Abstract
This study offers evidence that vitamin D deficiency could be a major public health burden among young Emirati adults, mostly because of sun deprivation in a sun-blessed country. This study included a random sample of 138 females and 70 males tested for serum 25-hydroxyvitamin D [25(OH)D] status. To further evaluate the predictors of vitamin D status in this population, the study examined diet, obesity and sun exposure. In summer, the mean serum 25(OH)D concentration for females was 20.9 ± 14.9 nmol/L, whereas that for males was 27.3 ± 15.7 nmol/L. Females scored significantly higher than males on the sun avoidance inventory (SAI), indicating that females avoid sun exposure to a greater extent than males, possibly explaining the lower vitamin D status. A significant negative correlation also existed between SAI and vitamin D status (Pearson's r = −0.33; p < 0.01), but no significant association was evident between vitamin D status and body mass index (Pearson's r = 0.03; p = 0.33) or low dietary intake of vitamin D-fortified foods (Pearson's r = 0.08; p = 0.13). The mean serum 25(OH)D concentration for females tested in winter was 31.3 ± 12.3 nmol/L while in the summer, it was 20.9 ± 14.9 nmol/L. This difference was statistically significant, suggesting that seasonal variation plays an important role in vitamin D status in the United Arab Emirates. Fortification of foods and drinks with vitamin D, supplementation and sensible sun exposure are important steps toward minimizing vitamin D deficiency.
Keywords: vitamin D deficiency, 25(OH)D, young adult emiratis, females, United Arab Emirates, sun avoidance inventory, high performance liquid chromatography, food frequency questionnaire
Introduction
The emergence of vitamin D deficiency as a factor in multiple illnesses has focused attention on vitamin D's preventive role and the importance of maintaining optimal concentrations in the body. Vitamin D deficiency has been recently noted in the Gulf region despite the area's sunny climate.1–3 A study conducted at Al-Ain, United Arab Emirates (UAE), showed that vitamin D deficiency (serum level, 25.3 ± 10.8 nmol/L) is common (36% frequency) in women of childbearing age (n = 33) in Arab communities residing in the UAE.2 Vitamin D deficiency is a common maternal-infant health problem in Arab communities residing in Al-Ain, UAE.4 Osteoporotic Emirati women suffer from mild to severe vitamin D deficiency.3 Arabian women and children have low serum vitamin D concentrations attributed to insufficient sunlight exposure and low dietary vitamin D intake.5
Vitamin D is essential for supporting bone integrity6,7 and has a protective role against several chronic morbidities8,9 such as low bone mass density,10 diabetes and cardiovascular diseases,11,12 and many types of cancer.13–15 A common assumption is that casual sun exposure can secure sufficient vitamin D status.2,8
In concordance, several research reports from other Gulf or Arab countries demonstrate a significant prevalence of vitamin D deficiency, particularly in women, owing to their cultural dress code.1,16–20 Paradoxically, in some sunny countries such as the UAE, residents tend to avoid exposure to sunlight because of excessive heat.2 The first study in adults from the region was conducted in university students and elderly from Saudi Arabia, revealing a mean 25(OH)D status ranging between 10 and 30 nmol/L.1 The mean 25(OH)D status was near 25 nmol/L in Lebanese, Saudi, Emirati and Iranian women.3,21–23 Because the UAE has not yet begun fortifying food with vitamin D, many groups in this population could be vulnerable for developing vitamin D deficiency.2,4 This supposition is particularly relevant to individuals who limit their outdoor activities or spend most of their time indoors, such as students, or cover the body extensively, such as Emirati females.2,6
One study selected 146 subjects (male, 22; female, 124) on the basis of established inclusion criteria. Of the women, 21 wore western-type dress styles (group 1); 80 wore dress styles covering the whole body but sparing the face and hands (group 2); and 23 wore dress styles covering the whole body, including the face and hands (group 3). The study was conducted in summer and winter. All volunteers underwent initial interviews, answered a food frequency questionnaire (FFQ) and underwent essential laboratory tests for serum 25(OH)D by radioimmunoassay and serum parathyroid hormone by chemiluminescent enzyme immunoassay. The 25(OH)D levels in groups 2 and 3 were significantly lower than those in the men (p < 0.05 in both comparisons). Dress styles covering the whole body, totally or nearly totally, have adverse effects on 25(OH)D status and may produce a state of secondary hyperparathyroidism over a long period. Although Jordan enjoys plentiful sunshine, these data suggest widespread hypovitaminosis D in that country.24 The prevalence of vitamin D deficiency among healthy Saudi men is between 28% and 37%.25 The objective of this research is to assess the prevalence of vitamin D deficiency among young-adult female and male Emirati students, a high-risk population group in the UAE. The secondary aim was to determine the variables that contribute to circulating 25(OH)D status.
