Abstract
Dehydrins (DHNs), or group 2 LEA (Late Embryogenesis Abundant) proteins, play a fundamental role in plant response and adaptation to abiotic stresses. They accumulate typically in maturing seeds or are induced in vegetative tissues following salinity, dehydration, cold and freezing stress. The generally accepted classification of dehydrins is based on their structural features, such as the presence of conserved sequences, designated as Y, S and K segments. The K segment representing a highly conserved 15 amino acid motif forming amphiphilic a-helix is especially important since it has been found in all dehydrins. Since more than 20 y, they are thought to play an important protective role during cellular dehydration but their precise function remains unclear. This review outlines the current status of the progress made toward the structural, physico-chemical and functional characterization of plant dehydrins and how these features could be exploited in improving stress tolerance in plants.
Key words: abiotic stress, dehydration stress, drought, cold acclimation, freezing tolerance, LEA proteins, dehydrins
Introduction
To face various abiotic stress factors, such as drought, heat, freezing or salinity, plants as sessile organisms, have evolved special mechanisms, to prevent loss of intracellular water, i.e., dehydration. The high accumulation of late embryogenesis abundant (LEA) proteins was described as the most common mechanism developed against water stress. The LEA proteins discovered in cotton more than two decades ago,1 were originally described to be expressed at high levels during the later stages of embryo development comprising up to 4% of cellular proteins.2 Dehydrins (DHNs) constitute a distinct biochemical group of LEA proteins, which is known as group 2 LEA (or LEA II) proteins3,4 or LEA-D11 proteins.5 These proteins are known to accumulate during late embryogenesis or can be induced in vegetative tissues by various stress factors that cause cell dehydration (i.e., drought, salinity, cold, heat etc.). Because the expression of many DHNs increases by the phytohormone abscisic acid (ABA), they are also referred as RAB proteins (Responsive to ABA). Like all LEA proteins, DHNs are ubiquitous among various plant species belonging to angiosperms and gymnosperms (reviewed in refs 6 and 7) and perhaps other photosynthetic organisms, including ferns, mosses, algae and cyanobacteria.8 They are believed to play an important protective role in plant cell during dehydration. This review will focus on the most relevant advances in understanding the structure-function relationship within DHNs and on their potential use in improving stress tolerance in plants.
Dehydrins: Undefined Structure for Multiple Functions
DHNs are characterized by wide range of molecular masses from 9–200 kD.9,10 As their sequence characteristics became available, DHNs were redefined on the basis of their motifs and newly defined as proteins possessing at least one copy of a conserved sequence known as K-segment in their molecules.6,8 The K-segment is a lysine-rich amino acid (aa) sequence (EKK GIM E/DKI KEK LPG) present in 1–11 copies near the C terminus of dehydrin molecules. DHNs can also possess other conserved motifs: the tyrosine-rich Y-segment [consensus (V/T)D(E/Q) YGNP] near the N-terminus and the serine-rich S-segment formed by a stretch of 4–10 serine residues, which are a part of a conserved sequence LHRSGS4–10(E/D)3. The S-segment can undergo phosphorylation by the casein kinase 2 (CK2).7,11 According to the presence of the K-, S- and Y-segments, DHNs can be divided into five structural subgroups: Kn, SKn, KnS, YnKn and YnSKn.6,8,12
