Abstract
As for other diseases of higher age, low birth weight was expected to be a risk factor for an altered bone metabolism and osteoporosis.
On the first glance this expectation appears to be confirmed by animal data: rats with intrauterine growth restriction following maternal protein malnutrition show a reduction of bone mineral density going in line with a decrease in serum vitamin D concentrations.
However, the situation is less clear in newborns with low birth weight: Some studies show a relation of birth weight and bone mineral density whereas others don't. The older the former low birth weight patients the fainter the effect seems to be. In fact young adults with idiopathic short stature have a low bone mineral density than the low birth weight group irrespective of whether they have experienced catch-up growth or not. As a consequence low birth weight is can not be identified as a relevant risk factor for hip fractures in menopausal women. Postmenopausal women with low birth weight even show higher vitamin D concentrations than normal birth weight individuals.
In conclusion, there is no consistent long term effect of low birth weight on bone mineral density or hip fracture risk later in life. Whether methodological weaknesses in the studies performed so far are causal or whether postnatal factors such as physical activity and nutrition are of higher importance can only be speculated upon at present.
Keywords: intrauterine growth restriction, low birth weight, bone mineral density, bone mineral content, fracture risk
Introduction
Low birth weight is associated with a number of diseases later in life. The best known examples are an increased prevalence of cardiovascular disease, metabolic diseases such as diabetes mellitus type 2, and renal disease.1,2 Despite the strong associations that have been found in large studies, there is a growing body of evidence that not low birth weight itself is the major risk factor for the increased morbidity but the intrauterine pathologies that lead to low birth weight. This includes placental failure and maternal diseases such as preeclampsia. On the other hand maternal conditions leading to an increased birth weight are also associated with higher morbidity later in life. Among these are maternal diabetes mellitus and obesity.
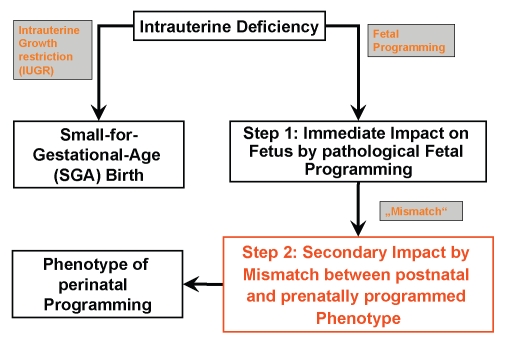
Therefore, the concept of fetal and perinatal programming of later disease has been developed (Fig. 1). It is the objective of the short review article to summarize the evidence for and impact of low birth weight and fetal programming, respectively, on bone metabolism and the risk for fractures later in life.
Figure 1.
Concept of perinatal programming of diseases of later age.
SGA, IUGR, Bone Mineral Density and Fracture Risk
Animal data.
Protein restriction has been the most widely used method for demonstrating how intrauterine growth restriction (IUGR) affects the cardiovascular system and the kidney.3–5 Although a number of mammalian models of protein restriction have been employed, most studies have been carried out in rats. In such work, pregnant rats are fed an isocaloric but protein restricted diet, varying from 10 to 40% of normal protein intake. This model mimics protein restriction, which is thought to be a frequent cause for intrauterine growth restriction in developing countries.
Animal data using the model depicted above suggest that there is a reduction in bone mineral density in animals with intrauterine growth restriction. These data were obtained by micro CT examinations.6 Interestingly, these animals also have reduced serum concentrations of vitamin D at 8, 12 and 20 weeks of life. Therefore, one might speculate that in the low protein model a lack of vitamin D might be the cause for the reduction of bone mineral density.
Human data.
Human data is restricted to children or adults with low birth weight or small for gestational age (SGA) birth. The causes of low birth weight in the clinical studies have not been well documented yet. They may include patients with real IUGR e.g., due to placental failure or preeclampsia or healthy infants with constitutional low birth weight. This may cause a bias to the studies that are described below.
In newborns with low birth weight a reduction of bone mineral density as measured by DEXA can be observed.7 However, DEXA bears the difficulty of an age-dependent systemic error. Therefore caution should be applied when looking at the data. At the age of 18–24 years the effect of low birth weight is indeed not statistically apparent any more. This is of particular interest since adolescents with idiopathic short stature have a reduction of bone mineral density in that study.8 In addition, catch-up growth, usually being a risk factor for enhanced morbidity after SGA birth did significantly reduce bone mineral density.
The hardest end point indicating an adverse effect of low birth weight on bone metabolism would be an increase fracture risk later in life. An epidemiological study, on 3,639 men and 3,447 women born between 1924 and 1933 of whom 112 had experienced hip fractures showed that birth weight, head circumference, ponderal index at birth, placental weight and length of gestation did not predict hip fracture risk.9 Men and women who were born less than 49 cm long tended to have a higher risk of hip fracture when compared with those who were born 51 cm in length or longer, but the difference just failed to attain statistical significance (hazard ratio 1.5; 95% CI 0.9–2.5). However, bearing in mind that birth length is a very crude measure even a statistical difference would have to be regarded with caution.