Results
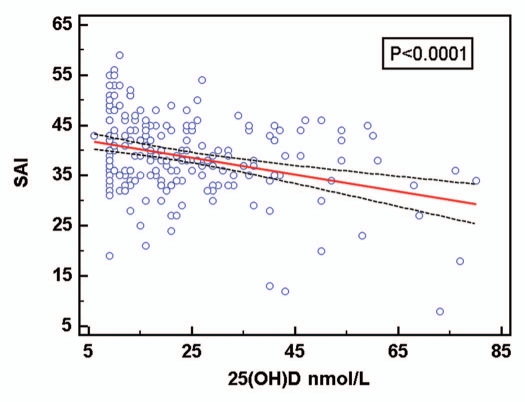
The mean age of students was 21.0 ± 4.6 years for males and 20.8 ± 4.0 years for females. Table 1 compares males and females according to season, age, BMI, SAI, FFQ and vitamin D status. Mean serum 25(OH)D concentrations in October were 20.9 ± 14.9 nmol/L for females and 27.3 ± 15.7 nmol/L for males, and for the total sample the value was 23.1 ± 15.5 nmol/L. Mean serum 25(OH)D concentration in April was 31.3 ± 12.3 nmol/L (females only). The majority of participants were either normal (52.83%) or underweight (22.17%). Those who were overweight accounted for 16.04%, while only 7.06% were obese. Very few participants were either severely underweight (0.47%) or severely obese (1.42%). The mean BMI for all females was 23.2 ± 5.0 and 23.7 ± 4.2 for males. Females scored significantly higher than males on the mean SAI score (39.4 ± 7.4 vs. 34.6 ± 7.0; p < 0.05), indicating that females avoid sun exposure to a greater extent than males, possibly explaining the lower vitamin D status among females (Table 1). Figure 1 shows the distribution of serum 25 (OH)D levels for participants according to sun avoidance score(SAI). Both females and males had a low score for mean FFQ, indicating that both are not getting adequate dietary vitamin D (12.5 ± 3.7 and 17.4 ± 5.8, respectively). A significant negative correlation also existed between SAI and vitamin D status (Pearson's r = −0.32; p < 0.01), but no significant association was evident between vitamin D status and BMI (Pearson's r = 0.03; p = 0.33) or dietary intake of vitamin D-rich foods (Pearson's r = 0.08; p = 0.13). Table 2 illustrates the mean ± SD values of vitamin D, SAI and BMI recorded during summer and winter for female students only. The mean vitamin D level for 70 female students during winter was 31.3 ± 12.3 nmol/L, whereas in summer the mean vitamin D status was 20.9 ± 14.9 nmol/L for 138 female students. A statistically significant difference existed in the levels of 25(OH)D according to seasonal variation (r = 0.03; p < 0.05).
Table 1.
Baseline characteristics of male and female students that participated in the study
| Characteristics | Males (n = 70) | Females (n = 208) | t-test 95% Confidence Interval of the Difference |
| Age (years) | 21.0 ± 4.6 | 20.8 ± 4.0 | 0.211 |
| BMI (kg/m2) | 23.7 ± 4.2 | 23.2 ± 5.0 | 0.057 |
| 25 (OH)D (nmol/L) | 27.3 ± 15.7 | 24.2 ± 14.9 | 0.0025* |
| SAI score | 34.6 ± 7.0 | 39.4 ± 7.4 | 0.000* |
| FFQ score | 17.4 ± 5.8 | 12.5 ± 4.9 | 0.064 |
Differences are significant; p < 0.05. All values are shown as mean ± SD; BMI, body mass index; SAI, sun avoidance inventory; FFQ, food frequency questionnaire.
Figure 1.
Group distribution of serum 25(OH)D status according to sun avoidance attitude: Scores for SAI are categorized as follows: 0–25, low avoidance; 26–35, moderate avoidance; 36–80, high avoidance.
Table 2.