In aqueous solutions, DHN molecules are present in the conformation of random coil, i.e., they form a maximum of hydrogen bonds with neighboring water molecules (intermolecular hydrogen bonds) and a minimum of hydrogen bonds between different aa residues (intramolecular hydrogen bonds). Due to low proportion of intramolecular hydrogen bonds, DHNs appear unstructured and share indeed many features with other types of intrinsically disordered/unstructured proteins (IDPs/IUPs).13,14 Accordingly they contain high proportions of hydrophilic aa and change their conformation according to the changes in their ambient microenvironment. Based on several experimental studies, it was confirmed that the decrease in dehydrin hydration status (loss of water molecules in their ambient microenvironment) or addition of high amounts of compatible solutes (i.e., glycerol), detergents (i.e., SDS) or salts (i.e., NaCl) into a dehydrin aqueous solution, leads to conformational changes which can be monitored by the technique of far-UV circular dichroism.15–20 Under reduced hydration, the K-segments adopt α-helical conformation similar to class A2 amphipathic α-helices found in apolipoproteins and α-synucleins.21 When α-helix is formed within a K-segment, negatively charged aa (with acidic pI, e.g., D and E) lie on one side of the helix, hydrophobic aa (nonpolar, e.g., I and L) lie on the opposite side of the helix, and positively charged aa (with basic pI, e.g., K and R) lie on the polar-non polar interface.7,22
The changes in protein conformation result also in changes in protein function. This phenomenon, which is characteristic for IDPs/IUPs, is called “moonlighting.”13,14 In the case of IDPs/IUPs, the changes in protein ambient microenvironment, such as availability of water molecules, result in protein conformational and functional changes. The amphipathic α-helices can interact with partly dehydrated surfaces of various other proteins and also with surfaces of biomembranes. It has been proposed by Ingram and Bartels4 that several K-segments in one DHN molecule can form bundles when present in α-helical conformation thus enhancing their amphipathic character in protein-protein or protein-biomembrane interactions. The binding of DHNs to the partly dehydrated surface of other proteins enhances formation of amphipathic α-helices in a DHN molecule and protects other proteins from further loss of water envelope (which can lead to irreversible changes in the protein conformation, i.e., protein denaturation). It has been suggested that these interactions between partly dehydrated surfaces of DHN molecules and other proteins and/or biomembranes (observed by Koag et al.23 in case of maize DHN1), present the basis of dehydrin protective functions. Kovacs et al.19 reported the protective activities of two dehydrin proteins isolated from Arabidopsis thaliana, ERD10 and ERD14, against thermal aggregation of citrate synthase, firefly luciferase, inactivation of lysozyme and thermal inactivation of alcohol dehydrogenase. Cryoprotective activity has been also reported for several DHNs, such as COR85 from spinach,24 WCS120 from common wheat,25 and PCA60 from peach.26 As shown by Reyes et al.27 the presence of K-segments is essential for dehydrin cryoprotective activity. In an opposite way, DHNs might also prevent heat inactivation and recently, Brini et al.28 showed that the wheat dehydrin DHN-5 improved the activity and/or thermostability of the fungal β-glucosidase (bglG) and glucose oxidase (GOD/POD) enzymes in vitro. It is therefore plausible to imagine that DHNs can act as chaperones on other proteins and help them to fold properly and/or prevent their aggregation under heat or freezing stress. However, classical chaperones not only prevent inappropriate protein aggregation but also form specific complexes with target proteins through interaction of hydrophobic patches.29 It is therefore rather difficult for DHNs to establish specific interactions with other proteins especially under dry state, that's why some authors described these dehydrin protective functions based on non-specific protein-protein interactions, as “molecular shield” effect.30
Moreover, when cells lose water, the relative distribution among intracellular complexes may change leading to undesirable interactions and aggregation/denaturation of several protein and membrane-associated complexes. As they can accumulate to relatively large amounts in various compartments inside the cells under dehydration, it has been previously proposed that DHNs may simply act as “space-fillers,” i.e., they can participate in keeping the original, non-harmful distances among intracellular complexes.11,30 Briefly, due to their unfolded state, higher accumulation and capability to bind water, DHNs can under dehydration, help in keeping the original cell volume, thus preventing cellular collapse.
