Abstract
Background:
Age is a major risk factor for herpes zoster (HZ) and its potential long-term complication post-herpetic neuralgia (PHN). Due to the significant burden of HZ and PHN on patients' quality of life, it is vital that effective and well-tolerated vaccines are available to prevent HZ in older adults. ZOSTAVAX® vaccine was developed to prevent HZ and PHN in individuals ≥50 years (y) of age, and its clinical efficacy and safety have been demonstrated.
Aims and Methods:
This phase 4, open-label, multicenter study was undertaken to assess the immunogenicity and safety of a single dose of ZOSTAVAX (refrigerator-stable formulation) given within 6 mo of its expiry date in individuals ≥50 y of age. The geometric mean fold rise (GMFR) from pre-vaccination to 4 weeks post-vaccination in varicella zoster virus (VZV) antibody titers was calculated. An acceptable antibody response was defined as a lower 95% confidence interval (CI) of GMFR >1.4. solicited and unsolicited injection-site reactions and systemic adverse events were recorded.
Results:
The GMFR in VZV antibody titers was 3.1 (95% CI: 2.6, 3.8), satisfying the criterion for an acceptable VZV antibody response to ZOSTAVAX (minimum requirement: 1.4 GMFR). An acceptable rise in VZV antibody titers was observed in individuals of 50–59 y of age (GMFR 3.9; 95% CI: 2.9, 5.1) and in those ≥60 y of age (GMFR 2.5; 95% CI: 1.9, 3.2). ZOSTAVAX was well tolerated; no serious adverse events were reported.
Conclusion:
ZOSTAVAX elicits an acceptable immune response in immunocompetent individuals ≥50 y of age when stored as directed and administered during the 6 mo prior to expiration.
Key words: elderly, expiry potency, herpes zoster, immunogenicity, clinical study, safety, vaccine, ZOSTAVAX
Introduction
Herpes zoster (HZ), or shingles, is the clinical manifestation of the reactivation of latent varicella zoster virus (VZV), which as a primary infection causes varicella or chickenpox.1–3 HZ is characterized by a unilateral, painful, vesicular rash that is usually limited to a single dermatome.4 While antiviral therapy reduces the severity and duration of HZ when administered within 72 h of rash onset, it does not prevent post-herpetic neuralgia (PHN),5–8 which is the most frequent and debilitating complication of HZ. PHN is a neuropathic pain syndrome that can persist for months, years or even decades after the HZ rash has gone.5,6,9–11 The treatment of PHN remains a clinical challenge, as no one treatment is uniformly effective.5–7
HZ, and particularly PHN, can have a devastating impact on patients' quality of life (QoL), interfering with the activities of daily living, with some patients becoming inactive or housebound, and also affecting psychological and social domains.5,12–19 Indeed, the impact of HZ on patients' QoL is at least as great as that observed with chronic medical conditions such as congestive heart failure and clinical depression.12
Changes in VZV-specific cell-mediated immunity play a pivotal role in the pathogenesis of HZ. Increasing age is associated with immunosenescense, the natural decline of the innate and adaptive immune systems to mount an effective immune response.20 As a consequence, the elderly are more susceptible to infectious diseases21,22 including HZ,23 due to the age-related decline in VZV-specific cell-mediated immunity.1 Therefore, the incidence of HZ and severity of PHN increase with age.3,9,24,25 One in four people in the general adult population will develop HZ during their lifetime.24,25 After 50 y of age the risk roughly doubles with each decade of life26 to one in two in people ≥85 y of age.5 The risk of PHN increases rapidly in those ≥60 y of age.3,9 Therefore, as the population ages, the number of cases of HZ and PHN is expected to rise.15,27
ZOSTAVAX® is a vaccine that was developed for the prevention of HZ and PHN in individuals ≥50 y of age. Clinical studies in patients aged ≥60 y have shown that ZOSTAVAX boosts VZV-specific cell-mediated immunity. Subsequently, the indication for ZOSTAVAX was extended to patients aged 50–59 y.28–33 The clinical efficacy of ZOSTAVAX was demonstrated in the large-scale Shingles Prevention Study, which included almost 40,000 immunocompetent participants of ≥60 y of age.34,35 In this study, ZOSTAVAX reduced the incidence of HZ by 51.3% (p < 0.001) and PHN by 66.5% (p < 0.001); it also reduced the burden of illness due to HZ by 61.1% (p < 0.001)—a measure that combined the incidence, severity and duration of HZ pain and discomfort.34,35 ZOSTAVAX was well tolerated.36
In Europe, ZOSTAVAX is marketed as a refrigerator-stable formulation33,37 and is indicated for the prevention of HZ and PHN in immunocompetent individuals ≥50 y of age.37 The refrigerator-stable formulation of ZOSTAVAX has a shelf-life of 18 mo and a potency not less than 19,400 plaque-forming units per dose (PFU).37 The release specifications of the vaccine were calculated assuming a linear decrease in the log-potency over time during storage, so that a minimum of 19,400 PFU is guaranteed at any time during the shelf-life. This study was undertaken after licensure to assess the immunogenicity and safety of the refrigerator-stable formulation of ZOSTAVAX at minimum-release specification approaching expiry potency in individuals ≥50 y of age.
