and other mammals exhibits a characteristic slow rhythm at <1 Hz (Fig. 1) [18]. This rhythm is considered to be the most fundamental of all sleep oscillations and acts to coordinate other electrographic sleep waves such as epochs of delta (δ; ~1–4 Hz) oscillations, sleep spindles and even higher-frequency gamma (γ; ~20–80 Hz) oscillations into recurring groups [12, 26, 38, 63, 64]. Although the precise function of the slow rhythm is much debated, a consensus is emerging that it plays an important role in memory consolidation [7]. Two main findings in humans have fuelled this idea. Firstly, it has been shown that, following a learning task involving specific brain regions, there is a localised increase in slow-wave activity that is associated with improved task performance [29]. This suggests that NREM sleep homeostasis, as measured by the amount of EEG slow-wave activity, has local components that are closely linked to the formation and strengthening of specific memories. That slow (<1 Hz) rhythm generation can be modulated and occur on a localised cortical basis has since been directly demonstrated in humans at the cellular level [52, 71]. Secondly, it has been established that an enhancement of slow waves via the transcranial application of appropriately timed oscillating potentials enhances the retention of hippocampal-dependent declarative memories [46]. These and related findings from both humans and other mammals [7] thus suggest a fundamental importance of the slow rhythm for human and animal physiology.
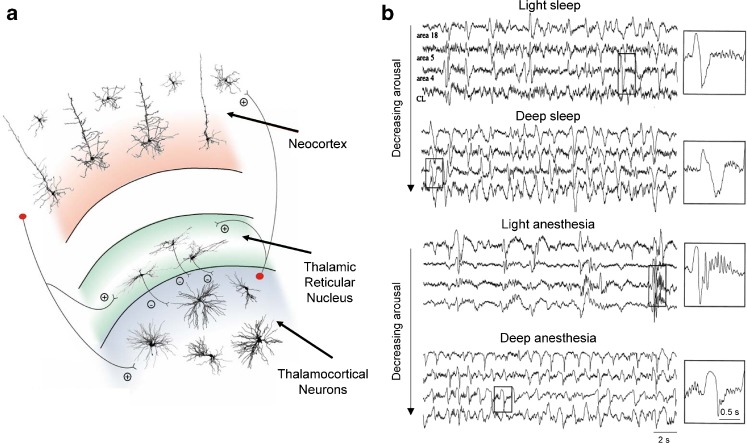
Fig. 1.
Schematic representation of corticothalamic circuitry and the slow (<1 Hz) rhythm. a Simplified schematic diagram of the corticothalamic circuitry involved in generating the EEG slow (<1 Hz) rhythm. The most relevant cellular components and synaptic connections are depicted (thalamic interneurons and neocortical neurons other than those in layers 4 and 5/6 have been omitted for clarity). Plus sign and minus sign indicate excitatory and inhibitory synapses, respectively. b Top EEG recordings during light and deep NREM sleep in freely moving cats showing the slow (<1 Hz) rhythm. Individual slow-wave complexes are enlarged to the right as indicated. Bottom recordings from the same animal during light and deep anaesthesia when the slow rhythm is also present (see also Fig. 2). Again, individual slow-wave complexes are enlarged to the right as indicated. Note how in both conditions the frequency of the slow rhythm increases with the deepening of natural sleep or anaesthesia. a adapted from [18]; b adapted from [1]
In light of the significance of the slow (<1 Hz) rhythm as highlighted above, it is essential to understand how this entity is generated at the neuronal level in relevant brain structures such as the neocortex and thalamus. In the rat and cat neocortex, it is now well established that the slow rhythm is correlated with alternating UP and DOWN membrane potential states [13, 48, 60, 61], with UP states consisting of periods of intense synaptic activity, depolarisation and action potential firing and DOWN states comprising neuronal hyperpolarisation, a lack of action potential output and network quiescence (Fig. 2a, b). This structured form of spontaneous activity is largely considered to represent the fundamental dynamic capacity of the neocortex in its most unconstrained state [44], thereby providing crucial insights into the basic nature of cortical circuit operations [25]. In terms of basic mechanisms, UP and DOWN states in the neocortex arise almost exclusively as an emergent property of the densely interconnected underlying network, with UP state initiation appearing to most often occur in layer 5 [55] and be the result of either a subset of spontaneously firing neurons [11, 37], the spontaneous and coincident release of glutamate onto different sites of large pyramidal cells [2, 9] or both [28]. UP states are subsequently sustained by intense network activity and thought to be eventually terminated by the ‘build up’ of activity-dependent (e.g. Ca2+-, Na+- and ATP-dependent) K+ channel conductances [2, 11, 22, 37, 55].
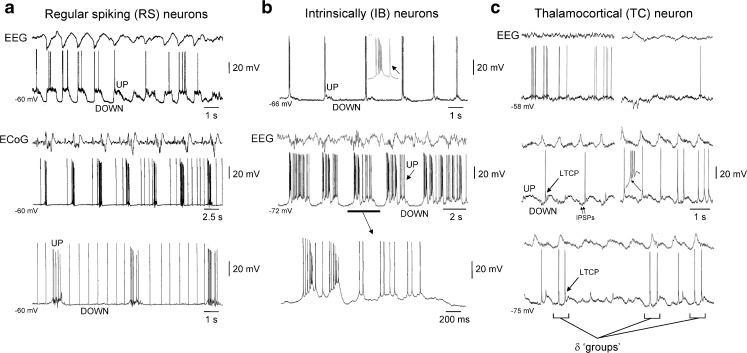
Fig. 2.
Reflection of the slow (<1 Hz) rhythm in neocortical and TC neurons in anaesthetised cats. a Top EEG and intracellular recording of an RS neuron in the cat neocortex during the slow rhythm. Note the presence of prominent UP and DOWN states, as indicated. Middle electrocorticogram (ECoG) recording and intracellular recording of a different neocortical RS neuron during the development of the slow rhythm (neuron recorded at a depth of 0.6 mm). In this neuron, DOWN states are characterised by low-frequency spontaneous firing. Bottom additional section of recording from the same neuron shown immediately above when the slow rhythm is fully developed. b Top intracellular recording of an IB neuron in the cat neocortex during the slow rhythm (recorded at a depth of 1.1 mm). Note how in this neuron, UP states start with a high-frequency burst of action potentials that is followed by little or no additional firing. Middle combined EEG and intracellular recording of a different IB neuron where UP states are initiated by a high-frequency burst that is followed by considerable additional firing (neuron recorded at a depth of 1.3 mm). The underlined section is enlarged below as indicated. c Top EEG and intracellular recording from a TC neuron in the ventrolateral nucleus during a period of low-amplitude EEG activity. Middle as EEG amplitude increases and the slow rhythm emerges, the TC neuron exhibits UP/DOWN states. Note how each UP state commences with an LTCP that often leads to a burst of action potentials and how the waveform of UP/DOWN states is well conserved from cycle to cycle. Note also the presence of rhythmic inhibitory postsynaptic potentials (IPSPs) during the DOWN state. Bottom upon further hyperpolarisation of the TC neuron, additional LTCPs are sometimes present during the DOWN state which are referred to as ‘grouped’ δ oscillations [15, 31, 33, 62, 73]. Top panel in a adapted from [1]. Middle and lower panels in a and all panels in b adapted from [61]. All panels in c adapted from [14]
An additional brain region that plays a central role in the generation of the slow (<1 Hz) rhythm is the thalamus [13, 18, 62]. In this structure, both glutamatergic, thalamocortical (TC) relay neurons and locally projecting GABAergic neurons in the nucleus reticularis thalami (NRT) exhibit pronounced UP and DOWN states that occur in synchrony with those in the neocortex [13–15, 18, 24, 62]. A striking feature of UP/DOWN states in both types of thalamic neurons, and a distinguishing factor from those in the neocortex, is their stereotypical nature from cycle to cycle [13, 62]. For example, in both types of cells, UP states invariably commence with a T-type Ca2+ channel-dependent burst of action potentials (i.e. low-threshold Ca2+ potential [LTCP]), whereas the duration and distinctive waveform of DOWN states are extremely well conserved (Fig. 2c) [13, 62]. Studies in reduced slice preparations have revealed that, in stark contrast to neocortical neurons where UP/DOWN states are primarily generated by synaptic mechanisms, the stereotypical nature of UP and DOWN states in thalamic neurons is due to powerful intrinsic mechanisms (Fig. 3a, b, lower panels) [5, 20, 21, 31, 33, 72, 73]. Such an intrinsic basis endows rhythmic UP/DOWN states in individual thalamic neurons with a robustness that may be crucial in generating a reliable timing signal for triggering and/or reinforcing UP states in the neocortex [18, 24].