One other factor is of interest in this context: Postmenopausal women tend to have higher concentrations of serum vitamin D if they were born in the lower birth weight range or if they had a lower weight at the age of one year. This implies that vitamin D the deficiency that is seen in the rats with intrauterine growth restriction and reduced bone mineral density is not a potential contributing factor in humans with low birth weight.10
Significance of Other Pre- and Post-Conceptional Factors than Birth Weight
Since birth weight does not appear to be the crucial factor influencing bone mineral density, the question arises, whether other pre- or post-conceptional factors may contribute to bone mineral density later in life. One factor correlating with bone mineral content at the age of nine years appears to be maternal vitamin D level.11 However, it cannot be proven whether this is causal or whether vitamin D is simply a surrogate of a better maternal nutritional status or better education also leading to more physical activity.
It is therefore interesting that also maternal intake of magnesium, potassium and folic acid during pregnancy are associated with a higher bone mineral density and bone mineral content.12 The data is based on 4,451 children with DEXA measurements.
But also the father can potential contribute to the bone mineral content of its male newborn offspring implying that at least for boys paternal genetic or epigenetic factors may play a role.13
Significance of Growth and Muscle Strength in Childhood for Bone Metabolism
The major contributor to bone mineral density is without doubt physical activity in childhood and later on. However, this will be covered elsewhere in this issue of the Journal. Interestingly, nonetheless, is the observation that body mass index gain in childhood is one determinant of hip fracture risk later in life.14 This was examined in 6,370 Finnish women aged 60–70 years. The lowest BMI gain quartile (between 1 and 12 years of age) carried a risk ratio of 8.2 fold as opposed to the highest BMI gain quartile. Interestingly, BMI gain in the first year of life did not influence fracture risk later in life.
Conclusions
In conclusion, there is no consistent long term effect of low birth weight on bone mineral density or hip fracture risk later in life. Probably, other factors influencing bone mineral density and fracture rate such as nutrition and physical activity later in life have a substantially more decisive effect.
Abbreviations
- IUGR
intrauterine growth restriction
- SGA
small for gestational age
- BMD
bone mineral density
- BMC
bone mineral content
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dötsch J. Renal and Extrarenal Mechanisms of Perinatal Programming after Intrauterine Growth Restriction. Hypertension Res. 2009;32:238–241. doi: 10.1038/hr.2009.4. [DOI] [PubMed] [Google Scholar]
- 2.Dötsch J, Plank C, Amann K, Ingelfinger J. The implications of fetal programming of glomerular number and renal function. J Mol Med. 2009;87:841–848. doi: 10.1007/s00109-009-0507-7. [DOI] [PubMed] [Google Scholar]
- 3.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Elmes MJ, Gardner DS, Langley-Evans SC. Fetal exposure to a maternal low-protein diet is associated with altered left ventricular pressure response to ischemiareperfusion injury. Br J Nutr. 2007;98:93–100. doi: 10.1017/S000711450769182X. [DOI] [PubMed] [Google Scholar]
- 5.Plank C, Östreicher I, Hartner A, Marek I, Struwe FG, Amann K, et al. Intrauterine growth retardation aggravates the course of acute mesangioproliferative glomerulonephritis in the rat. Kidney Int. 2006;70:1974–1982. doi: 10.1038/sj.ki.5001966. [DOI] [PubMed] [Google Scholar]
- 6.Lanham SA, Roberts C, Perry MJ, Cooper C, Oreffo RO. Intrauterine programming of bone. Part 2: alteration of skeletal structure. Osteoporos Int. 2008;19:157–167. doi: 10.1007/s00198-007-0448-3. [DOI] [PubMed] [Google Scholar]
- 7.Beltrand J, Alison M, Nicolescu R, Verkauskiene R, Deghmoun S, Sibony O, et al. Bone mineral content at birth is determined both by birth weight and fetal growth pattern. Pediatr Res. 2008;64:86–90. doi: 10.1203/PDR.0b013e318174e6d8. [DOI] [PubMed] [Google Scholar]
- 8.Leunissen RW, Stijnen T, Hokken-Koelega AC. Influence of birth size on body composition in early adulthood: the programming factors for growth and metabolism (PROGRAM)-study. Clin Endocrinol (Oxf) 2009;70:245–251. doi: 10.1111/j.1365-2265.2008.03320.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Eriksson JG, Forsén T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int. 2001;12:623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 10.Arden NK, Syddall HE, Javaid MK, Dennison EM, Swaminathan R, Fall C, Cooper C. Early life influences on serum 1,25 (OH) vitamin D. Paediatr Perinat Epidemiol. 2005;19:36–42. doi: 10.1111/j.1365-3016.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 11.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 12.Tobias JH, Steer CD, Emmett PM, Tonkin RJ, Cooper C, Ness AR, ALSPAC study team Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos Int. 2005;16:1731–1741. doi: 10.1007/s00198-005-1912-6. [DOI] [PubMed] [Google Scholar]
- 13.Harvey NC, Javaid MK, Poole JR, Taylor P, Robinson SM, Inskip HM, et al. Southampton Women's Survey Study Group Paternal skeletal size predicts intrauterine bone mineral accrual. J Clin Endocrinol Metab. 2008;93:1676–1681. doi: 10.1210/jc.2007-0279. [DOI] [PubMed] [Google Scholar]
- 14.Javaid MK, Eriksson JG, Kajantie E, Forsén T, Osmond C, Barker DJ, Cooper C. Growth in childhood predicts hip fracture risk in later life. Osteoporos Int. 2011;22:69–73. doi: 10.1007/s00198-010-1224-3. [DOI] [PubMed] [Google Scholar]