Abstract
The root system is particularly affected by unfavorable conditions because it is in direct contact with the soil environment. Casparian strips, a specialized structure deposited in anticlinal walls, are characterized by the impregnation of the primary wall pores with lignin and suberin. The Casparian strips in the endo- and exodermis of vascular plant roots appear to play an important role in preventing the non-selective apoplastic bypass of salts into the stele along the apoplast under salt stress. However, only a few investigations have examined the deposition and function of these apoplastic barriers in response to salt stress in higher plants.
Key words: Casparian strip, chemical components, development, root
Introduction
Roots are exposed to the soil directly to enable the efficient uptake of water and dissolved nutrients. However, the simultaneous uptake of unwanted or toxic solutes, such as during elevated salt stress, from the cortex into the stele must be prevented. To prevent this uptake, the vascular tissues must be protected from various environmental stresses, and the developmental changes that occur in response to environmental changes are still poorly understood. Therefore, better understanding the developmental and morphological changes in root structure that protect them against the excessive influx of salt is of great importance. This may also be promising for screening or generating salt-tolerant varieties of important crop plants.
Solutes and water move radially through the roots via a combination of apoplastic, symplastic and transcellular pathways.1 The apoplastic pathway is blocked by hydrophobic barriers in both the endo- and exodermis,2,3 while the latter two pathways require solutes to cross plasma membranes at least twice. Furthermore, the dense cytoplasm in the meristematic root zone in the root tip also forms a major barrier to solutes.
Casparian strips are specially modified primary carbohydrate cell walls, in which the radial parts of the cell walls are characterized by the deposition of lignin and suberin in the primary cell wall and middle lamella (Fig. 1A–H).4,5 Consequently, the primary walls become encrusted and sometimes thickened by the continuous deposition of phenolic and lipophilic biopolymers, blocking the submicroscopic capillaries in the primary cell wall and reducing apoplastic transport. Most importantly, the cell membrane of the endodermal cell is tightly attached to the primary cell wall at the Casparian strips, and it does not detach from the cell wall even if the tissue is exposed to severe plasmolysis. Therefore, the endodermal membrane together with the Casparian strips forms a tight barrier, which regulates the apoplastic pathway, and solutes must move through the selectively permeable plasma membrane into the cytoplasm.
Figure 1.
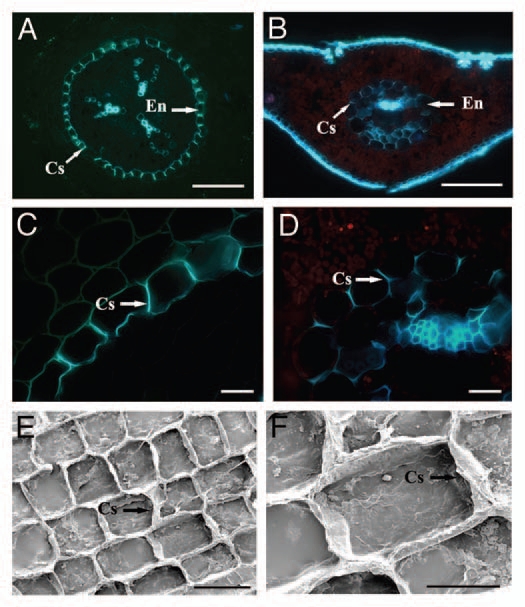
Fluorescence and scanning electron micrographs of freehand cross-sections of 2-y-old Pinus bungeana primary roots (A, C, E and F) and needles (B and D). (A) Fluorescence micrographs of freehand cross-sections of a developing primary root (3 mm behind the root tip) of Pinus bungeana sequentially stained with berberine hemisulfate and toluidine blue. Scale bar = 100 µm. (B) Cross-section of a wounded needle treated with berberine hemisulfate and potassium thiocyanate. Scale bar = 200 µm. (C) Detail of the cross-section of a developing primary root, showing the Casparian strips already occupying most of the radial walls. Scale bar = 20 µm. (D) Detail of the cross-section of a wounded needle. Scale bar = 20 µm. (E) Isolated Casparian strips from a primary root of a P. bungeana sapling. Radial and transversal walls have resisted the enzymatic digestion. Scale bar = 100 µm. (F) Magnification of (E), showing the characteristic undulation of the Casparian strips. Scale bar = 20 µm. Cs, Casparian strip; En, endodermis.