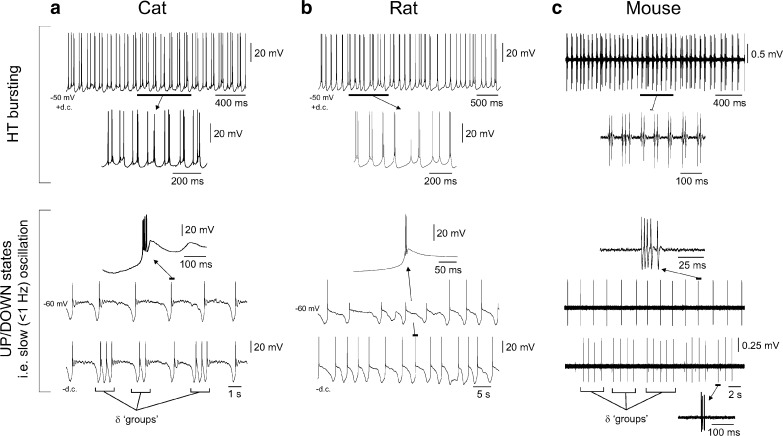
Fig. 3.
Activity of TC neurons in thalamic slices from the cat, rat and mouse. a Top intracellular recording of a TC neuron from a slice preparation of the cat LGN (i.e. visual thalamus) in the presence of the mGluR agonist, trans-ACPD, and following the injection of steady depolarising current reveals HT bursting at ~8 Hz. The underlined section is expanded below as indicated. Bottom a different LGN TC neuron, also recorded in a slice preparation in the presence of trans-ACPD but in the absence of steady current, shows spontaneous UP/DOWN states (upper trace). Note how UP states commence with an LTCP-mediated burst and how the waveform of UP/DOWN states is conserved from cycle to cycle. A single LTCP-mediated burst is enlarged above as indicated. As occurs in vivo, when this TC neuron is hyperpolarised with steady current, additional LTCPs are generated during the DOWN state, leading to ‘grouped’ δ oscillations (lower trace; cf. Fig. 2c, bottom). b Top as in a except the recording is from a slice preparation of the rat ventrobasal thalamus. Bottom spontaneous UP/DOWN states recorded from the same neuron shown above. The lower trace was obtained following the injection of a small amount of steady hyperpolarising current. c Top: as in a and b except extracellular recording of a TC neuron in a slice preparation of the mouse LGN in the presence of a high concentration of trans-ACPD (200 μM). Bottom upper and lower traces show extracellular recordings of rhythmic LTCP-mediated bursts (i.e. corresponding to UP states) from two distinct neurons at a more moderate concentration of trans-ACPD (100 μM). Note the presence of ‘grouped’ δ activity in the lower trace. Top and bottom panels in a and bottom panel in c adapted from [73]
In anaesthetised cats, UP/DOWN states in TC neurons are reliant on intact corticothalamic projections [66]. Because such projections are known to activate the group I metabotropic glutamate receptor (mGluR) [47], mGluR1a, on TC neurons, we earlier hypothesised that such activation is essential for the expression of UP/DOWN states in these cells [31]. This was later confirmed in studies in thalamic slices where UP/DOWN states only became apparent in TC neurons following either the pharmacological or synaptic activation of mGluR1a [31], leading to the view that TC (and NRT) neurons behave as conditional oscillators during the slow rhythm [18]. Interestingly, when TC neurons in this condition are either tonically depolarised in the presence of a small amount of mGluR1a activation or when mGluR1a is more intensely activated, UP/DOWN states can be replaced by a novel form of rhythmic burst firing, termed high-threshold (HT) bursting (Fig. 3a–c, upper panels) [31, 33]. Whilst there is substantial evidence from extracellular recordings from the cat lateral geniculate nucleus (LGN) that HT bursting also occurs in vivo [33, 42], this phenomenon has yet to be unequivocally observed with intracellular recordings in the intact brain.
Up to now, the majority of cellular level investigations into the neuronal basis of the slow (<1 Hz) rhythm have largely been restricted to cats (e.g. [13, 60–62]) and rats (e.g. [35, 48, 54, 56]), with a limited number of key studies performed in humans using depth recordings (e.g. [8, 38, 52]). Whilst in the main, these studies have uncovered a consistent picture with regard to the correlation between slow waves and UP/DOWN states in specific neuronal groups, the applicability of these findings to other mammals is largely assumed on the basis that sleep occurs ubiquitously across different species [57]. Whilst this assumption may well turn out to be justified, one species where it is becoming increasingly important to definitively establish that the mechanisms of slow rhythm generation are conserved is the mouse. Given the ever-increasing application of genetic manipulation in these animals as well their growing use in basic sleep research, it is paramount to show that the basic features of UP/DOWN states in this species are equivalent to those observed in higher mammals. To this end, we have now obtained in vivo intracellular recordings of UP and DOWN states in neocortical and thalamic neurons in anaesthetised mice. In doing so, we demonstrate that neurons from both regions exhibit UP/DOWN state properties that are broadly equivalent to those previously described in other mammals [18]. We also show that tonic depolarisation of TC neurons that are exhibiting UP/DOWN states can reveal rhythmic HT bursting at ~5 Hz, as predicted by in vitro studies [31, 33]. The importance of these findings is discussed within the context of slow (<1 Hz) rhythm-related memory processes and basic cortical and TC circuit operations.
Methods
All procedures were carried out in accordance with local ethical committee guidelines and the UK Animals (Scientific Procedure) Act, 1986. All efforts were made to minimise the suffering and number of animals used in each experiment.
Surgical procedures and in vivo electrophysiology
Adult mice (C57BL/6, P30-60, Harlan, UK) were anaesthetised with urethane (0.8 g/kg) and supplemented with ketamine and xylazine (20 and 2 mg/kg, respectively) to maintain stable anaesthesia as required (note: we did not observe any notable differences in the appearance of the slow oscillation across the age range of animals used). Body temperature was maintained at constant levels using a heating plate (Supertech Ltd., Pecs, Hungary). After exposing the skull, a small hole was drilled above the target area at stereotaxic coordinates (0.5 mm anterior of bregma; 3.5 mm lateral to midline) [53]. The dura was then punctured and a glass electrode lowered into the cortex. Stainless steel screws (0.8 mm diameter) were placed into the skull over the cerebellum and the frontal cortex, which served as a ground and reference, respectively, and were used to monitor the EEG. Extracellular single units were recorded using glass pipettes filled with 0.5 M NaCl (impedance, 5–15 MΩ) connected to a Neurolog 104 differential amplifier (Digitimer Ltd., Welwyn Garden City, UK). Intracellular recordings were obtained using glass micropipettes filled with 1 M potassium acetate (impedance, 60–80 MΩ) connected to an Axoclamp-700B amplifier (Molecular Devices, Sunnyvale CA, USA). All extracellular and intracellular recordings were obtained from a depth corresponding to layer 5 (0.6–0.8 mm). Data were digitally acquired using a Digidata 1440A interface (Molecular Devices) and stored on a PC for offline analysis. At the end of the experiment, animals were given a lethal dose of urethane.
Data analysis
Average subthreshold UP states were calculated for a period of at least 1,000 ms following the detected start of an UP state and were constructed from at least 10 consecutive UP states. Average firing rate plots were computed from at least 20 individual UP states. For a given neuron, the voltage difference between UP and DOWN states was taken as the difference between the two peaks in the membrane potential histogram. For examining the frequency components of subthreshold UP states, we averaged the power spectra for at least 20 consecutive UP states. Unless otherwise stated, all quantitative data are expressed as the mean±SEM and statistical significance was assessed with Student’s t test.