Results
Study population.
A total of 97 individuals ≥50 y of age were screened of which one individual was not vaccinated due to participating in another study with an investigational compound. Consequently, 96 individuals were included in the study which was conducted between 14 and 28 May 2008. All received a single dose of ZOSTAVAX and completed the study. The safety set and the full-analysis set (FAS) comprised all 96 participants, and the per-protocol set (PPS) comprised 92 participants (95.8%). Four participants were identified as having one or more protocol deviations or conditions which may have interfered with the immunogenicity evaluation and were excluded from the PPS.
Demographics and baseline characteristics in the safety set (same set as the FAS) were similar to those in the PPS. The mean age of participants was 62.2 y (range 51.0–82.4 y) and 47.9% were ≥60 y of age; 52.1% of participants were female.
The medical histories of the participants were typical of this age group: 54.2% had vascular or cardiovascular disorders (primarily hypertension), 43.8% had metabolic and nutritional disorders (comprising mainly hypercholesterolemia and diabetes mellitus), 40.6% had musculoskeletal and connective tissue disorders (primarily osteoarthritis), and 37.5% had psychiatric disorders (primarily depression). The prior and concomitant medications (on the day of vaccination) were linked to the participants' medical history, with the most commonly reported concomitant medications being cardiovascular agents (67.7%), central nervous system agents (42.7%), and agents for gastrointestinal or metabolic disorders (29.2%).
Immunogenicity.
In the PPS, geometric mean titers (GMT) for VZV antibodies increased from 215.8 [95% confidence interval (CI) 178.1, 261.4] VZV glycoprotein enzyme-linked immunosorbent (gpELISA) units/mL pre-vaccination, to 674.0 (95% CI: 565.4, 803.5) gpELISA units/mL 4–5 weeks after vaccination. The geometric mean fold rise (GMFR) from pre-vaccination to 4 weeks post-vaccination in VZV antibody titers was 3.1 (95% CI: 2.6, 3.8; Table 1). The primary objective of the study—an acceptable increase in VZV antibody titers in response to ZOSTAVAX at minimum release specification approaching expiry potency (lower bound of 95% CI: >1.4)—was met.
Table 1.
Geometric mean titer and geometric mean fold rise from pre- to post-vaccination of varicella zoster virus antibody titers (measured by gpELISA ) in the per-protocol set (primary analysis) and the full analysis set (supportive analysis) after vaccination with ZOSTAVAX at minimum-release specification approaching expiry potency
| GMT, gpELISA units/mL [95% CI] | GMFR* [95% CI] | ||
| Pre-vaccination | 28–35 d after ZOSTAVAX | ||
| Per-protocol set | |||
| All (N = 92) | 215.8 [178.1, 261.4] | 674.0 [565.4, 803.5] | 3.1 [2.6, 3.8] |
| 50–59 y (n = 48) | 224.6 [166.5, 302.9] | 868.1 [692.8, 1087.7] | 3.9 [2.9, 5.1] |
| ≥60 y (n = 44) | 206.6 [161.7, 263.9] | 511.4 [395.5, 661.2] | 2.5 [1.9, 3.2] |
| Full-analysis set | |||
| All (N = 96) | 218.4 [181.0, 263.5] | 700.1 [588.7, 832.5] | 3.2 [2.6, 3.9] |
| 50–59 y (n = 50) | 228.6 [170.0, 307.3] | 891.1 [713.8, 1112.3] | 3.9 [3.0, 5.1] |
| ≥60 y (n = 46) | 207.8 [164.3, 262.8] | 538.6 [416.9, 695.9] | 2.6 [2.0, 3.4] |
The VZV antibody response was defined as acceptable if the lower limit of the 95% CI of the GMFR was >1.4. CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; gpELISA, glycoprotein enzyme-linked immunosorbent assay; VZV, varicella zoster virus.
