Abstract
The majority of understanding of root gravity responses comes from the study of primary roots, even though lateral roots make a far greater contribution to root system architecture. The focus of this report is the analysis of gravitropic responses in lateral roots of wild-type background and pgm-1 mutants. Despite the significant reduction in gravitropic response of primary roots of pgm-1 mutants, the lateral roots of this mutant demonstrate wild-type rates of gravitropism, suggesting a significant difference in gravity signal transduction between primary and lateral roots.
Key words: gravitropism, lateral roots, pgm-1, root system architecture
Plants are extremely sensitive to numerous environmental stimuli, including touch, gravity, light and humidity, among many others. As a pervasive signal on Earth, gravity exerts a persistent influence on plant morphogenesis by directing the primary roots and shoots of most species to align parallel with the gravity vector. The vertical orientations obtained by primary organs has provided for a simple assay of gravitropic responses, and much of our understanding of gravity stimulus perception, signal transduction and differential growth response has been gained by a focus on primary organ systems.
With respect to gravity stimulus perception, there is strong evidence that the movement of starch-filled plastids plays a primary role in the detection of a change in the orientation of an organ relative to gravity.1 Consistent with this evidence, we have recently demonstrated that roots of the starchless mutant of Arabidopsis, pgm-1, respond to gravity at approximately 30% the rate of wild-type roots, and that they lack the wild-type relationship between cap angle and response rate.2 Furthermore, pgm-1 roots lack the gravity-induced gradient of auxin reported by DR5-GFP expression, found in wild-type roots, linking plastid sedimentation with the differential auxin transport thought to mediate the differential growth response.3
While our understanding of root gravitropism has grown in sophistication and detail, the emerging picture has been compiled almost entirely from observations of primary organ behavior. The degree to which our model of signaling involved in primary root gravitropic responses applies to the behavior of lateral roots is an almost entirely open question, with only a handful of studies investigating lateral root gravitropic responses.4–6 Toward that end, we have begun to explore the question of lateral root gravitropism in the overall context of root system architecture, and wish to report here on the gravitropic response of lateral roots in wild-type and pgm-1 genetic backgrounds.
Lateral Roots of pgm-1 Mutants Show Normal Gravitropism
As part of an ongoing project in our lab investigating the interaction of nutrient sensing and gravitropic responsiveness, we performed a gravity response assay on lateral roots of wild-type (Col-0) and starchless (pgm-1) genotypes. To collect data on entire root systems, we grew seedlings on 100 mm petri plates containing standard growth media, consisting of ½-strength Murashige & Skoog media,7 0.8% (w/v) agarose and 1% (w/v) sucrose. We captured images of 10- to 12-d-old seedling root systems at 20 min intervals with digital cameras under dimly lit conditions consisting of 1–5 µmol m−2 s−1 fluorescent white light. Root tip angles were determined with the ImageJ image analysis software.8
Due to the lack of starch production in the pgm-1 mutant and its dramatic effect on gravitropism in primary roots, we predicted a similar reduction in gravitropism of lateral roots. However, we observed no difference in gravitropism in lateral roots of pgm-1 compared to the wild-type (Fig. 1). One possible explanation for this result is that the lateral roots of pgm-1 contain starch-filled plastids, in contrast with primary roots, but we were unable to detect any starch in pgm-1 lateral root caps by I2KI staining (data not shown), nor was any difference in plastid sedimentation reported between primary and lateral roots of pgm-1 mutants.4 Another possibility is that the ambient lighting necessary for image capture was inducing a phototropic response, causing us to overestimate the gravitropic response. However, analysis of the gravitropic response of the primary roots in these and other experiments is inconsistent with this explanation. Thus, while we cannot entirely eliminate the possibility that phototropism may be contributing to the gravitropic response of lateral roots reported here, the results imply that lateral roots would need to be more sensitive to light than primary roots, a prerequisite for which there is no evidence.
Figure 1.
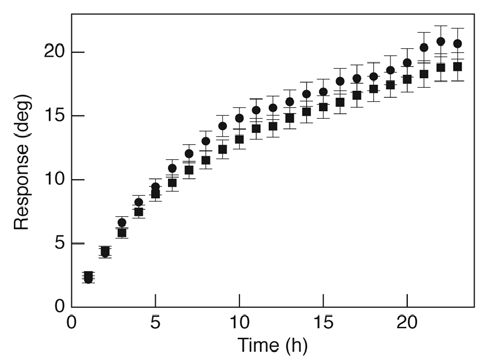
Gravitropic responses of Col-0 (squares) and pgm-1 (circles) lateral roots. Seedlings (10- to 12-d-old) were gravistimulated by 45° and images of entire root systems were captured and analyzed as described in the text. Each point represents the mean lateral root tip angle for the given time point (n = 397 for Col-0; n = 438 for pgm-1). Error bars represent standard error of the mean.
Previous studies have investigated lateral root gravitropism in pgm-1 mutants, but not in the absence of a strong phototropic signal.4 In addition, the data presented here constitute the average of all actively-elongating lateral roots, rather than a subset within a particular size class.4 Taken together with our recent findings,2 the data reported here argue strongly for an alternate pathway for gravity perception in roots, one that persists in the absence of starch and therefore plastid sedimentation. Moreover, the finding that lateral root gravitropism appears to be completely intact in the pgm-1 mutant indicates that plastid sedimentation may play little or no role in gravity perception in lateral roots.
Acknowledgments
The authors acknowledge support from the Ohio Wesleyan University Summer Science Research Program and the American Society of Plant Biologists Summer Undergraduate Research Fellowship program.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- 2.Wolverton C, Paya A, Toska J. Root cap angle and gravitropic response rate are uncoupled in the Arabidopsis pgm-1 mutant. Physiol Plant. 2011;141:373–382. doi: 10.1111/j.1399-3054.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 3.Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA. 2003;100:2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiss JZ, Miller KM, Ogden LA, Roth KK. Phototropism and gravitropism in lateral roots of Arabidopsis. Plant Cell Physiol. 2002;43:35–43. doi: 10.1093/pcp/pcf017. [DOI] [PubMed] [Google Scholar]
- 5.Mullen JL, Wolverton C, Hangarter RP. Apical control, gravitropic signaling and the growth of lateral roots in Arabidopsis. Adv Space Res. 2005;36:1211–1217. [Google Scholar]
- 6.Kuya N, Kato M, Sato Y, Kaneta T, Sato S. Comparative study of cellular structures implicated in gravisensing in statocytes of primary and lateral roots of Vigna angularis. Protoplasma. 2006;229:83–91. doi: 10.1007/s00709-006-0188-9. [DOI] [PubMed] [Google Scholar]
- 7.Murashige T, Skoog FK. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 8.Rasband WS. ImageJ. Bethesda, MD USA: US National Institutes of Health; Available at: imagej.nih.gov/ij/ [Google Scholar]