Results
Properties of the slow rhythm in mice under anaesthesia
Under anaesthesia, the EEG of mice exhibited a distinctive slow rhythm as described for other species (Fig. 4a) [18]. The mean frequency of this rhythm was 0.88 ± 0.07 Hz (range, 0.4–2.1 Hz; n = 40). Interestingly, in 68% (n = 27 of 40) of our recordings, this slow rhythm was modulated by an additional slower oscillation occurring at 0.13 ± 0.02 Hz (range, 0.05–0.3 Hz; n = 27; Fig. 4a). As observed previously in ferrets [26], cats [63, 64] and humans [38], in local field potential (LFP) recordings obtained with glass electrodes at a cortical depth corresponding to layer 5 (0.6–0.8 mm), the negative component of the slow rhythm, which corresponds to neuronal UP states, was often accompanied by prominent oscillations in the γ frequency (~20–80 Hz) band (88%; n = 35 of 40 recordings; Fig. 4b). The mean peak frequency of γ oscillations was 67.5 ± 2.4 Hz (range, 35–98 Hz; n = 35 recordings).
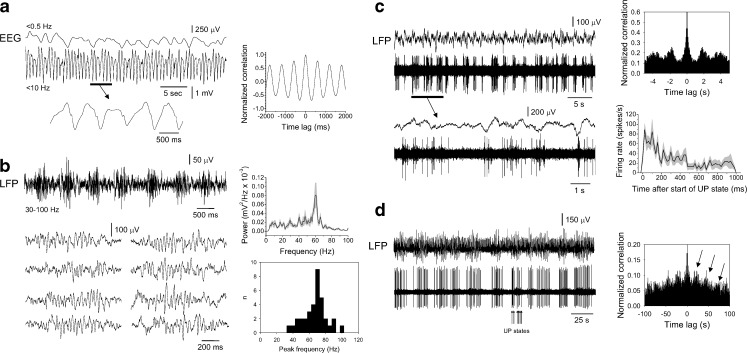
Fig. 4.
Properties of the slow (<1 Hz) rhythm in the anaesthetised mouse. a EEG recording from the anaesthetised mouse that has been low-pass filtered at <0.5 Hz (top) and <10 Hz (bottom) shows rhythmic slow waves at ~1.7 Hz (see bottom trace) that are modulated by a slower oscillation at ~0.3 Hz (see top trace). The corresponding autocorrelogram for the bottom trace is shown to the right. b LFP recording from layer 5 of the mouse neocortex filtered between 30 and 100 Hz, showing that the slow rhythm is associated with recurrent epochs of γ oscillations. The traces below show eight individual γ oscillation epochs. The plot to the top right shows the average power spectra for 20 such epochs and reveals a clear peak in power at ~60 Hz (in this and all subsequent plots the grey shading gives the standard error). The histogram below shows the distribution of peak γ frequencies for 35 different recordings. c Simultaneously acquired LFP (top) and multiunit (bottom) recordings from the mouse neocortex during the slow rhythm. The underlined section is enlarged below as indicated. The plot to the top right shows the corresponding autocorrelogram which reveals clear periodicity at ~0.5 Hz. The plot below shows the average firing rate across 20 UP states. d Simultaneously acquired LFP (top) and unit (bottom) recordings reveal that neocortical neuronal activity is also modulated by a slower rhythm, in this case with a periodicity of ~20 s (as indicated by the arrows in the corresponding autocorrelogram shown to the right)
Recordings of extracellular multiunit activity (MUA) obtained in combination with depth LFP recordings revealed that, as expected, the negative component of the LFP (i.e. UP state) was associated with neuronal firing (mean frequency, 45.6 ± 3.3 Hz; duration of epochs, 552 ± 65 ms; n = 200 epochs from 10 recordings), whilst the positive phase (i.e. DOWN state) was mostly related to neuronal silence (duration of silent epochs, 779 ± 85 ms; n = 200 epochs from 10 recordings; Fig. 4c, d). Examination of the average firing during UP states revealed that action potential output was at a maximum near the start of the UP state, decreased to a stable level from ~200 to 400 ms after UP state commencement and then gradually declined (Fig. 4c, right). The slower modulation of the slow rhythm as noted in the EEG was also apparent in MUA recordings (Fig. 4d).
Extracellular single-unit recordings of neocortical neurons during the slow rhythm reveal two main types of neuronal activity
Extracellular single-unit recordings from layer 5 of the neocortex during the slow rhythm revealed two separate groups of cells that exhibited distinct types of firing during UP states (Fig. 5). In the smaller of these two groups (n = 8 of 31; 26%), UP states were mainly characterised by seemingly sporadic action potential firing that did not show a consistent pattern across consecutive UP states (Fig. 5a, b), with spike frequency being essentially constant for the first ~200–300 ms before gradually declining (mean frequency during first 200 ms, 20.6 ± 2.2 Hz; n = 100 UP states from 5 recordings; Fig. 5a, b, bottom right plots). Interestingly, in this group of cells, UP states sometimes emerged from a baseline of low-frequency (~4–6 Hz) regular firing (Fig. 5b), as has previously been described in the intact brain of both cats [61] and humans [52, 70], as well as in brain slices from the ferret neocortex [12, 55]. In the other group of neurons (n = 23 of 31; 74%), UP states nearly always commenced with a high-frequency burst of spikes (spikes per burst, 3.4 ± 0.2; peak frequency, 86.4 ± 5.5 Hz; n = 200 bursts from 10 neurons) that was followed by varying degrees of additional firing that ranged from a few isolated (e.g. one to four) spikes (Fig. 5c) to further prominent firing (Fig. 5d). On average, the overall action potential output during UP states in these cells was highest at the start of UP states, diminished to a stable plateau that lasted from around 50 to 250 ms and then gradually decreased thereafter (Fig. 5c, d, bottom right plots). In these cells, low-frequency firing was never observed between UP states.
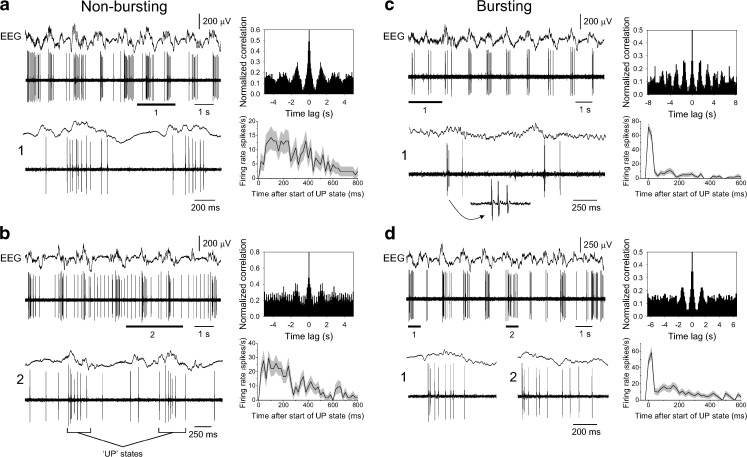
Fig. 5.