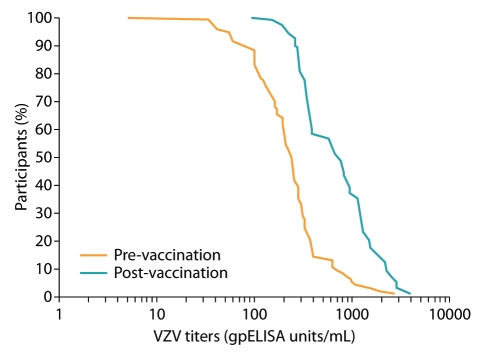
The acceptable VZV antibody response to ZOSTAVAX was further supported in the PPS by the shift in the distribution of VZV antibody titers between the pre- and post-vaccination blood samples in the reverse cumulative distribution curves of antibody titers to VZV (Fig. 1). Supportive analysis in the FAS provided similar results: the GMFR was 3.2 (95% CI: 2.6, 3.9; Table 1).
Figure 1.
Reverse cumulative distribution of varicella zoster virus (VZV) antibody titers (log scale) before vaccination and 28–35 d following vaccination with ZOSTAVAX at minimum-release specification approaching expiry potency (per-protocol set; n = 92). BS1, blood sample 1 (before vaccination); BS2, blood sample 2 (28–35 d following vaccination).
When the immunogenic response was analyzed by age group (50–59 y and ≥60 y), the GMT for VZV antibodies in response to ZOSTAVAX were numerically higher in participants of 50–59 y of age than in those ≥60 y of age (Table 1). Of note, the pre-vaccination VZV antibody levels were similar in both groups. As a result, the GMFR was numerically higher in the younger group [3.9 (95% CI: 2.9, 5.1) in the PPS and 3.9 (95% CI: 3.0, 5.1) in the FAS] compared with the older group [2.5 (95% CI: 1.9, 3.2) in the PPS and 2.6 (95% CI: 2.0, 3.4) in the FAS]. Despite the limited sample sizes when the results were split by age group, the lower bound of the 95% CI for the GMFR was >1.4 for both age groups in both the PPS and the FAS (Table 1).
Safety.
At least one adverse event (AE) was reported in 57 of the 96 participants (59.4%) during the 28 d following vaccination, with 52 (54.2%) having an event considered by the investigator to be related to the vaccination or vaccine. No serious AEs were reported during the study.
At least one solicited injection-site reaction (erythema, swelling or pain) occurring during the 4 d following vaccination was reported by 50 participants (52.1%; Table 2). Injection-site reactions occurred mostly on the day of vaccination or the following day and were mainly mild in intensity. All resolved within 14 d. Two participants had severe injection-site reactions: one presented with severe injection-site erythema that lasted for 6 d, the other had severe injection-site pain lasting for 5 d.
Table 2.
Injection-site reactions and systemic AE s related to ZOSTAVAX at minimum-release specification approaching expiry potency in the 28 d following vaccination (safety set; N = 96)
| n (%)* | |
| Any injection-site reactions or systemic AEs related to the vaccine | 52 (54.2%) |
| Injection-site reactions (day 0–28) | 50 (52.1) |
| Solicited (day 0–4) | 50 (52.1) |
| Erythema 36 | (37.5) |
| Swelling 21 | (21.9) |
| Pain | 39 (40.6) |
| Spontaneously reported (day 0–28) | 4 (4.2) |
| Pruritus | 4 (4.2) |
| Systemic AEs related to the vaccine (day 0–28) | 8 (8.3) |
| Asthenia | 2 (2.1) |
| Headache | 2 (2.1) |
| Keratitis | 1 (1.0) |
| Pyrexia (body temperature ≥38.3°C) | 1 (1.0) |
| Rash of interest† | 2 (2.1) |
| Herpes zoster | 1 (1.0) |
| Vesicular rash | 1 (1.0) |
| Paresthesia | 1 (1.0) |
Number and percentage of participants reporting at least one adverse event (AE )
specific descriptions were required for rashes, including number and type of lesion, location (injection-site or non-injection-site) and confirmation of diagnosis.