Baseline characteristics of only female students during summer and winter
| Characteristic | Females (summer) (n = 138) | Females (winter) (n = 70) | t-test 95% Confidence Interval of the Difference |
| Age (years) | 21.1 ± 4.6 | 20.3 ± 1.9 | 0.106 |
| BMI (kg/m2) | 22.6 ± 4.6 | 24.4 ± 5.6 | 0.30 |
| 25 (OH)D (nmol/L) | 20.9 ± 14.9 | 31.3 ± 12.3 | 0.0005* |
| SAI score | 41.4 ± 7.4 | 35.1 ± 5.4 | 0.000* |
| FFQ score | 13.2 ± 5.4 | 11.0 ± 3.2 | 0.003 |
Differences are significant; p < 0.05. All values are shown as mean ± SD; BMI, body mass index; SAI, sun avoidance inventory; FFQ, food frequency questionnaire.
Discussion
Mean serum 25(OH)D concentrations were higher in April, which marks the end of the short, cool winter season and lower in October, which marks the end of the hot summer season. Although many studies have reported the inverse association between BMI and serum 25(OH)D concentrations, few have also demonstrated the absence of correlation in some populations. In general, males had a higher 25(OH)D status and spent more time in the sun, consumed a similar level of dietary vitamin D, and had a comparable BMI.
Table 1 shows the results of predictive models of serum 25(OH)D in all the participants by sex. The inverse association (negative correlation) between SAI and circulating 25(OH) D level was statistically significant. Sex differences with regard to 25(OH)D concentrations and SAI scores were significant. However, no significant differences occurred between males and females for BMI and FFQ.
In this study, women had lower 25(OH)D concentrations than those of men, but women had similar BMI and intake of dietary vitamin D. However, women had a higher measure of sun avoidance attitude, suggesting that the sex differences in 25(OH) D concentrations are attributed to behavior toward sun avoidance or exposure. Emirati women and men usually cover most of the body for cultural and religious reasons; however, our data show that women tend to avoid the sun more than do men. The mean serum 25(OH)D concentration for females tested in winter was significantly higher than that in summer. This seasonal pattern is the opposite of what is reported for many countries. This finding could be attributed to the fact that winter in Abu Dhabi is cool yet sunny, hence residents tend to engage highly in outdoor activities—in contrast to the summer, which is humid and extremely hot, a time when people significantly limit their outdoor activities. The observed differences in serum 25(OH) D by season are in concordance with the findings reported by Saadi et al.26 who indicated that optimal levels were obtained in April compared with August and October. There was a seasonal variation in the blood status of 25(OH)D due to increased sun exposure. The exposure time was noted between 11 in the morning and 3 in the afternoon.
Other researchers reported the lack of association between diet and 25(OH)D levels revealed that Canadian aboriginal women have a higher prevalence of vitamin D deficiency than non-aboriginal women, despite similar dietary vitamin D intake.3,27 Another study showed that Arab women residing in the UAE had low serum 25(OH)D status that did not correlate with their dietary intake of vitamin D.3 The lack of association between diet and vitamin D status could be attributed to the observation that the recommended daily intake of dietary vitamin D is not sufficient to maintain a nominal vitamin D status if sunlight exposure is limited. Moreover, the content of vitamin D in natural foods such as mushrooms, eggs and oily fish often varies, resulting in poor documentation of such foods' vitamin D content. For instance, wild salmon has up to 90% more vitamin D than farmed salmon.28 Even fortified foods are subject to this fluctuation because of the fortification procedures such as storage conditions. The absence of association between BMI and 25(OH)D status in the examined participants may be attributed to the fact that the sample was underpowered to show an association between 25(OH)D and increasing BMI indexes, since most participants were normal weight and only 7% were obese. This lack of association can not be generalized to the total UAE national population because the sample represents younger university students, who are often conscious about their body image and hence pay great attention to weight. Although the UAE is a sun-blessed country, Emiratis are often not exposed to sunlight, owing to avoidance of excessive heat and cultural reasons, for which women cover most of their bodies. This factor adds an extra burden to vitamin D status for this population, which becomes at high risk of developing deficiency. Only three male students were found with 25(OH)D >75 nmol/L, not taking any vitamin D supplements but had low SAI meaning that their exposure to sun was high. This is worth further investigation as it confirms that sun will provide adequate levels in the absence of supplementation or with low food intake of vitamin D.