Single-unit activity of layer 5 neocortical neurons during the slow rhythm. a Combined EEG and unit recording of a layer 5 neocortical neuron that exhibits UP states consisting of mainly single-spike activity. The corresponding autocorrelogram is shown to the top right, whereas the plot below shows the average firing rate across 20 UP states. Note that the firing rate is more or less constant for the first ~200 ms of UP states before gradually declining. b As in a except UP states emerge from a baseline of low-frequency firing (cf. Fig. 2a, bottom). Again, the plot of average firing rate across 20 UP states given at the bottom right shows that firing rate is essentially stable for the first ~200 ms before gradually declining. c, d Simultaneous EEG and unit recordings of layer 5 neocortical neurons that exhibit UP states which consistently commence with a high-frequency burst of action potentials. Underlined sections are enlarged as indicated. In c, these bursts are followed by very little additional firing, whereas in d, bursts are followed by substantial additional action potential output. For both c and d, the plots to the top right show the corresponding autocorrelogram, whereas the plots below show the average firing rate for 20 UP states. Note how firing rate is transiently very high at UP state commencement due to the presence of bursts but is then essentially constant for the following ~200 ms at a lower rate
Intracellular recordings of neocortical neurons during the slow rhythm
To investigate the nature and diversity of UP states in neocortical neurons in more detail, we obtained intracellular recordings, again at a cortical depth corresponding to layer 5 (0.6–0.8 mm). In full agreement with extracellular recordings, we observed two basic types of UP states. In 11 of 27 neurons (41%), UP states consisted of sporadic action potential firing (mean frequency during first 200 ms, 25.3 ± 1.5 Hz; n = 80 UP states from 4 neurons) that did not show a consistent pattern between consecutive UP states (Fig. 6a, b). These cells responded to the injection of brief (600–800 ms) positive current pulses with regular firing (not illustrated) and were, therefore, classified as regular spiking (RS) neurons [58]. The difference in membrane potential between UP and DOWN states in RS cells was 14.7 ± 0.4 mV (n = 10), whereas the average durations of UP and DOWN states were 390 ± 11 ms (n = 80 UP states from 4 recordings) and 575 ± 35 ms (n = 80 DOWN states from 4 recordings), respectively. Steady hyperpolarisation of these neurons to prevent action potential firing during UP states revealed that these events were composed of prominent barrages of synaptic activity (Fig. 6b, bottom). This activity exhibited a mean peak frequency of 36.3 ± 3.4 Hz (range, 22.1–61.8 Hz; n = 11 neurons; Fig. 6b, bottom right). Notably, in RS cells, average subthreshold UP states were of a similar duration and form to ‘full-blown’ UP states (i.e. those involving action potential firing), exhibiting an almost ‘step’-like profile (Fig. 6c, top and middle panels). In addition to conventional synaptic activity, a subset of RS neurons (n = 3 of 11) also exhibited a distinct type of subthreshold event that was reminiscent of so-called spikelets [6, 32, 33, 41, 43]. These events were remarkably well conserved within any given cell and had considerably faster rise and decay times (time to peak, 1.1 ± 0.1 ms; τ decay, 3.0 ± 0.1 ms; n = 30 events from 3 neurons) than those that would be expected for conventional excitatory postsynaptic potentials (EPSPs; Fig. 6d).
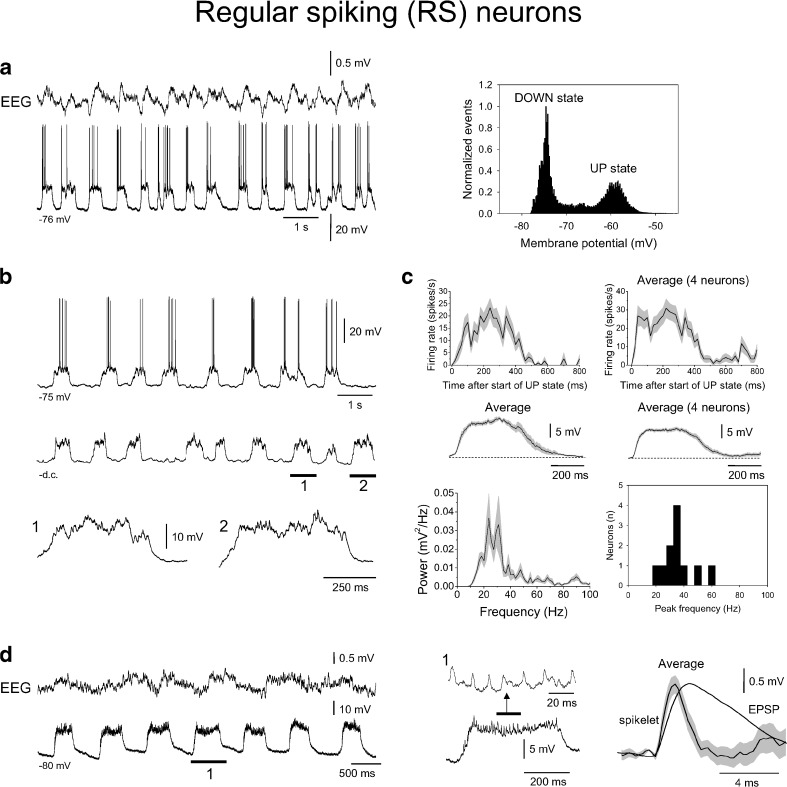
Fig. 6.
Intracellular recordings of layer 5 RS neurons during the slow rhythm. a Combined EEG and intracellular recording of a layer 5 RS neuron during the slow rhythm, showing clear UP and DOWN states (cf. Fig. 2a, top). The plot to the right shows the related membrane potential distribution which exhibits a bimodal form with two clear peaks corresponding to UP and DOWN states. b Intracellular recording of a different RS neuron from layer 5 during the slow rhythm which also shows prominent UP and DOWN states. The trace below shows the activity of the same neuron after injection of steady hyperpolarising current to prevent action potential firing. The underlined sections are enlarged below as indicated and show the presence of intense synaptic activity during UP states. c Top left average firing rate for the neuron shown in b for 20 UP states. Top right average UP state firing for four different RS neurons. Note how for these neurons firing is essentially stable for ~400 ms (i.e. the duration of an UP state) before declining. Middle row average waveforms for subthreshold UP states for the neuron shown in b (left) and for four different RS neurons (right). Note how these waveforms qualitatively match the time-course of the average firing rates shown above. Bottom left average power spectrum for 20 subthreshold UP states from the neuron depicted in b shows a peak around 20–30 Hz. Bottom right histogram showing the mean peak frequencies for subthreshold activity during UP states from 11 RS neurons. d Combined EEG and intracellular recording of a layer 5 RS neuron in the presence of steady hyperpolarising current to prevent action potential firing. The underlined section is enlarged to the immediate right and shows the presence of spikelets during UP states. The traces to the far right show an average spikelet overlaid by an average EPSP (constructed from a different neocortical RS neuron). Note the considerably faster rise and decay times for spikelets compared to EPSPs
In the remainder of neurons (n = 16 of 27; 59%), UP states commenced with a prominent, high-frequency burst of action potentials (spikes per burst, 4.4 ± 0.2; peak frequency, 129.6 ± 13.6 Hz; n = 100 bursts from 5 cells; Fig. 7). As with extracellular recordings, ensuing activity during UP states in these neurons ranged from a few isolated action potentials (Fig. 7a) to considerable additional firing (Fig. 7b). These cells responded to the injection of brief (600–800 ms) positive current pulses with bursts of action potentials (not illustrated) and were, therefore, classified as intrinsically bursting (IB) neurons [58]. The difference in membrane potential between UP and DOWN states in IB cells was 12.2 ± 0.4 mV (n = 100 UP states from 5 cells), whereas the average durations of UP and DOWN states were 343 ± 23 ms (n = 100 UP states from 5 neurons) and 501 ± 20 s (n = 100 DOWN states from 5 neurons), respectively. As observed with extracellular recording, action potential output was on average greatest at the beginning of UP states and then decreased at a variable rate as the UP state progressed (Fig. 8a, b). As with RS cells, steady hyperpolarisation of IB neurons revealed that UP states were also underpinned by barrages of synaptic activity (Fig. 8a, b, d), the time-course and form of which was markedly different to that observed in RS cells but which largely mirrored the action potential output generated by these cells during ‘full-blown’ UP states (Fig. 8a–c). The mean peak frequency of synaptic activity in these cells was 27.9 ± 2.3 Hz (n = 16; Fig. 8d, e).