Of the 22 participants who reported at least one systemic AE (22.9%), 8 (8.3%) had an event considered by the investigator to be related to the vaccine (Table 2), including two participants with a non-injection site rash of interest: one presented with a vesicular varicella-like rash, and the other with HZ (no lesion specimen was obtained to determine the viral strain involved—wild-type or vaccine type). All vaccine-related systemic AEs were of mild or moderate intensity, except one report of severe asthenia. All vaccine-related systemic AEs occurred within 4 d of vaccination, except the HZ rash, which was reported 5 d after vaccination. Most systemic AEs resolved within 3 d (range: 1–14 d with the exception of HZ infection in one individual, which was reported for 16 d).
Discussion
This study was undertaken to assess the immunogenicity and safety of ZOSTAVAX at minimum-release specification given when approaching expiry potency in individuals ≥50 y of age. The results show that an acceptable increase in VZV antibodies was achieved based on the pre-specified criterion for acceptability that was used: the lower limit of the 95% CI: of GMFR in VZV antibody titers pre- to post-vaccination was >1.4 and a GMFR of 3.1 (95% CI: 2.6, 3.8) was achieved.
The GMFR results achieved in the current study were higher than those achieved in the Gilderman et al. study in which ZOSTAVAX at the minimum release specification was administered throughout the 18-mo shelf-life to individuals ≥50 y of age; the GMFR was 2.6 (95% CI: 2.2, 3.0).33
Younger participants (50–59 y) had greater increases in GMTs for VZV antibodies and therefore higher GMFRs than those ≥60 y of age. This was expected and is probably due to the age-related decline in VZV-specific cell-mediated immunity.38 The responses achieved in both age groups in the current study were slightly higher than those achieved in the Gilderman et al. study presented above: GMFR 50–59 y, 3.9 (95% CI: 2.9, 5.1) vs. 3.1 (95% CI: 2.3, 4.2), respectively; GMFR ≥60 y, 2.5 (95% CI: 1.9, 3.2) vs. 2.3 (95% CI: 1.9, 2.8), respectively.33
The response achieved in participants ≥60 y of age was also higher in the current study than in the pivotal Shingles Prevention Study, which used a frozen formulation of ZOSTAVAX [GMFR 1.7 (95% CI: 1.6, 1.8)].34 In the Shingles Prevention Study antibody responses were measured at 6 weeks post-vaccination compared with 4 weeks in the current study.
However, the response achieved in participants ≥60 y of age in the current study was lower than those achieved in another study undertaken by MacIntyre et al.39 The GMFR was 3.1 (95% CI: 2.8, 3.5) when ZOSTAVAX was administered alone to individuals ≥60 y of age within the first year of its shelf-life with a release potency well above the minimum release specification.39
ZOSTAVAX at minimum-release specification was well tolerated when approaching expiry potency with no serious AEs reported in this study. AE reports were consistent with those in previous clinical trials.33,36,40 One case of HZ was clinically diagnosed during the study (no lesion specimen was obtained to determine the viral strain involved—wild-type or vaccine type). The investigator deemed this event unrelated to ZOSTAVAX.
The results of this study demonstrate that ZOSTAVAX elicits an acceptable immune response in individuals ≥50 y of age when stored as directed and administered during the 6 mo prior to expiration.
Materials and Methods
Study design and participants.
This was a phase 4, open-label, single-arm, multicenter study (ClinicalTrials.gov identifier: NCT00681031), undertaken in six centers in France.
The study was conducted in accordance with applicable national and local requirements. Before commencement, the study protocol was approved by the appropriate ethics committees and Health Authorities. The study was conducted in compliance with informed consent regulations, the 2000 Declaration of Helsinki41 and International Conference of Harmonization Good Clinical Practice Guidelines.42 All study participants gave signed, informed consent at the time of study entry.
Individuals ≥50 y of age were enrolled, with a history of varicella or resident for more than 30 y in a country with endemic VZV infection, and who were affiliated to a health social security system. Investigators were instructed to recruit an equal number of individuals of 50–59 y of age and of ≥60 y of age. Exclusion criteria included a history of HZ diagnosed by a physician, previous varicella or HZ vaccine, fever (oral temperature ≥38.3°C) during the 72 h before vaccination, or exposure to varicella or HZ in the 4 weeks before vaccination. Individuals were also excluded if they had received a live-virus vaccine during the 4 weeks prior to study entry, an inactivated vaccine during the previous 2 weeks, or if they expected to receive one during the study period.