Limitations in this study must be considered, including the relatively few male participants compared with females, because of the local students' demographics. In addition, this study did not assess parathyroid hormone, ALP, Ca and PO4 levels to exclude any cases of secondary hyperparathyroidism. Nevertheless, several studies have shown that such serum biomarkers may not reflect the true status of an individual's vitamin D status. Moreover, seasonal variation in serum 25(OH)D status was investigated only for females. Other factors to consider when studying the variables that affect vitamin D status include skin pigmentation and intestinal malabsorption diseases. However, most participants had a similar skin color and none reported any diseases such as Crohn's or cystic fibrosis.
Further exploration of how to remedy vitamin D deficiency in Emiratis is needed. When a nutritional public health need is identified, the solution is to ensure that all people can benefit. Staple foods are hence good targets for food fortification, provided that the right recommended intake is really attained. Jordan recently issued a food policy that mandates the fortification of bread with vitamin D.29,30 However, the amount of vitamin D in food would have to be about 1,000–3,000 IU/day to be effective in increasing serum 25(OH)D status to the 100 nmol/L that optimal health requires.31–37
This study documents the prevalence of vitamin D deficiency due mostly to sun deprivation in a sun-blessed country among young-adult Emiratis. The results of all these studies emphasize the need for urgent measures in our part of the world to avoid long-term complications related to vitamin D deficiency; these measures include vitamin D supplementation and fortification of some highly consumed food, milk and other dairy products. Educational endeavors about sensible sun exposure should be implemented to improve vitamin D status among this population. Whether Emiratis are predisposed to vitamin D deficiency by inability to maintain adequate vitamin D status (due mainly to sun avoidance) or the possible existence of polymorphism in the vitamin D receptor gene or other related genes is worth investigating.
We note that the Institute of Medicine (IOM) recently stated that a 25(OH)D level of 20 ng/ml (50 nmol/l) was adequate for all people since the only benefit of vitamin D strongly supported by the evidence was bone health.38 However, this finding flies in the face of overwhelming evidence of numerous benefits in reducing the risk of many diseases including cancer, cardiovascular disease, diabetes, bacterial and viral infectious diseases, autoimmune diseases and central nervous system diseases as well as adverse pregnancy outcomes. The IOM report has been strongly criticized in numerous journal publications.39,40 Based on a more scientific review of the evidence, serum 25(OH)D levels of 30–40 ng/ml (75–100 nmol/L) are recommended for optimal health.41
Subjects and Methods
Study population and design.
A total of 278 participants (female, 208; male, 70) were randomly selected from a computer-generated sequence from the list of Zayed University students who were willing to participate in the study. Data collection involved a blood test to measure serum 25(OH)D concentration and a questionnaire that had several components pertaining to sociodemographic (residential, educational and occupational), medical history, nutritional (FFQ),3 and psychosocial (personality traits, depression, anthropometry) aspects. A project coordinator and two research assistants were trained to administer the survey questionnaire regarding nutritional and lifestyle factors. The study recruitment was conducted in October 2009 (n = 138 females and n = 70 males) and in April 2010 (n = 70 females) to document seasonal variation in vitamin D status. All participants completed questionnaires with the help of the research assistants to ensure accuracy of collected information. The study was approved by the Zayed University Human Subjects Committee and the Sheikh Khalifa Medical City institutional review board. Written informed consent was obtained from all participants before study enrollment.
Analysis of serum 25(OH)D.
