Abstract
Signal transduction through MAPK cascades is essential for eukaryotic cell response to various extracellular stimuli, such as the induction of innate immune responses. Arabidopsis thaliana relies in particular on three of its 20 MAPKs, MPK3, -4, -6, for a proper immune response. Recently we showed that one MPK4-substrate, MKS1, is required for basal resistance against the virulent Pseudomonas syringae and the oomycete Hyaloperonospora arabidopsidis. Overexpression of MKS1 (35S-MKS1) led to increased resistance to the same pathogens but also to an increased susceptibility toward the fungi Botrytis cinerea. MKS1 interacts with the transcription factor WRKY33, which in turn controls the regulation of PAD3 and CYP71A13, two genes, required for proper resistance to B. cinerea. Therefore, we tested if the increased susceptibility toward B. cinerea from 35S-MKS1 was due to deregulation of WRKY33 targets. PAD3 and CYP71A13 expression is similar in 35S-MKS1 and WT after B. cinerea treatment suggesting another mechanism controls 35S-MKS1 susceptibility.
Key words: Arabidopsis, MAPK cascade, innate immune response, Botrytis cinerea, lesion mimic mutant, resistance genes, transcription
MPK4 is part of a signaling cascade that consists of MEKK1 and MKK1/MKK2. Deletion mutants of mekk1,1,2 mkk1/mkk2,3 double knockout and mpk4,4 share a dwarf phenotype and MEKK1 and MKK1/MKK2 are required for proper MPK4 activation.1–3 MKS1 was identified in a yeast-two-hybrid screen for interactors of MPK4 and found to be an MPK4 substrate and in vivo partner.5 Importantly, deletion of MKS1 abrogates some of the mpk4 phenotypes.6,7
We recently found that MPK4-MKS1-WRK33 exist in a nuclear localized complex. Activation of MPK4, for example by bacterial elicitors, leads to MKS1 phosphorylation and dissociation of WRKY33 from MPK4 complexes. Subsequently, WRKY33 binds to the promoter of PAD3 and promote its expression.7 A recent publication confirmed that WRKY33 binds the PAD3 promoter.8 In addition, Mao et al. find that WRKY33 is a substrate of especially MPK6 and to a lower extent MPK3 and MPK4 (most strongly in the absence or MPK3 and MPK6).8
Expression of PAD3 and CYP71A13 is required for the production of the antimicrobial compound camalexin, necessary for proper defense against B. cinerea.9 Because overexpression of MKS1 leads to increased susceptibility to B. cinerea6 and MKS1 and WRKY33 interact in planta and are regulated by MAPK activity, it is possible that 35S-MKS1 susceptibility is caused by deregulation of WRKY33 targets.
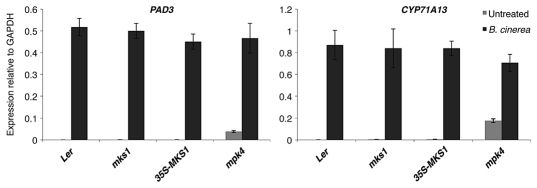
We therefore tested the expression of the WRKY33 targets PAD3 and CYP71A13 in Ler (wild type control), 35S-MKS1, mks1 and mpk4 before and 48 h after infection with B. cinerea. Interestingly, there was no difference in the expression of these two genes between 35S-MKS1 and wild type (Fig. 1). In accordance with previously published data, mpk4 accumulates PAD3 and CYP71A13 transcripts even in absence of infection.5,7
Figure 1.
Real-time PCR detection of PAD3 and CYP71A13 on untreated controls or plants treated with B. cinerea for 48 h. Samples were tested in triplicate and normalized to GAPDH. Means ± SD is shown.
Since MPK4 is activated within minutes and releases WRKY33 to bind the promoter of PAD3 within hours and not days, we tested whether a difference in the early response to B. cinerea might explain the different susceptibilities. Real time PCR on cDNA from RNA extracted at time points between 2–12 h after B. cinerea treatment did not reveal a strong consistent differential response in 35S-MKS1 compared with Ler and mks1 (data not shown).
It is perhaps not surprising that expression of the 35S-MKS1 construct does not lead to reduced PAD3 and CYP71A13. The model proposed by Qiu et al.7 would project that excess MKS1 leads to less WRKY33 sequestered in a complex with MPK4. With recent results from Mao et al. one might suspect more WRKY33 to be readily available for MPK3,(4),6 phosphorylation. However, we do not see consistently higher PAD3, CYP71A13 transcript levels upon induction in 35S-MKS1. Moreover higher levels of PAD3, CYP71A13 in 35S-MKS1 would not explain its susceptibility phenotype.