Individuals were screened up to 7 d before vaccination (visit 0). Each participant received a single dose of live attenuated HZ vaccine (ZOSTAVAX®, sanofi pasteur MSD, 0.65 mL) by subcutaneous injection into the deltoid region on day 0 (visit 1). Blood samples were taken during the 7 d prior to vaccine administration (visit 0) or on the day of vaccination (visit 1), and 4 weeks (28–35 d) following vaccination (visit 2).
ZOSTAVAX refrigerator-stable formulation vaccine is supplied as a powder and solvent for suspension for injection. Powder and solvent were stored in a refrigerator (+2.0°C to +8.0°C) in the original package protected from light. The ZOSTAVAX lot used in the study was within 6 mo of its expiry date.
Endpoints and assessments.
The primary objective of the study was to demonstrate that ZOSTAVAX at minimum-release specification approaching expiry potency elicited an acceptable increase in VZV antibody titers 4 weeks after vaccination in those ≥50 y of age. VZV antibody titers were measured using gpELISA.43 The primary endpoint was the GMFR in VZV antibody titers from pre- to post-vaccination.
The secondary objectives of the study were to describe the immunogenic response to ZOSTAVAX in different age groups (50–59 y and ≥60 y), and to describe the safety profile of ZOSTAVAX.
During the development of ZOSTAVAX, VZV-specific immune response was evaluated extensively using several assays. Both the gpELISA and VZV interferon-gamma enzyme-linked immunospot (IFNγ ELISPOT) post-vaccination responses correlated with protection against HZ.44 Since the Shingles Prevention Study, the gpELISA assay (Data from Merck and Co., Inc.) has been extensively used to assess the immune response to ZOSTAVAX and was thus chosen for this study.
Participants were monitored by the investigator for at least 20 min following vaccination for immediate AEs. Solicited injection-site reactions (erythema, swelling and pain) were recorded by participants in a diary card from day 0 to day 4 following vaccination. Unsolicited injection-site reactions and systemic AEs were recorded spontaneously in the diary card for up to 28 d following vaccination. Serious AEs were recorded throughout the study. Serious AEs were defined as per EU directive,45 as any untoward medical occurrence or effect that at any dose results in death, is life-threatening, requires in-patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a congenital anomaly or birth defect, or another medically important event, or any cancer or overdose with or without adverse event. Any temperature ≥38.3°C (oral or equivalent) was considered to be an AE. The investigator measured the participant's temperature before vaccination and the participant measured it any time during the following 28 d if they felt feverish. The intensity of measurable and non-measurable AEs were ranked as mild: <5 cm or awareness of sign and symptom but easily tolerated; moderate: ≥5 cm and <10 cm or discomfort enough to cause interference with usual activity; or severe: ≥10 cm or incapacitating with inability to work or undertake usual activities, respectively.
Statistical methods.
The primary hypothesis of the study was that ZOSTAVAX at minimum-release specification approaching expiry potency elicits an acceptable GMFR in VZV antibody titers. An acceptable rise in GMFR was demonstrated if the lower bound of the two-sided 95% CI of the GMFR was >1.4, corresponding to a one-sided test at a type I error rate of 2.5%. The acceptability threshold of 1.4 was based on the efficacy results observed in the Shingles Prevention Study34 and current experience with the vaccine.
The PPS comprised participants who received vaccination excluding those with HZ onset before visit 2 (second blood sample) and those with protocol violations that may interfere with immune response. The FAS comprised all participants who received vaccination and for whom any immunogenicity data were available. The safety set comprised all participants who received vaccination.
The primary analysis was undertaken in the PPS and a supportive analysis in the FAS. Two-sided 95% CIs for GMFR and GMT were calculated using the Student's t distribution based on log-transformed data. The safety analysis was undertaken in the safety set.
The sample size was calculated based on the assumption that up to 10% of the participants enrolled in the study would not be evaluable for the primary GMFR analysis, i.e., were either lost to follow-up or deviated from the protocol. Consequently, 96 vaccinated participants would provide at least 86 evaluable participants for the primary analysis. Given 86 evaluable participants, and assuming that the standard deviation of the natural log-transformed fold rise of VZV antibody titers was 1.0 and that the true GMFR was at least 2.0, the study would have 90% power to demonstrate an acceptable increase in the GMFR in VZV gpELISA antibody titers in response to ZOSTAVAX.