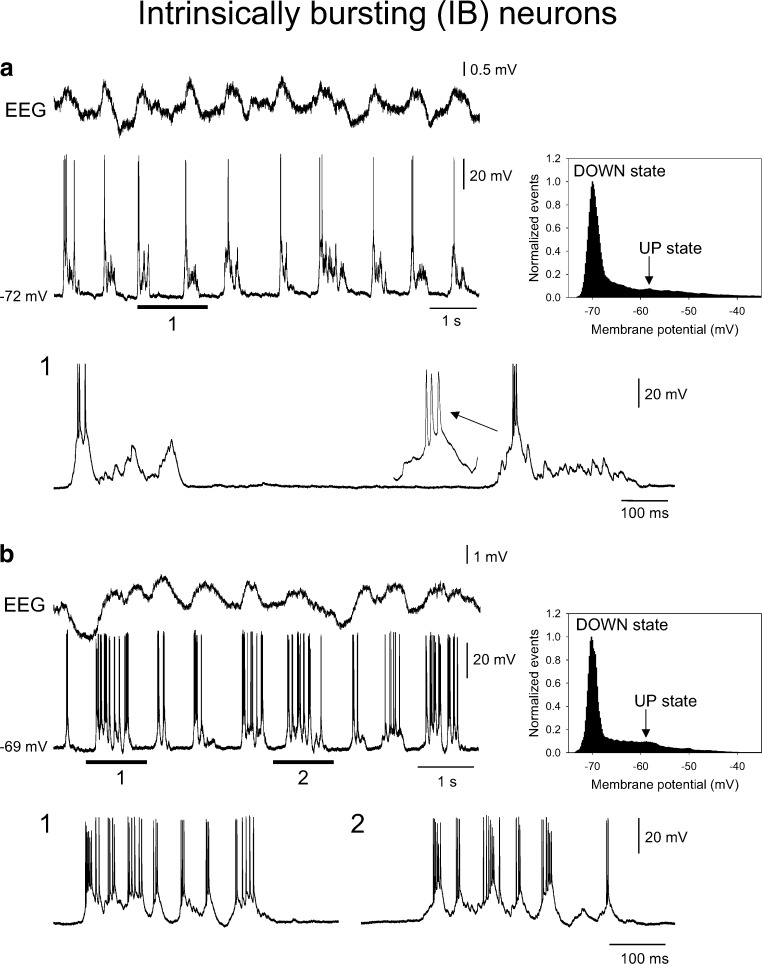
Fig. 7.
Intracellular recordings of layer 5 IB neurons during the slow rhythm. a Combined EEG and intracellular recording of a layer 5 IB neuron during the slow rhythm. The underlined section is enlarged below as indicated. In this neuron, UP states commence with a high-frequency burst that is followed by little or no additional firing (cf. Fig. 2b, top). The plot to the right shows the corresponding membrane potential distribution which exhibits a skewed rather than clear bimodal form. b As in a except that in this case UP states commence with a high-frequency burst that is followed a considerable amount of additional firing (cf. Fig. 2b, bottom). Note: in both a and b, the arrow indicates the UP state values used to calculate the voltage difference between UP and DOWN states in the respective cells
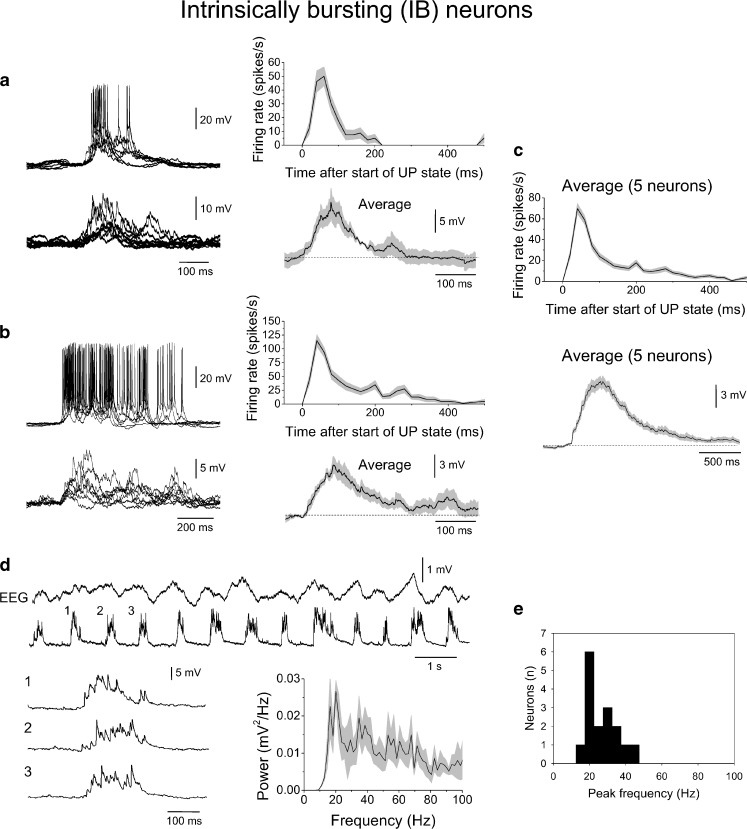
Fig. 8.
Subthreshold activity of layer 5 IB neurons during the slow rhythm. a Left overlay of suprathreshold (top) and subthreshold (bottom) UP states from the same neuron depicted in Fig. 7a. The corresponding average firing rate and average subthreshold UP state waveform are shown to the right. Note the distinct form compared to RS cells (i.e. Fig. 6c) but how there is good qualitative correspondence between firing rate and subthreshold activity. b As in a except for the neuron depicted in Fig. 7b. c Average firing rate (top) and average subthreshold waveform (bottom) for five different IB neurons. d Combined EEG and intracellular recording of a different layer 5 IB neuron in the presence of steady hyperpolarising current to prevent action potential firing. The numbered sections are enlarged below as indicated. The plot to the bottom right gives the average power spectrum for 20 subthreshold UP states which shows a peak at around 20 Hz but also substantial power across the 20–100 Hz band. e Histogram showing the mean peak frequencies for subthreshold activity during UP states from 16 IB neurons
Intracellular recordings of thalamocortical neurons during the slow rhythm
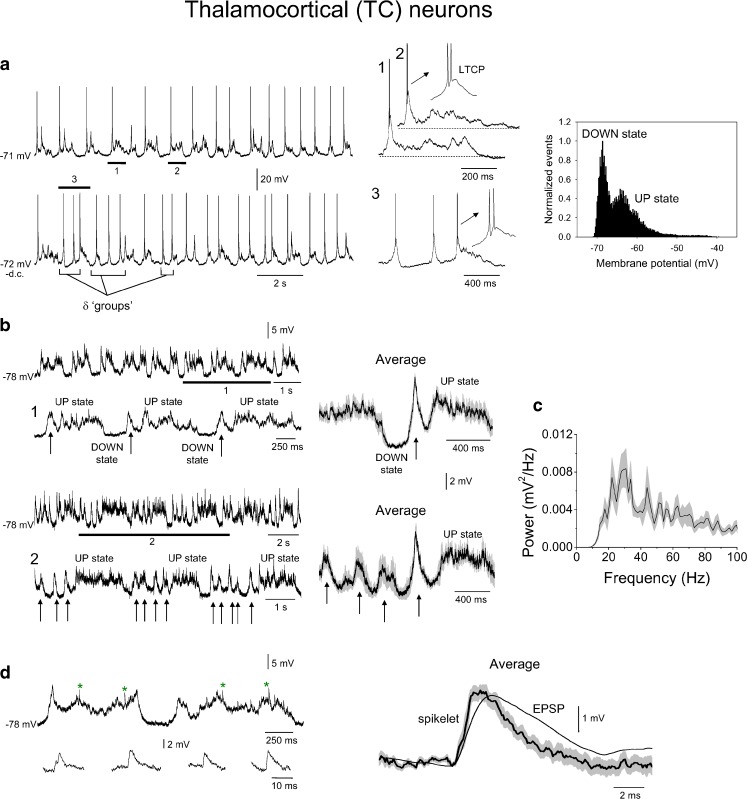
Previous intracellular recordings obtained from TC neurons in the cat during the slow (<1 Hz) rhythm have shown that UP states are invariably initiated by an LTCP-mediated burst of action potentials [13, 62] that is followed by a depolarised membrane potential but (usually) not by additional firing. An identical pattern of activity can also be observed in recordings from TC neurons in thalamic slice preparations and has been shown to depend on intrinsic mechanisms that mainly involve the interaction of a T-type Ca2+ current and a K+ leak current [18, 20, 21, 31, 72]. Because these ionic currents are a conserved property of TC neurons across several species [16, 19, 27, 36, 40, 49–51, 65], it is likely that a similar form of TC neuron UP state to that observed in cats will also be present in mice. Indeed, extracellular recordings from TC neurons in slices of the mouse thalamus strongly support this assertion [73]. To fully investigate this issue, we obtained intracellular recordings from TC neurons of the ventral posterolateral nucleus in anaesthetised mice (n = 4). Similar to cats, UP states in these cells always commenced with an LTCP (Fig. 9a; see also Fig. 10a, b, bottom panels). Following these LTCPs, the membrane potential of TC neurons remained depolarised for a brief period but additional firing was never observed. Thus, as also largely observed in mouse thalamic slices [73], LTCP-mediated bursts in these neurons were the only type of action potential output that occurred during the slow rhythm. Overall, the average difference in membrane potential between UP and DOWN states in TC neurons was 6.3 ± 0.3 mV (n = 4), whereas the average durations of UP and DOWN states were 406 ± 36 ms (n = 80 UP states from 4 recordings) and 482 ± 43 ms (n = 80 DOWN states from 4 recording), respectively.