Blood samples were taken from all subjects to analyze serum 25(OH)D as an indicator of vitamin D status. Status of 25(OH)D was measured at Sheikh Khalifa Medical City with a Waters HPLC 2695 separation module with UV detection using Chromsystems kits (Chromsystems Instruments & Chemicals GmbH, Heimburgstrasse, Munich, Germany) by using a modified high-performance liquid chromatography (HPLC) method.42,43 In brief, 500 µL of serum (standard, controls or specimen) and 50 µL of internal standard were mixed into the labeled, light-protected reaction vial. We then added 500 µL of precipitation reagent and mixed this in a vortex for 20 seconds. Reaction vials were then incubated for 10 min at 4°C. Vials were then centrifuged for 5 min at 13,000 rpm (Centrifuge 5430/5430R, Eppendorf AG, 22331, Hamburg, Germany). Immediately the supernatant was applied to a labeled sample cleanup column and drawn through by centrifugation at 1,500 rpm for 1 min followed by discarding the effluent. Sample cleanup columns were then washed two times by using 1 mL of Wash Buffer I each time by centrifuging at 1,500 rpm for 1 min and discarding the effluent. Column washing was repeated by using 75 µL of Wash Buffer II by centrifuging at 1,500 rpm for 1 min and discarding the effluent. Two hundred microliters of elution buffer was applied to each column, and the eluates were collected by centrifugation at 1,500 rpm for 1 min. The eluants were collected into glass vials and then diluted with 20 µL of distilled water. 50 µL of the sample was finally injected into the HPLC system. The prepared controls were included in every analytical series to monitor accuracy and precision within the system. The chromatographic separation was completed in about 10 min. All values of 25(OH)D were recorded in nanomoles per liter. This HPLC assay includes the measurement of both 25(OH)D2 and 25(OH)D3 separately, and the reported values represent the combined concentrations. The intra-assay coefficient of variation was 4% and the interassay coefficient of variation was 5.8%. Reference ranges used in this study based upon Chromsystems kits were as deficiency: <25 nmol/L, sufficiency: 50–200 nmol/L and toxicity: >200 nmol/L. According to the new IOM recommendations corresponding to a serum 25(OH) D status of at least 20 ng/ml (50 nmol/liter), meet the requirements of at least 97.5% of the population.38
Exposure variables.
Dietary data were collected using a modified FFQ, a self-administered instrument that considers self-reported recall of the intake of vitamin D-rich foods (naturally and fortified) for the last 4-week period. The modified FFQ was based on previous studies that included local food items.44 The sun avoidance inventory (SAI) assesses sun avoidance attitudes and behaviors across six factors: cosmetic/aesthetic, health and safety, transport, occupational, recreational and sartorial. Sun exposure was evaluated by using a modified version of the SAI, which is a questionnaire designed to assess attitude toward sun avoidance.45 Body mass index (BMI) was calculated as weight in kilograms divided by the height in square meters. Height and weight were measured using a digital height and weight scale. BMI results were interpreted according to the following: BMI <16 severely underweight, 16–19 underweight, 20–25 normal range, 26–30 overweight, 31–40 obese and >41 severely obese.2,4 The reference range for vitamin D was adopted from Grant et al.31,32,35–37 and Sabetta et al.46 and included deficiency, insufficiency and sufficiency (<50, 50–75 and >75 nmol/L, respectively). The inclusion criterion required being an enrolled university student, and the exclusion criterion was all participants taking any form of vitamin D supplementation.
Statistical data analyses.
All analyses were conducted by using SPSS version 16.0 (Chicago, IL). Means and standard deviations were used to describe vitamin D status. The p values were calculated by using SPSS for categorical variables and linear regression for continuous variables. Differences in 25(OH) D concentrations by sex and season were analyzed with Lavene's test.47 Comparisons of serum 25(OH)D concentrations were performed by using the Student t test (1-tailed) and p values less than 0.05 were considered to be statistically significant. Correlation between 25(OH)D and the independent variables (FFQ, BMI and SAI) were determined by the Pearson correlation coefficient.
Acknowledgments
This study was supported by research funds from Zayed University and the Emirates Foundation for Philanthropy granted to F. Al Anouti. We thank Dr. Ali Djawad Khalili, from Primary Health Care Services of Sheikh Khalifa Medical City for his cooperation throughout this study and Ms. Sarra Al Hasani for statistical analysis of the data. Ms. Sharifa Al Adawi and Ms. Mariam Mansouri for their valuable help in recruitment and data entry and Mr. Nafiz Nimer Hussein for his technical expertise in the HPLC analysis.
Disclosure of Potential Conflicts of Interest
W.B.G. receives or has received funding from the UV Foundation (McLean, VA), the Sunlight Research Forum (Veldhoven), Bio-Tech-Pharmacal (Fayetteville, AR), the Vitamin D Council (San Luis Obispo, CA) and the Danish Sunbed Federation (Middelfart).