Acknowledgments
The authors take full responsibility for the content of the paper and thank Communigen Ltd., Oxford, UK (supported by sanofi pasteur MSD, Lyon, France) for their assistance in preparing the manuscript, and Isabelle Bertrand for her comments and critical review of the drafts.
The authors would like to thank the principal investigators Michel Lambert, Philippe Igigabel, Jacques Tondut, Daniel Gombaud, Pierre Leroy and co-investigators Philippe Remaud, Alain Palomba and Didier Nourry for their participation in this study. They would also like to thank Julie Boyer-Tran for the global coordination of the study, and Catherine Bosc, Xavier Cornen, Cecile Neyret and Sandrine Gilhet-Mailfait for their contribution to the conduct of the study.
Abbreviations
- AE
adverse event
- CI
confidence interval
- FAS
full-analysis set
- GMT
geometric mean titer
- GMFR
geometric mean fold rise
- gpELISA
glycoprotein enzyme-linked immunosorbent assay
- HZ
herpes zoster
- IFNγ ELISPOT
interferon-gamma enzyme-linked immunospot
- PFU
plaque-forming units
- PHN
post-herpetic neuralgia
- PPS
per-protocol set
- QoL
quality of life
- VZV
varicella zoster virus
Financial Support
Research sponsored by sanofi pasteur MSD. ClinicalTrials.gov identifier: NCT00681031.
Conflicts of Interest
R. Arnou has received consulting fees/honoraria/travel grants from sanofi pasteur MSD. A. Fiquet, S. Thomas and C. Sadorge are employees of sanofi pasteur MSD.
Trademark Statement
ZOSTAVAX is a trademark of Merck and Co., Inc., Whitehouse Station, NJ.
References
- 1.Arvin AM. Varicella-zoster virus. In: DM Knipe, PM Howley., editors. Fields Virology. 4th. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2731–2768. [Google Scholar]
- 2.Lungu O, Annunziato PW, Gershon A, Staugaitis SM, Josefson D, LaRussa P, et al. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc Natl Acad Sci USA. 1995;92:10980–10984. doi: 10.1073/pnas.92.24.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier A, Breuer J, Carrington D, Martini M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137:38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 4.Gnann JW, Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347:340–346. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- 5.Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32:1481–1486. doi: 10.1086/320169. [DOI] [PubMed] [Google Scholar]
- 6.Schmader K, Gnann JW, Jr, Watson CP. The epidemiological, clinical and pathological rationale for the herpes zoster vaccine. J Infect Dis. 2008;197:207–215. doi: 10.1086/522152. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RW, Wasner G, Saddier P, Baron R. Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging. 2008;25:991–1006. doi: 10.2165/0002512200825120-00002. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Chen N, Yang J, Zhou M, Zhou D, Zhang Q, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2009;15:6866. doi: 10.1002/14651858.CD006866.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–575. [PMC free article] [PubMed] [Google Scholar]
- 10.Scott FT, Leedham-Green ME, Barrett-Muir WY, Hawrami K, Gallagher WJ, Johnson R, et al. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol. 2003;70:24–30. doi: 10.1002/jmv.10316. [DOI] [PubMed] [Google Scholar]
- 11.Meister W, Neiss A, Gross G, Doerr HW, Höbel W, Malin JP, et al. A prognostic score for postherpetic neuralgia in ambulatory patients. Infection. 1998;26:359–363. doi: 10.1007/BF02770836. [DOI] [PubMed] [Google Scholar]
- 12.Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: a self-limited disease with severe impact. Neurology. 1995;45:52–53. doi: 10.1212/wnl.45.12_suppl_8.s52. [DOI] [PubMed] [Google Scholar]
- 13.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342–348. doi: 10.1086/421942. [DOI] [PubMed] [Google Scholar]
- 16.Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6:356–363. doi: 10.1016/j.jpain.2005.01.359. [DOI] [PubMed] [Google Scholar]
- 17.van Seventer R, Sadosky A, Lucero M, Dukes E. A cross-sectional survey of health state impairment and treatment patterns in patients with postherpetic neuralgia. Age Ageing. 2006;35:132–137. doi: 10.1093/ageing/afj048. [DOI] [PubMed] [Google Scholar]