Like constitutive-defense/lesion-mimic mutants, 35S-MKS1 plants are semi-dwarfed with occasional lesions, accumulate PR1 transcript accompanied with increased levels of the plant hormone salicylic acid (SA) and exhibit increased resistance toward Pseudomonas syringae pv. tomato DC3000.5,6
Although important, camalexin is not the only determinant in B. cinerea susceptibility.10 Other factors include e.g., JA/ethylene signaling.11 Methyl-JA treatment of 35S-MKS1 leads to increased levels of the JA marker gene PDF1.2.5 Although PDF1.2 induction appears to be slightly impaired upon B. cinerea infection6 there is no block in JA/ethylene signaling and this modest decrease is probably not the reason for the enhanced susceptibility.
It has been observed that induction of cell death through the hypersensitive response can facilitate B. cinerea infection.12 This suggests that the lesions sometimes observed in 35S-MKS1 might be the cause of the enhanced susceptibility and that perhaps the susceptibility is an indirect effect. Suppressor of salicylic acid insensitive 2 (ssi2) is a lesion-mimic and B. cinerea-susceptible mutant. SSI2 encodes a stearoyl-ACP desaturase.13 Additionally, like 35S-MKS1, ssi2 is smaller than wild type, accumulates PR1 transcript and shows enhanced resistance to virulent strains of Pseudomonas syringae.13,14 Surprisingly, B. cinerea susceptibility in ssi2 seems to be independent of its lesions. The double mutants ssi2/pad4 and ssi2/eds5 partially rescue ssi2 size but still have spontaneous cell death and are much more resistant to B. cinerea than the single mutant.14
The ssi2 mutant has low levels of oleic acid (18:1) and its phenotype can be rescued by addition of oleic acid. Recently, it has been shown that the low levels of oleic acid leads to induction of Resistance-gene (R-gene) expression dependent on EDS1 and SA production (through SID2).15,16 The authors propose that the accumulation of various R-genes and activation thereof is responsible for ssi2 altered defense-related phenotype.
Whether 35S-MKS1-phenotype is due to aberrant R-gene activation remains to be tested. If R-gene activation causes both the 35S-MKS1 and ssi2 phenotypes presumably the properties of the specifically activated R-gene(s) result in their secondary phenotypes such as B. cinerea susceptibility.
Abbreviations
- SA
salicylic acid
- B. cinerea
Botrytis cinerea
- Pst DC3000
Pseudomonas syringae pv tomato DC3000
- MAPK
mitogen activated protein kinase
- R-gene
resistance genes
- JA
jasmonate
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J Biol Chem. 2006;281:36969–36976. doi: 10.1074/jbc.M605319200. [DOI] [PubMed] [Google Scholar]
- 2.Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H. A Mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 3.Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, et al. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4 and MKS1. Plant Physiol. 2008;148:212–222. doi: 10.1104/pp.108.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 5.Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NH, Zhu S, et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005;24:2579–2589. doi: 10.1038/sj.emboj.7600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen K, Qiu JL, Lutje J, Fiil BK, Hansen S, Mundy J, et al. Arabidopsis MKS1 is involved in basal immunity and requires an intact N-terminal domain for proper function. PLoS One. 2010;5:14364. doi: 10.1371/journal.pone.0014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008;27:2214–2221. doi: 10.1038/emboj.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23:1639–1653. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomma BP, Nelissen I, Eggermont K, Broekaert WF. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999;19:163–171. doi: 10.1046/j.1365-313X.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313X.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- 11.Mengiste T, Laluk K, AbuQamar S. Mechanisms of Induced Resistance Against B. cinerea. In: Prusky D, Gullino ML, editors. Postharvest Pathology. Netherlands: Springer; 2010. pp. 13–30. [Google Scholar]
- 12.Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:7517. doi: 10.1016/S0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 13.Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nandi A, Moeder W, Kachroo P, Klessig DF, Shah J. Arabidopsis ssi2-conferred susceptibility to Botrytis cinerea is dependent on EDS5 and PAD4. Mol Plant Microbe Interact. 2005;18:363–370. doi: 10.1094/MPMI-18-0363. [DOI] [PubMed] [Google Scholar]
- 15.Chandra-Shekara AC, Venugopal SC, Barman SR, Kachroo A, Kachroo P. Plastidial fatty acid levels regulate resistance gene-dependent defense signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:7277–7282. doi: 10.1073/pnas.0609259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venugopal SC, Jeong RD, Mandal MK, Zhu S, Chandra-Shekara AC, Xia Y, et al. Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet. 2009;5:1000545. doi: 10.1371/journal.pgen.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]