References
- 1.Sedrani SH, Elidrissy AW, El Arabi KM. Sunlight and vitamin D status in normal Saudi subjects. Am J Clin Nutr. 1983;38:181–185. doi: 10.1093/ajcn/38.1.129. [DOI] [PubMed] [Google Scholar]
- 2.Dawodu A, Absood G, Patel M, Agarwal M, Ezimokhai M, Abdulrazzaq Y, et al. Biosocial factors affecting vitamin D status of women of child bearing age in the United Arab Emirates. J Biosoc Sci. 1998;30:431–437. doi: 10.1017/s0021932098004313. [DOI] [PubMed] [Google Scholar]
- 3.Saadi HF, Nagelkerke N, Benedict S, Qazaq HS, Zilahi E, Mohamadiyeh MK, et al. Predictors and relationships of serum 25 hydroxyvitamin D concentration with bone turnover markers, bone mineral density and vitamin D receptor genotype in Emirati women. Bone. 2006;39:1136–1143. doi: 10.1016/j.bone.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Dawodu A, Dawson KP, Amirlak I, Kochiyil J, Agarwal M, Badrinath P. Diet, clothing, sunshine, exposure and micronutrient status of Arab infants and young children. Ann Trop Paediatr. 2001;21:39–44. [PubMed] [Google Scholar]
- 5.Saadi H, Dawodu A. Vitamin D deficiency in Arabian women and children: It is time for action. Emirates Med J. 2007;25:201–207. [Google Scholar]
- 6.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80:1678–1688. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739–2748. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080–1086. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 10.Barnes MS, Robson PJ, Bonham MP, Strain JJ, Wallace JM. Effect of vitamin D supplementation on vitamin D status and bone turnover markers in young adult. Eur J Clin Nutr. 2006;60:727–733. doi: 10.1038/sj.ejcn.1602374. [DOI] [PubMed] [Google Scholar]
- 11.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and metaanalysis. Maturitas. 2010;65:225–236. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 13.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. AmJPublic Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26:2687–2699. [PubMed] [Google Scholar]
- 15.Grant WB. How strong is the evidence that solar ultraviolent B and vitamin D reduce the risk of cancer? An examination using Hill's criteria for causality. Dermatoendocrinol. 2009;1:17–24. doi: 10.4161/derm.1.1.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedrani SH. Low 25-hydroxyvitamin D and normal serum calcium concentrations in Saudi Arabia: Riyadh region. Ann Nutr Metab. 1984;38:181–185. doi: 10.1159/000176801. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui AM, Kamfar HZ. Prevalence of vitamin D deficiency rickets in adolescent school girls in western region, Saudi Arabia. Saudi Med J. 2007;28:441–444. [PubMed] [Google Scholar]
- 18.Molla AM, Al Badawi M, Hammoud MS, Molla AM, Shukkur M, Thalib L, et al. Vitamin D status of mothers and their neonates in Kuwait. Pediatr Int. 2005;27:649–652. doi: 10.1111/j.1442-200x.2005.02141.x. [DOI] [PubMed] [Google Scholar]
- 19.Fuleihan GE. Vitamin D deficiency in the Middle East and its health consequences. In: Holick MF, editor. Vitamin D; Physiology, Molecular Biology and Clinical Applications, Second Edit. New York: Humana Press, Springer; 2010. pp. 469–494. Ch. 24. [Google Scholar]
- 20.El-Kaissi S, Sherbeeni S. Vitamin D deficiency in the Middle East and its health consequences for adults. In: Holick MF, editor. Vitamin D; Physiology, Molecular Biology and Clinical Applications, Second Edit. New York: Humana Press, Springer; 2010. pp. 495–503. Ch. 25. [Google Scholar]
- 21.Fuleihan GE, Deeb M. Hypovitaminosis D in a Sunny Country. New England J Med. 1999;340:1840–1841. doi: 10.1056/NEJM199906103402316. [DOI] [PubMed] [Google Scholar]
- 22.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: Relation to lifestyle and bone markers. J Bone Miner Res. 2000;15:1856–1862. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- 23.Ghannam NN, Hammami MM, Bakheet SM, Khan BA. Bone mineral density of the spine and femur in healthy Saudi females: Relation to vitamin D status, pregnancy and lactation. Calcif Tissue Int. 1999;65:23–28. doi: 10.1007/s002239900652. [DOI] [PubMed] [Google Scholar]
- 24.Mishal AA. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporosis Int. 2001;12:931–935. doi: 10.1007/s001980170021. [DOI] [PubMed] [Google Scholar]