Fig. 9.
Intracellular activity of TC neurons during the slow rhythm. a Top left intracellular recording of a TC neuron during the slow rhythm. The underlined sections are expanded to the immediate right as indicated and show that UP states commence with an LTCP-mediated burst that is followed by synaptic activity but not additional firing (cf. Fig. 2c, middle). Bottom left same cell but following the injection of a small amount of steady hyperpolarising current. Note how additional LTCPs are now present during the DOWN state leading to so-called ‘grouped’ δ oscillations (cf. Fig. 2c, bottom). The marked section is enlarged to the immediate right. Far right corresponding membrane potential histogram has a bimodal nature, although this is not as pronounced as for RS cells. b Top left activity of the neuron shown in a following the injection of sufficient steady hyperpolarising current to prevent both action potential firing and LTCP generation. The underlined section is expanded below as indicated and shows how subthreshold UP states are initiated by a stereotypical depolarising event (indicated by arrows) that is followed by a barrage of synaptic activity. The trace on the immediate right shows the corresponding average subthreshold activity triggered by the peak of the stereotypical depolarising events and reveals a form which mirrors ‘full-blown’ UP/DOWN states in these neurons (i.e. panel a, top). Bottom left subthreshold activity of the same neuron recorded under the same conditions as above but a later point in the recording. Note how stereotypical depolarising events now occur rhythmically during the DOWN state (indicated by arrows). Again, the trace on the immediate right shows the corresponding average subthreshold activity triggered by the peak of the stereotypical depolarising event which immediately precedes an UP state and reveals a form which mirrors full-blown UP/DOWN states in these neurons when ‘grouped’ δ activity is present (i.e. a, bottom). c Average power spectrum for 20 subthreshold UP states from the cell shown in a and b which not only shows a peak at around 20 Hz but also substantial power across the 20–100 Hz band. d An enlargement of two subthreshold UP states, again from the neuron in a–c, reveals the presence of occasional fast events which resemble spikelets (indicated by asterisks and enlarged below as indicated). The traces to the far right show an average spikelet overlaid by an average EPSP. Note the considerably faster rise and decay times for spikelets compared to EPSPs
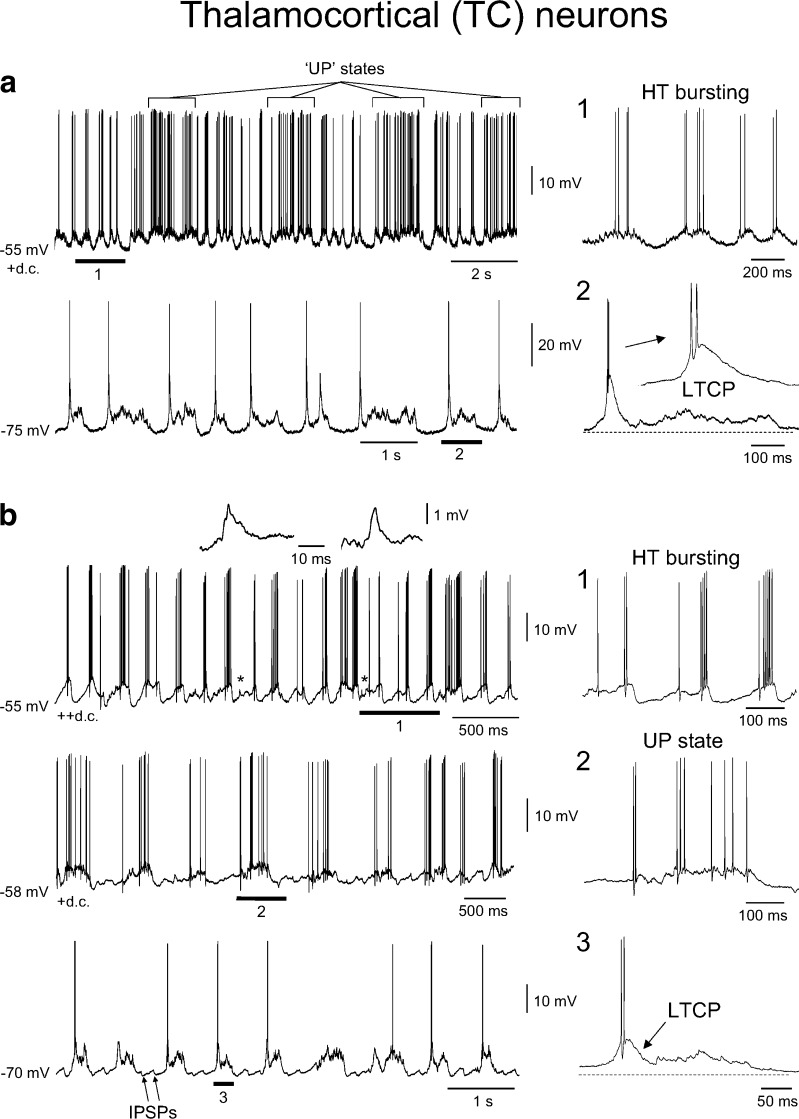
Fig. 10.
Presence of HT bursting in intracellular recordings of TC neurons in vivo. a Top intracellular activity of a TC neuron during the slow rhythm following injection of steady depolarising current. Note how UP states are still apparent as occasional increases in firing rate (as indicated) but that in between these UP states the neuron exhibits rhythmic HT bursting at ~5 Hz. The underlined section is expanded to the right as indicated and more clearly shows the nature of this HT bursting (cf. Fig. 3b, top). Bottom in the absence of steady depolarising current, the same neuron shows rhythmic UP/DOWN states of the same type as those shown in Fig. 9a, i.e. UP states which commence with an LTCP-mediated burst and are followed by a barrage of synaptic activity but no further firing. Again, the underlined section is expanded to the immediate right. b Top intracellular recording of a different TC neuron during the slow rhythm which also shows HT bursting when subjected to steady depolarising current. However, note how in this cell HT bursting completely overwhelms UP/DOWN state generation. The asterisks indicate spikelets which are enlarged above. The underlined section is enlarged to the immediate right and shows more clearly the nature of HT bursting in this neuron (cf. Fig. 3a, top). Middle following the injection of a smaller amount of steady current, UP states become apparent. However, unlike UP states recorded in the absence of steady current, these consist solely of single-spike activity which occurs for the full duration of the UP state (cf. Fig. 2c, top). The underlined section is enlarged to the immediate right as indicated. Bottom in the absence of any steady depolarising current, the same neuron shows rhythmic UP/DOWN states of the same type as those shown in Fig. 9a and the lower part of a above. In this neuron, we also observed IPSPs during the DOWN state (cf. Fig. 2c, middle). Again, the underlined section is expanded to the immediate right
Interestingly, when TC neurons were subjected to a small amount of steady hyperpolarising current, additional LTCPs were often observed during the DOWN state in a manner reminiscent of that previously shown for cat TC neurons, recorded both in vivo [15, 62] and in vitro [31, 33, 73], where it has been referred to as a ‘grouped’ δ oscillation (Fig. 9a, lower traces; cf Figs. 2c and 3a–c, bottom panels). Further steady hyperpolarisation of TC neurons to prevent both action potential firing and LTCP generation revealed a striking pattern of subthreshold activity consisting of barrages of synaptic activity (mean peak frequency, 31.4 ± 2.3 Hz; n = 3) that were preceded by a stereotypical depolarising event that had the appearance of a ‘subthreshold LTCP’ (indicated by arrows in Fig. 9b). Interestingly, at times, these events occurred rhythmically in groups during DOWN states in a manner that was essentially identical to the grouping of ‘full-blown’ LTCPs described above (i.e. Fig. 9a, lower traces). These events appeared to be non-synaptic in nature because their amplitude did not markedly change when cells were depolarised or hyperpolarised (not illustrated). As with neocortical RS cells, 2 out of the 4 recorded TC neurons also exhibited spikelets (time to peak, 1.2 ± 0.4 ms; τ decay, 2.2 ± 0.2 ms; n = 20 events from 2 neurons; Fig. 9d).