Chemical Composition and Basic Functions of Casparian Strips
The compounds deposited in the Casparian strips are suberin, lignin and some structural proteins, which are capable of reducing the diffusive apoplastic flow of water and solutes into the stele. The endodermis of all vascular plants and the exodermis of many angiosperms develop Casparian strips that are deposited in the transverse and radial walls.3,5,6 This specialized structure is also found in the needles of gymnosperms, but they are more solute-permeable than the endodermal transport barriers in roots (Fig. 1B and F).7
Several approaches have been used to better understand the structure and chemical composition of these specialized cell walls in many plant species.8–10 A gas chromatography study showed that lignin was one of the major biopolymers in the Casparian strips of Clivia miniata roots,11 while a different study comparing rice with corn demonstrated that rice roots show reduced apoplastic water permeability than corn roots, although the amount of suberin deposited in the root cell walls was not correlated with the differences in water and ion transport.12 The characterization of the chemical composition of endodermal and hypodermal cell walls isolated from seven monocotyledonous and three dicotyledonous plant species indicated that isolated Casparian strips (primary endodermis) were strongly lignified. In addition, they contained a high carbohydrate content and significant amounts of cell wall protein,9 and the amounts of these biopolymers varied with the endodermal developmental stage. The endodermal and hypodermal cell walls of roots also contained varying amounts of suberin, lignin, cell wall proteins and carbohydrates depending on the species. In histochemical and biochemical studies of apoplastic barriers in rice (Oryza sativa L.) roots, Kotula et al. examined the correlation between radial oxygen loss and the root porosity of plants grown under either aerated or deoxygenated (stagnant) conditions. They found that the radial oxygen loss was effectively reduced by the formation of a suberised exodermis or lignified sclerenchyma in the outer part of the rice root.13 The relative contributions of suberin and lignin to barrier formation, however, are still unclear.
A few efforts have been made to analyze how the chemical composition of apoplastic barriers changes in response to environmental changes. Castor bean plants (Ricinus communis L.) were shown to reinforce their apoplastic transport barriers in roots in response to NaCl stress,14 while the development of Casparian bands was also observed in response to salinity in maize and cotton.5,15 In addition, the barrier to radial oxygen loss and to Fe2+ uptake in rice increased after exposing the roots to organic acids and sulphide.16,17 Since bypass flow is reported to be the major pathway of Na+ entry into the shoots in rice,18–20 characterization of the apoplastic barriers in rice roots is of additional importance. Using the enhanced suberin1 (esb1) mutant, Baxter et al. correlated suberin in the root with both water movement through the plant and solute accumulation in the shoot in Arabidopsis thaliana. Esb1 mutants had reduced daytime transpiration rates and increased water-use efficiency during their vegetative growth period. Paralleling these changes in suberin and water transport, reduced amounts of ions accumulated (Na+, Ca2+, K+, Zn2+ and others) in the shoot, providing direct genetic evidence of the critical roles of suberin in controlling both water and mineral ion uptake and transport to the leaves.21
Potential Functions of the Casparian Strip in Salt Tolerance
Salinity is one of the major environmental stresses influencing crop yields worldwide, and keeping the apoplastic Na+ uptake low is crucial for the survival of most plants.6,19 Accumulating evidence indicates that plants have developed different strategies to minimise the access of salt to the stelar tissues by sequestering/excreting Na+ into the intracellular and extracellular compartments away from the cytosol.19,22,23 In parallel, plants react to different environmental factors (such as salinity and drought), reinforcing the level of their apoplastic barriers in roots.15,24
In terms of the relationship between the development of endo- and exodermal Casparian strips and the growth of roots under stress, studies have demonstrated that the distance of Casparian strip formation from the root tip decreases significantly in maize roots under osmotic stress and in cotton roots under salt stress.15,24 This may be attributable to the decrease in cell number and length of cells in the endo- and exodermis between the root tip and the earliest position of strip formation, although the mechanism leading to Casparian strip development closer to the root tip under stress remains unknown. Reinhardt and Rost (1995) concluded that salinity can accelerate the formation of Casparian strips, but the length and number of cells may depend on the cell division rate, cell elongation rate and time required for formation of the strip in individual cells. Therefore, one cannot simply conclude that Casparian strip development is accelerated exclusively based on the observation that the strip forms closer to the root tip.