Some DHNs, which contain relatively large amounts of H, R and other reactive aa residues on their surface, exhibit also reactive oxygen species (ROS) scavenging and metal ion binding properties. Both functions are mediated by direct interactions between the aa residue and the ROS species (superoxide anion radical O2−; singlet oxygen 1O2; hydroxyl radical HO−; Hydrogen peroxide H2O2) or the metal ions (Co2+; Cu2+; Fe2+; Fe3+; Ni2+; Zn2+). The interactions of the aa residue with ROS lead to oxidation of the residue, whereas the interactions with metal ions lead to the formation of covalent bonds. Binding of free metal ions prevents the intracellular compounds from excessive ROS formation since free metal ions act as catalyzers of synthesis of various ROS. DHNs can thus function also as antioxidants (e.g., CuCOR15 and CuCOR19 in Citrus unshiu),18,31 ion sequestrants [e.g., VCaB45 in celery (Apium graveolens) vacuoles where it binds Ca2+],32 or metal ion transporters in plant phloem sap [e.g., ITP protein from castor bean (Ricinus communis) which binds Fe2+ and Fe3+].33
Induction and Modification of Dehydrins in Response to Abiotic Stresses
Like other LEA proteins, DHNs accumulate to high amounts in plant embryos in later stages of their development (embryo maturation and desiccation). In vegetative tissues, they are hardly detected and their presence is limited to young plant organs and those exhibiting rapid cell division or cell elongation, e.g., root tips, elongating stems, petioles, etc.34,35 Whereas once plants are exposed to various stresses related to cellular dehydration (e.g., drought, osmotic stress, salinity, temperature), DHNs accumulate to high amounts in all vegetative tissues.36,37 Stress-inducible DHN encoding genes contain ABA-responsive elements (ABRE), C-repeat/drought-responsive/low-temperature-responsive elements (CRT/DRE/LTRE), myeloblastosis (MYB) and myelocytomatosis (MYC) regulatory elements in their promoter regions. Their expression is regulated by ABA-dependent and ABA-independent signaling pathways. ABA-dependent signaling pathways include either bZIP transcription activators named ABFs or AREBs (ABRE binding factors), which bind to ABRE elements, homologs of A. thaliana CBF4/DREB1D transcription activator, which bind to CRT/DRE/LTRE elements, and MYBFs and MYCFs, which bind to MYB and MYC promoter elements. ABA-independent signaling pathways include homologs of A. thaliana DREB2A and DREB2B transcription activators, which bind to CRT/DRE/LTRE elements (reviewed in refs 38–42).
On the other hand, some DHNs are able to undergo under stress conditions, posttranslational modifications and mainly phosphorylation.43–47 This phosphorylation occurring on the S-segment of several DHNs, was shown to be controlled in some cases by casein kinase II (CK2)-type kinases and seems to be associated to their translocation from the cytoplasm to the nucleus (e.g., RAB17 of Zea mays).43 However, nuclear localization has also been reported for some dehydrins that lack the S-segment (e.g., wheat WCS120 or peach PCA60).25,26 Nonetheless, the phosphorylation of the S segment may have significance in stress tolerance since the wheat stress-inducible dehydrin DHN-5 (YSK2) shows a differential phosphorylation pattern in two Tunisian durum wheat cultivars with contrasting tolerance to drought and salt stress.47 In other cases, phosphorylation is necessary for the cation binding properties of DHNs. The phosphorylated forms of the acidic dehydrins COR47, ERD10 and ERD14 of Arabidopsis and of the vacuole-located dehydrin VCaB45 of celery (Apium graveolens) bind Ca2+ much more efficiently than the dephosphorylated ones.32,48,49
Dehydrins as Molecular Markers of Plant Abiotic Stress Tolerance
Several comparative studies on varieties or cultivars (showing marked difference in stress tolerance) of economically important crops provide evidence for a positive correlation between DHN gene expression or DHN protein accumulation, and plant stress tolerance.
It is hence becoming evident that DHNs can be used as plant molecular marker for stress tolerance.50 Ismail et al.51 who studied chilling tolerance during the process of seedling emergence in the tropical legume crop Vigna unguiculata, revealed a positive correlation between the accumulation of DHN1 protein (Y2K-type dehydrin of 35 kDa) in seeds of a chillingtolerant line 1393-2-11, and seedling emergence at 14°C. In contrast, DHN1 was absent in the seeds of a genetically related, but chilling-sensitive line 1393-2-1. Quantitative differences in dehydrin gene expression and dehydrin protein accumulation with respect to the low-temperature stress (cold and frost) have been also largely studied in Triticeae, which are grown in temperate climates. In bread wheat (T. aestivum), Houde et al.52 have already described a correlation between the accumulation of DHN proteins belonging to the WCS120 family, and the level of plant-acquired frost tolerance. Furthermore, Vítámvás et al.53 were able to distinguish at WCS120 protein level, 20 cultivars of different frost-tolerant winter wheat's grown at 17°C or 9°C. Thus, it seems possible to use the level of WCS120 accumulation in wheat plants grown under mild cold temperatures (17–9°C) as a means for estimation of plant winter survival, hence improving the pre-screening procedures in the breeding programmes aimed at improving wheat frost tolerance.