- 18.Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 19.Weinke T, Edite A, Schmitt S, Lukas K. Impact of herpes zoster and post-herpetic neuralgia on patients' quality of life: a patient-reported outcomes survey. J Public Health. 2010;18:367–374. doi: 10.1007/s10389-010-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/S1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 22.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RW. Herpes Zoster and Postherpetic Neuralgia: a review of the effects of vaccination. Aging Clin Exp Res. 2009;21:236–243. doi: 10.1007/BF03324909. [DOI] [PubMed] [Google Scholar]
- 24.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 25.Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–314. doi: 10.1017/S0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. doi: 10.1001/archinte.155.15.1605. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin RH, Gnann JW, Jr, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9:37–44. doi: 10.1016/j.jpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Levin MJ, Murray M, Rotbart HA, Zerbe GO, White CJ, Hayward AR. Immune response of elderly individuals to a live attenuated varicella vaccine. J Infect Dis. 1992;166:253–259. doi: 10.1093/infdis/166.2.253. [DOI] [PubMed] [Google Scholar]
- 29.Oxman MN. Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurology. 1995;45:41–46. doi: 10.1212/wnl.45.12_suppl_8.s41. [DOI] [PubMed] [Google Scholar]
- 30.Levin MJ, Barber D, Goldblatt E, Jones M, LaFleur B, Chan C, et al. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis. 1998;178:109–112. doi: 10.1086/514264. [DOI] [PubMed] [Google Scholar]
- 31.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 32.Trannoy E, Berger R, Holländer G, Bailleux F, Heimendinger P, Vuillier D, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose-response trial. Vaccine. 2000;18:1700–1706. doi: 10.1016/S0264410X(99)00510-1. [DOI] [PubMed] [Google Scholar]
- 33.Gilderman LI, Lawless JF, Nolen TM, Sterling T, Rutledge RZ, Fernsler DA, et al. ZOSTAVAX Protocol 010 Study Group, author. A double-blind, randomized, controlled, multicenter safety and immunogenicity of a refrigerator stable formulation of Zostavax®. Clin Vaccine Immunol. 2008;15:314–319. doi: 10.1128/CVI.00310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 35.Oxman MN, Levin MJ Shingles Prevention Study Group, author. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008;197:228–236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simberkoff MS, Arbeit RD, Johnson GR, Oxman MN, Boardman KD, Williams HM, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:545–554. doi: 10.7326/0003-4819-152-9-201005040-00004. [DOI] [PubMed] [Google Scholar]
- 37.ZOSTAVAX® Summary of Product Characteristics. May 2006. Available at: http://www.ema.europa.eu/humandocs/PDFs/EPAR/zostavax/emea-combinedh674en.pdf.
- 38.Arvin A. Aging, immunity and the varicella-zoster virus. N Engl J Med. 2005;352:2266–2267. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- 39.MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old. Hum Vaccin. 2010;6:894–902. doi: 10.4161/hv.6.11.12852. [DOI] [PubMed] [Google Scholar]
- 40.Sanford M, Keating GM. Zoster vaccine (ZOSTAVAX®). A review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs Aging. 2010;27:159–176. doi: 10.2165/10489140. [DOI] [PubMed] [Google Scholar]
- 41. [07 Jan 2010];World Medical Association Declaration Of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. doi: 10.1191/0969733002ne486xx. at: http://www.wma.net/en/30publications/10policies/b3/index.html. [DOI] [PubMed] [Google Scholar]
- 42. [07 Jan 2010];Note For Guidance On Good Clinical Practice. 1997 (CPMP/ICH/135/95), at: http://www.ema.europa.eu/pdfs/human/ich/013595en.pdf. [Google Scholar]
- 43.Krah DL, Cho I, Schofield T, Ellis RW. Comparison of gpELISA and neutralizing antibody responses to Oka/Merck live varicella vaccine (VARIVAX®) in children and adults. Vaccine. 1997;15:61–64. doi: 10.1016/S0264-410X(96)00107-7. [DOI] [PubMed] [Google Scholar]
- 44.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontaine N, Rosengren B. The European Parliament and the council of the European Union. Directive 2001/20/EC relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use Luxembourg, 4 April 2001. [June 3 2011]. Available at: http://www.eortc.be/Services/Doc/clinical-EU-directive-04-April-01.pdf.