Studies have shown that different unfavorable conditions may induce potential alterations in root architecture and structure.9,15,25 Azaizeh et al. reported that salinity reduced the hydraulic conductivity of roots, which could have been attributable to the symplastic and transcellular pathway (cell-to-cell pathway). However, hydrostatic experiments in maize have shown that the radial flow of water appears to be predominantly apoplastic.26 Therefore, the importance of the apoplastic path in water and NaCl uptake cannot be ignored.27 Changes in the morphology and development of the Casparian strip might also be important for regulating hydraulic conductivity in roots. Differences in the hydraulic conductivity of maize roots caused by a variety of growth conditions were shown to be due to differences in the development of exodermal Casparian strips and suberin lamellae, and concomitant changes in the suberin and lignin levels were also detected.28
Shannon et al. showed that salinity leads to an increase in suberisation levels in the hypo- and endodermis paralleled by Casparian strip formation.25 Similar results were also found in castor bean, in which the degree of suberisation in the endodermal and hypodermal cell walls increased significantly in the presence of NaCl.14 In addition, salinity can induce exodermis formation in cotton seedling roots.15 The effects of different unfavorable environmental factors, such as salt (NaCl), osmotic (PEG) and heavy metals (Cd) stress, on the chemical composition of the exodermal cell walls of hydroponically grown corn showed that the qualitative composition of the exodermal suberin and lignin was not altered.9 Nevertheless, in all cases, the absolute amounts of suberin and lignin increased by 1.5- to 3-fold compared with the control, indicating that elevated levels of suberisation and lignification in the exodermis can lead to greater resistance, thereby reducing the uptake of salts or other toxic solutes into the stele. Furthermore, salt stress was also reported to activate lignin deposition and reduce the apoplastic transport in soybean roots, a phenomenon that may either strengthen the Casparian strip or provide an alternative hydrophobic barrier to bypass flow.29 Using ion element analysis and X-ray microanalysis, Peng et al. reported the K+/Na+ selectivity of potassium channels and the existence of an apoplastic barrier in the salt-tolerance mechanism of alkali grass (Puccinellia tenuiflora) compared with wheat (Triticum aestivum L.). The Casparian bands in the endodermis led to a lateral gradient in K+ and Na+ across root tissues, resulting not only in a high [K+] in the shoot, but also to a large [Na+] gradient between the root and shoot.30
Conclusions
Higher plants have developed various mechanisms to deal with Na+ uptake and transport into shoots under saline stress. Casparian strips form a barrier to the apoplastic flux, forcing ions to pass through the selectively permeable plasma membrane into the cytoplasm, rather than move along the cell wall. Since the strip density, amounts of suberin and lignin and hydrophobic components of the strip are thought to affect the efficacy of Casparian strip as a barrier, further efforts should be expended to determine whether modifications in the endodermal cell walls in response to salt stress affect the radial transport properties of roots. Investigating how the width of the Casparian strips is determined in response to salinity and which proteins are deposited in Casparian strips would also be useful. This could be relevant for biotechnological improvements in crop productivity in various stressful habitats.
Acknowledgments
This work was supported by an International Science and Technology Cooperation Program from Ministry of Science and Technology of China (2007DFA30770), an international cooperation project from Sino-German Center (GZ616), grants from Ministry of Agriculture of China (2009ZX08009-011B and 2009ZX08009-095B) and a grant from the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCXR-EW-J-1).
References
- 1.Steudle E. Water uptake by roots: effects of water deficit. J Exp Bot. 2000;51:1531–1342. doi: 10.1093/jexbot/51.350.1531. [DOI] [PubMed] [Google Scholar]
- 2.Enstone DE, Peterson CA, Ma F. Root endodermis and exodermis: structure, function and responses to the environment. J Plant Growth Regul. 2002;21:335–351. doi: 10.1007/s00344-003-0002-2. [DOI] [Google Scholar]
- 3.Ma F, Peterson CA. Current insights into the development, structure and chemistry of the endodermis and exodermis of roots. Can J Bot. 2003;81:405–421. doi: 10.1139/b03-042. [DOI] [Google Scholar]
- 4.Yokoyama M, Karahara I. Radial widening of the Casparian strip follows induced radial expansion of endodermal cells. Planta. 2001;213:474–477. doi: 10.1007/s004250100536. [DOI] [PubMed] [Google Scholar]