Lastly, when TC neurons exhibiting UP/DOWN states were subjected to sufficient steady depolarising current, we observed HT bursting at 5.3 ± 0.4 Hz (n = 3). This HT bursting could occur simultaneously with UP/DOWN states (Fig. 10a, top) or could overwhelm UP/DOWN state generation to fully define the output of these cells (Fig. 10b, top). In all senses, the HT bursting observed in vivo was indistinguishable from that previously noted in vitro (i.e. Fig. 3a–c, upper panels) [30, 31, 33, 34, 41–43].
Discussion
The main findings of this study are: (1) under anaesthesia, the mouse EEG exhibits a prominent slow rhythm as has been observed in other mammalian species [18]; (2) this slow rhythm can be modulated by an additional slower oscillation at ~0.05–0.3 Hz; (3) the slow rhythm is accompanied by epochs of γ oscillations during UP states; (4) extracellular and intracellular recordings of neocortical neurons in layer 5 reveal two types of cells which exhibit distinct patterns of firing during UP states, namely, RS and IB neurons; (5) in RS cells, action potentials during UP states are seemingly sporadic, synaptic activity occurs in the γ frequency range and fast events resembling so-called spikelets are sometimes apparent; (6) in IB cells, UP states commence with a high-frequency burst that is followed by additional firing, synaptic activity occurs at a lower peak frequency than in RS cells and spikelets are not evident; and (7) intracellular recordings of TC neurons show that UP states in these cells always commence with an LTCP and associated burst of action potentials, which is not followed by additional firing, exhibit subthreshold events which resemble LTCPs and also display spikelets. Thus, neocortical and TC neurons in the mouse brain exhibit UP/DOWN states that possess broadly similar properties to those observed in other species [18].
Similarities between UP and DOWN states in different species
In the main, the results of the current study conducted in mice are in good agreement with those obtained from investigations in other mammals. Firstly, in all previous studies, the slow rhythm that is observed under anaesthesia is a highly rhythmic entity with a frequency close to, or slightly below, 1 Hz [35, 54, 56, 60, 61]. Although the mean frequency in mice is slightly greater than that noted for rats [35, 54, 56], and especially cats [60, 61], it clearly represents the same phenomenon. This is most evident from examining intracellular recordings of neocortical and TC neurons where the properties of UP and DOWN states are extremely similar to those seen previously in higher mammals [60, 61]. In particular, the sculpting of neocortical UP states by recurrent barrages of synaptic activity, the presence of spikelets during both neocortical and thalamic UP states, the commencement of UP states in TC neurons by LTCP-mediated bursts and the presence of so-called ‘grouped’ δ activity in TC neurons are also consistent findings in other species [15, 31, 33, 62, 73]. Secondly, during UP states in mice, we observed prominent γ frequency network oscillations as also observed during UP states in the ferret [12, 26], cat [63, 64] and human [38] neocortex. Thirdly, the observation that some UP states in the neocortex emerge from a baseline of low-frequency firing again fits well with work carried out in neocortical slices from the ferret [12, 55] and in the intact brain of cats [61] and humans [52, 70].
In addition to the above similarities, we also noted that the slow rhythm in mice was modulated by an additional slower oscillation at ~0.04–0.3 Hz. Whilst a cyclic modulation of brain state with a periodicity of ~10 min has previously been shown to occur under urethane anaesthesia [10], to our knowledge, a rhythmic modulation of slow waves on the timescale of several seconds has not been described. A further novel and striking finding in this study was that, following tonic depolarisation, UP/DOWN states in TC neurons could be replaced by rhythmic HT bursting at ~5 Hz. This is entirely consistent with findings in thalamic slices [31, 33] and shows unequivocally that HT bursting occurs in vivo (see also [33, 42]).
Potential insights into slow rhythm mechanisms
The current study provides several potential insights into the mechanisms of slow rhythm generation. Most notably, the finding that UP states in TC neurons always start with a robust LTCP-mediated burst supports our previous suggestion that this event may be a key trigger for initiating activity in the neocortex [18, 24]. Indeed, evidence from cats clearly shows that, in the absence of thalamic input, UP states in the neocortex become much less frequent and robust [66, 68]. An additional noteworthy finding in this study is the extent to which a large proportion of layer 5, presumably pyramidal, neurons also exhibit prominent bursts at the beginning of an UP state. This phenomenon is also apparent in intracellular recordings from the cat neocortex (see Fig. 2b) [60, 61] and strongly implies that these neurons may also play a key role in reinforcing UP states and maintaining rhythmicity in the neocortical network.
An often overlooked finding from earlier studies performed in cats is the presence of so-called spikelets (sometimes referred to as fast prepotentials) that are present during UP states in both neocortical [17] and TC neurons [59, 67]. In these studies, such spikelets were proposed to represent regenerative dendritic potentials. However, an alternative interpretation is that spikelets are action potentials from electrotonically coupled neurons, i.e. communicated via gap junctions (GJs) [6, 32, 33, 41, 43]. Whilst evidence for GJ coupling between neocortical pyramidal neurons in mature animals is not extensive (but see [45]), we have previously shown that spikelets in TC neurons recorded from slices of the cat LGN maintained in vitro are almost certainly due to GJ coupling with other TC neurons [6, 32, 33, 41]. If GJ coupling is indeed operational between TC neurons in the mouse during UP states in vivo, the low-pass nature of this coupling predicts that LTCP events should be efficiently propagated between cells. In fact, upon steady hyperpolarisation, subthreshold UP states in TC neurons commence with a slow event that intuitively has the appearance one would expect from an LTCP that has been electrotonically communicated from a distinct TC neuron (see arrows in Fig. 9b). Thus, as indicated previously [18, 33], the possibility exists that a certain amount of synchronisation of the slow rhythm may occur locally in the thalamus via electrotonic coupling. The possibility that such coupling is also involved in synchronising neocortical activity during UP states should also not be discounted.
Functional implications
A host of studies in both humans and animals now support the idea that the slow rhythm of NREM sleep is involved in memory consolidation [7]. However, the manner in which such consolidation takes place at the cellular and network level is still not well understood. One line of thought suggests that the coordinated replay of spike sequences during UP states in NREM sleep that have occurred in prior wakefulness may form the basis for strengthening connections between relevant groups of neurons [39]. In terms of the synaptic changes that take place, several studies suggest that sleep is associated with a net synaptic depression [3, 69], indicating that long-term depression (LTD) may be central to understanding the cellular changes that are linked to sleep-related memory processes. This is interesting because it has been demonstrated that the pairing of EPSPs with burst firing in layer 5 pyramidal neurons is an effective way to bring about LTD in these cells [4, 23]. As we have shown here, the combination of EPSPs and burst firing is commonly observed in layer 5 cells during the slow rhythm (i.e. mainly at the start of UP states; e.g. Figs. 5c, d and 7a, b), thereby potentially setting up the ideal context for the induction of LTD. Of course, whether bursting in layer 5 neocortical neurons occurs to the same extent during the slow rhythm of natural sleep as it does during anaesthesia remains to be established. However, it is tempting to speculate that, were this to be the case, it may play a key role in mediating synaptic plasticity and, ultimately, the consolidation of memory. Aside from intracortical processes, the robust burst of action potentials that consistently marks the beginning of an UP state in TC neurons may also play an important role in inducing plasticity during sleep. For example, apart from acting as a potential trigger for initiating widespread UP states in the neocortex, the significant Ca2+ entry associated with these bursts, both directly (i.e. in TC neurons) and indirectly (i.e. in neocortical neurons) may establish a window of opportunity for subsequent modifications in synaptic strength in either cell type [24].