- 25.Sadat-Ali M, Al Elq A, Al-Turki H, Al-Mulhim F, Al-Ali A. Vitamin D levels in healthy men in eastern Saudi Arabia. Ann Saudi Med. 2009;29:378–382. doi: 10.4103/0256-4947.55168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadi HF, Reed RL, Carter AO, Dunn EV, Qazaq HS, Al-Suhaili AR. Quantitative ultrasound of the calcaneus in Arabian women: relation to anthropometric and lifestyle factors. Maturitas. 2003;44:215–223. doi: 10.1016/s0378-5122(02)00339-0. [DOI] [PubMed] [Google Scholar]
- 27.Weiler HA, Leslie WD, Krahn J, Steiman PW, Metge CJ. Canadian Aboriginal women have a higher prevalence of vitamin D deficiency than non-aboriginal women despite similar dietary vitamin D intakes. J Nutr. 2007;137:461–465. doi: 10.1093/jn/137.2.461. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D status: measurement, interpretation and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natri AM, Salo P, Vikstedt T, Palssa A, Huttunen M, Kärkkäinen MU, et al. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J Nutr. 2006;136:123–127. doi: 10.1093/jn/136.1.123. [DOI] [PubMed] [Google Scholar]
- 30.Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, et al. Long-term effects of giving nursing home residents bread fortified with 125 microg (5,000 IU) vitamin D(3) per daily serving. Am J Clin Nutr. 2009;89:1132–1137. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- 31.Grant WB, Cross HS, Garland CF, Gorham ED, Moan J, Peterlik M, et al. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog Biophys Mol Biol. 2009;99:104–113. doi: 10.1016/j.pbiomolbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Grant WB. In defense of the sun: An estimate of changes in mortality rates in the United States if mean serum 25-hydroxyvitamin D levels were raised to 45 ng/mL by solar ultraviolet-B irradiance. Dermato-Endocrinology. 2009;1:207–214. doi: 10.4161/derm.1.4.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vatanparast H, Calvo MS, Green TJ, Whiting SJ. Despite mandatory fortification of staple foods, vitamin D intakes of Canadian children and adults are inadequate. J Steroid Biochem Mol Biol. 2010;121:301–303. doi: 10.1016/j.jsbmb.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 34.Zittermann A. The estimated benefits of vitamin D for Germany. Mol Nutr Food Res. 2010;54:1164–1171. doi: 10.1002/mnfr.200900494. [DOI] [PubMed] [Google Scholar]
- 35.Grant WB, Schwalfenberg GK, Genuis SJ, Whiting SJ. An estimate of the economic burden and premature deaths due to vitamin D deficiency in Canada. Mol Nutr Food Res. 2010;54:1127–1133. doi: 10.1002/mnfr.200900420. [DOI] [PubMed] [Google Scholar]
- 36.Grant WB, Schuitemaker G. Health benefits of higher serum 25-hydroxyvitamin D levels in The Netherlands. J Steroid Biochem Molec Biol. 2010;121:456–458. doi: 10.1016/j.jsbmb.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 37.Grant WB, Juzeniene A, Moan JE. Health benefit of increased serum 25(OH)D levels from oral intake and ultraviolet-B irradiance in the Nordic countries. Scandinavian J Public Health. doi: 10.1177/1403494810382473. Published online before print September 3, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–457. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 40.Holick MF. The IOM D-lemma. Public Health Nutrition. 2011;14:939–941. doi: 10.1017/S1368980011000590. [DOI] [PubMed] [Google Scholar]
- 41.Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9:709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Haq A, Rajah J, Abdel-Wareth LO. Routine HPLC analysis of vitamin D3 and D2. DIALOG, Germany. 2007;2:1–2. [Google Scholar]
- 43.Rajah J, Abdel-Wareth L, Haq A. Failure of alphacalcidol(1alpha-hydroxyvitamin D3) in treating nutritional rickets and the biochemical response to ergocalciferol. J Steroid Biochem Mol Biol. 2010;121:273–276. doi: 10.1016/j.jsbmb.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 44.Musaiger AO. Breast feeding patterns in the Arabian Gulf Countries. World Rev Nutr Diet. 1995;78:164–190. doi: 10.1159/000424480. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes LE, Webb AR, Fraser HI, Kift R, Durkin MT, Allan D, et al. Recommended summer sunlight exposure levels can produce sufficient (20 ngml/L) but not the proposed optimal (32 ngml/L) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010;130:1411–1418. doi: 10.1038/jid.2009.417. [DOI] [PubMed] [Google Scholar]
- 46.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levene H. In: In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling I. Olkin, et al., editors. Stanford University Press; 1960. pp. 278–279. [Google Scholar]