- 5.Karahara I, Ikeda A, Kondo T, Uetake Y. Development of the Casparian strip in primary roots of maize under salt stress. Planta. 2004;219:41–47. doi: 10.1007/s00425-004-1208-7. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.) Planta. 2009;230:119–134. doi: 10.1007/s00425-009-0930-6. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Lin JX, Lin QQ, Wang J, Schreiber L. Casparian strips in needles are more solute-permeable than endodermal transport barriers in roots of Pinus bungeana. Plant Cell Physiol. 2005;46:1799–1808. doi: 10.1093/pcp/pci194. [DOI] [PubMed] [Google Scholar]
- 8.Zeier J, Schreiber L. Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata. Plant Physiol. 1997;113:1223–1231. doi: 10.1104/pp.113.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeier J, Schreiber L. Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of 5 monocotyledoneous species: chemical composition in relation to fine structure. Planta. 1998;206:349–361. doi: 10.1007/s004250050410. [DOI] [Google Scholar]
- 10.Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: composition of endodermal and hypodermal cell walls. J Exp Bot. 1999;50:1267–1280. doi: 10.1093/jexbot/50.337.1267. [DOI] [Google Scholar]
- 11.Schreiber L. Chemical composition of Casparian strips isolated from Clivia miniata Reg. roots: evidence for lignin. Planta. 1996;199:596–601. doi: 10.1007/BF00195192. [DOI] [Google Scholar]
- 12.Schreiber L, Franke R, Hartmann KD, Ranathunge K, Steudle E. The chemical composition of suberin in apoplastic barriers affects radial hydraulic conductivity differently in the roots of rice (Oryza sativa L. cv. IR64) and corn (Zea mays L. cv. Helix) J Exp Bot. 2005;56:1427–1436. doi: 10.1093/jxb/eri144. [DOI] [PubMed] [Google Scholar]
- 13.Kotula L, Ranathunge K, Schreiber L, Steudle E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot. 2009;60:2155–2167. doi: 10.1093/jxb/erp089. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber L, Franke R, Hartmann K. Effects of NO3 deficiency and NaCl stress on suberin deposition in rhizo- and hypodermal (RHCW) and endodermal cell wall (ECW) of castor bean (Ricinus communis L.) roots. Plant Soil. 2005;269:333–339. doi: 10.1007/s11104-004-0721-6. [DOI] [Google Scholar]
- 15.Reinhardt DH, Rost TL. Salinity accelerates endodermal development and induces an exodermis in cotton seedling roots. Environ Exp Bot. 1995;35:563–574. doi: 10.1016/0098-8472(95)00015-1. [DOI] [Google Scholar]
- 16.Armstrong J, Armstrong W. Rice and Phragmites: effects of organic acids on growth, root permeability and radial oxygen loss to the rhizosphere. Am J Bot. 2001;88:1359–1370. doi: 10.2307/3558443. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong J, Armstrong W. Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake and lateral root emergence. Ann Bot. 2005;96:625–638. doi: 10.1093/aob/mci215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo AR, Yeo ME, Flowers TJ. The contribution of an apoplastic pathway to sodium uptake by rice roots in saline conditions. J Exp Bot. 1987;38:1141–1153. doi: 10.1093/jxb/38.7.1141. [DOI] [Google Scholar]
- 19.Anil VS, Krishnamurthy P, Kuruvilla S, Sucharitha K, Thomas G, Mathew MK. Regulation of the uptake and distribution of Na+ in shoots of rice (Oryza sativa) variety Pokkali: role of Ca2+ in salt tolerance response. Physiol Plant. 2005;124:451–464. doi: 10.1111/j.1399-3054.2005.00529.x. [DOI] [Google Scholar]
- 20.Gong HJ, Randall DP, Flowers TJ. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 2006;29:1970–1979. doi: 10.1111/j.1365-3040.2006.01572.x. [DOI] [PubMed] [Google Scholar]
- 21.Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz J, Muthukumar B, et al. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet. 2009;5:1000492. doi: 10.1371/journal.pgen.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochica H, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 2005;10:615–620. doi: 10.1016/j.tplants.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Perumalla CJ, Peterson CA. Deposition of Casparian bands and suberin lamellae in the exodermis and endodermis of young corn and onion roots. Can J Bot. 1986;64:1873–1878. doi: 10.1139/b86-248. [DOI] [Google Scholar]
- 25.Shannon MC, Grieve CM, Francois LE. Whole plant response to salinity. In: Wilkinson RE, editor. Plant environment interactions. New York USA: Marcel Dekker; 1994. pp. 199–244. [Google Scholar]
- 26.Frensch J, Steudle E. Axial and radial hydraulic resistance to roots of maize (Zea mays L.) Plant Physiol. 1989;91:719–726. doi: 10.1104/pp.91.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azaizeh H, Gunse B, Steudle E. Effects of NaCl and CaCl2 on water transport across root cells of maize (Zea mays L.) seedlings. Plant Physiol. 1992;99:886–894. doi: 10.1104/pp.99.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann HM, Hartmann K, Schreiber L, Steudle E. Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of maize roots (Zea mays L.) Planta. 2000;210:302–311. doi: 10.1007/PL00008138. [DOI] [PubMed] [Google Scholar]
- 29.Goyal SS, Sharma SK, Rains DW. Crop production in saline environments: global and integrative perspectives. New York: Haworth Press; 2003. pp. 131–162. [Google Scholar]
- 30.Peng YH, Zhu YF, Mao YQ, Wang SM, Su WA, Tang ZC. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+ J Exp Bot. 2004;55:939–949. doi: 10.1093/jxb/erh071. [DOI] [PubMed] [Google Scholar]