Concluding remarks
In this study, we have shown that UP/DOWN states in neocortical and thalamic neurons in mice possess broadly equivalent properties to those observed in other mammals. This suggests that the cellular basis of slow rhythm generation is a conserved property of corticothalamic networks across different species. The main consequence of this is that insights garnered from mice into both the basic mechanisms of slow sleep rhythms as well as the fundamental dynamics of the neocortex are likely to be highly translatable to higher organisms, including humans.
Acknowledgements
This work was supported by the Wellcome Trust grants 71436, 78403, 91882 awarded to VC and 78311 awarded to SWH.
Conflicts of interest
The authors declare that they do not have any conflicting interests regarding the findings or interpretations of this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This article is published as part of the Special Issue on Sleep.
An erratum to this article is available at http://dx.doi.org/10.1007/s00424-011-1067-6.
References
- 1.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82(3):671–686. doi: 10.1016/S0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 2.Bazhenov M, et al. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci. 2002;22(19):8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann TO, et al. A local signature of LTP- and LTD-like plasticity in human NREM sleep. Eur J Neurosci. 2008;27(9):2241–2249. doi: 10.1111/j.1460-9568.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- 4.Birtoli B, Ulrich D. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci. 2004;24(21):4935–4940. doi: 10.1523/JNEUROSCI.0795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blethyn KL, et al. Neuronal basis of the slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J Neurosci. 2006;26(9):2474–2486. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blethyn KL, Hughes SW, Crunelli V. Evidence for electrical synapses between neurons of the nucleus reticularis thalami in the adult brain in vitro. Thalamus Relat Syst. 2008;4(1):13–20. doi: 10.1017/S1472928807000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Born J. Slow-wave sleep and the consolidation of long-term memory. World J Biol Psychiatry. 2010;11(Suppl 1):16–21. doi: 10.3109/15622971003637637. [DOI] [PubMed] [Google Scholar]
- 8.Cash SS, et al. The human K-complex represents an isolated cortical down-state. Science. 2009;324(5930):1084–1087. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20(11):2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clement EA, et al. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS One. 2008;3(4):e2004. doi: 10.1371/journal.pone.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compte A, et al. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89(5):2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 12.Compte A, et al. Spontaneous high-frequency (10–80 Hz) oscillations during up states in the cerebral cortex in vitro. J Neurosci. 2008;28(51):13828–13844. doi: 10.1523/JNEUROSCI.2684-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15(1 Pt 2):604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras D, Steriade M. State-dependent fluctuations of low-frequency rhythms in corticothalamic networks. Neuroscience. 1997;76(1):25–38. doi: 10.1016/S0306-4522(96)00392-2. [DOI] [PubMed] [Google Scholar]
- 15.Contreras D, Steriade M. Synchronization of low-frequency rhythms in corticothalamic networks. Neuroscience. 1997;76(1):11–24. doi: 10.1016/S0306-4522(96)00393-4. [DOI] [PubMed] [Google Scholar]
- 16.Coulter DA, Huguenard JR, Prince DA. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crochet S, et al. Selective amplification of neocortical neuronal output by fast prepotentials in vivo. Cereb Cortex. 2004;14(10):1110–1121. doi: 10.1093/cercor/bhh071. [DOI] [PubMed] [Google Scholar]
- 18.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13(1):9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crunelli V, Lightowler S, Pollard CE. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crunelli V, et al. The ‘window’ T-type calcium current in brain dynamics of different behavioural states. J Physiol. 2005;562(Pt 1):121–129. doi: 10.1113/jphysiol.2004.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40(2):175–190. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham MO, et al. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci U S A. 2006;103(14):5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578(Pt 2):471–479. doi: 10.1113/jphysiol.2006.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Destexhe A, et al. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30(7):334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62(2):171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasenstaub A, et al. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47(3):423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Cruz A, Pape HC. Identification of two calcium currents in acutely dissociated neurons from the rat lateral geniculate nucleus. J Neurophysiol. 1989;61(6):1270–1283. doi: 10.1152/jn.1989.61.6.1270. [DOI] [PubMed] [Google Scholar]
- 28.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93(3):1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 29.Huber R, et al. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 30.Hughes SW, Crunelli V. Just a phase they’re going through: the complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int J Psychophysiol. 2007;64(1):3–17. doi: 10.1016/j.ijpsycho.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes SW, et al. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33(6):947–958. doi: 10.1016/S0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 32.Hughes SW, et al. Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience. 2002;110(3):395–401. doi: 10.1016/S0306-4522(01)00577-2. [DOI] [PubMed] [Google Scholar]
- 33.Hughes SW, et al. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42(2):253–268. doi: 10.1016/S0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- 34.Hughes SW, et al. Novel modes of rhythmic burst firing at cognitively-relevant frequencies in thalamocortical neurons. Brain Res. 2008;1235:12–20. doi: 10.1016/j.brainres.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isomura Y, et al. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52(5):871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Kim D, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31(1):35–45. doi: 10.1016/S0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 37.Le Bon-Jego M, Yuste R. Persistently active, pacemaker-like neurons in neocortex. Front Neurosci. 2007;1(1):123–129. doi: 10.3389/neuro.01.1.1.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Van Quyen M, et al. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci. 2010;30(23):7770–7782. doi: 10.1523/JNEUROSCI.5049-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36(6):1183–1194. doi: 10.1016/S0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 40.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95(6):3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 41.Lorincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8–13 Hz) rhythms in sensory thalamic nuclei in vitro. J Neurosci. 2008;28(3):660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorincz ML, et al. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63(5):683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorincz ML, et al. ATP-dependent infra-slow (<0.1 Hz) oscillations in thalamic networks. PLoS One. 2009;4(2):e4447. doi: 10.1371/journal.pone.0004447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron. 2009;62(3):413–425. doi: 10.1016/j.neuron.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacVicar BA, et al. Paradoxical effects of lithium on field potentials of dentate granule cells in slices of rat hippocampus. Neuropharmacology. 1981;20(5):489–496. doi: 10.1016/0028-3908(81)90183-0. [DOI] [PubMed] [Google Scholar]
- 46.Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 47.McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci U S A. 1992;89(7):2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meuth SG, et al. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23(16):6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meuth SG, et al. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006;69(4):1468–1476. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- 51.Musset B, et al. Effects of divalent cations and spermine on the K+ channel TASK-3 and on the outward current in thalamic neurons. J Physiol. 2006;572(Pt 3):639–657. doi: 10.1113/jphysiol.2006.106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- 54.Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical UP states. Proc Natl Acad Sci U S A. 2008;105(24):8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3(10):1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 56.Seamari Y, et al. Robust off- and online separation of intracellularly recorded up and down cortical states. PLoS One. 2007;2(9):e888. doi: 10.1371/journal.pone.0000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegel JM. Do all animals sleep? Trends Neurosci. 2008;31(4):208–213. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steriade M. Neocortical cell classes are flexible entities. Nat Rev Neurosci. 2004;5(2):121–134. doi: 10.1038/nrn1325. [DOI] [PubMed] [Google Scholar]
- 59.Steriade M, et al. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991;88(10):4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13(8):3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steriade M, et al. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13(8):3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16(1):392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steriade M, et al. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 1996;16(8):2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki S, Rogawski MA. T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc Natl Acad Sci U S A. 1989;86(18):7228–7232. doi: 10.1073/pnas.86.18.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76(6):4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 67.Timofeev I, Steriade M. Fast (mainly 30–100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J Physiol. 1997;504(Pt 1):153–168. doi: 10.1111/j.1469-7793.1997.153bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timofeev I, et al. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10(12):1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 69.Vyazovskiy VV, et al. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 70.Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vyazovskiy VV, et al. Local sleep in awake rats. Nature. 2011;472(7344):443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams SR, et al. The ‘window’ component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol. 1997;505(Pt 3):689–705. doi: 10.1111/j.1469-7793.1997.689ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu L, et al. Nucleus- and species-specific properties of the slow (<1 Hz) sleep oscillation in thalamocortical neurons. Neuroscience. 2006;141(2):621–636. doi: 10.1016/j.neuroscience.2006.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]