In studies dealing with drought stress, Pelah et al.54 found a correlation between drought tolerance and accumulation of dehydrin proteins in Populus popularis. Park et al.55 have found a correlation between Dhn3 and Dhn4 transcript accumulation and several traits associated with drought tolerance (relative water content RWC, Drought yield index) in a set of Korean barley cultivars. Similarly, Labhilili et al. found a correlation between the level of dehydrin transcript accumulation and drought tolerance in two differently tolerant cultivars of durum wheat (T. turgidum ssp. durum).
Use of Dehydrins for Improvement of Plant Tolerance to Abiotic Stress
Numerous transgenic studies revealed a positive effect of dehydrin gene expression on plant stress tolerance (Table 1). Studies performed by Saavedra et al.22 on the moss Physcomitrella patens, have shown that a P. patens knockout mutant, which has its only dehydrin gene, PpdhnA, disrupted, reveals an impaired ability to recover after salt and osmotic stress. Peng et al.57 have shown that Arabidopsis transgenic plants overexpressing RcDHN5 (an SK2 acidic dehydrin from frost-tolerant Rhododendron catawbiense) show enhanced frost tolerance. Similarly, Yin et al.58 concluded that the expression of DHN24 protein from wild potato Solanum sogarandinum in cucumber (Cucumis sativus) resulted in improved frost tolerance. Similarly, studies that used a dehydrin transgene expressed in a stress-sensitive plant, have reported enhanced tolerance to stress in the transformed plant. For example, Hara et al.59 have shown that the expression of CuCOR19 from Citrus unshiu in tobacco mitochondria led to a reduced lipid peroxidation. Houde et al.60 have found out that expression of WCOR410 from common wheat in strawberry led to the enhancement of strawberry frost tolerance. Kaye et al.61 reported that tobacco plants expressing spinach CAP85 and CAP160 DHNs revealed a lower level of electrolyte leakage after a frost test, which indicates a reduction of freezing injury in the transformants. Brini et al.62 have observed that the expression of the durum wheat (Triticum turgidum ssp. durum) DHN-5 in A. thaliana led to an increase in salt and osmotic stress tolerance. Likewise, RoyChoudhury et al.63 observed enhanced tolerance to drought and salt stress in tobacco plants transformed with Rab16A (=Rab21) gene from salt-tolerant Indica rice variety Pokkali. Similarly, Cheng et al.64 have shown that overexpression of the wheat dehydrin PMA80 (as well as the LEAI protein PMA1959) enhances rice tolerance to drought and salt stress. Figueras et al.65 have also reported that A. thaliana plants transgenic for the maize Rab17 are more tolerant to osmotic stress. Park et al.55 have observed that Arabidopsis plants overexpressing barley Dhn3 and Dhn4 genes stayed green and viable on a growth medium containing 500 mM mannitol, a concentration at which wild-type plants are unable to grow.
Table 1.
Possible functions for selected plant DHNs
| Host | DHNs | Domains | Reported activity | Reported phenotype | Reference |
| Wheat | WCOR410 | SK3 | Membrane binding | Increased tolerance to cold stress | 16, 60 |
| Maize | DHN1 | YSK2 | Lipid vesicle binding (PA, PS, PG) | Increased tolerance to ABA and water stress | 23 |
| Arabidopsis | ERD10/ERD14 | SK3/SK2 | Phospholipid vesicles binding | Increased tolerance to dehydration | 19 |
| Arabidopsis | ERD10/ERD14 | SK3/SK2 | Heat protection of ADH, Luciferase, lysozyme, citrate synthase | Increased tolerance to dehydration | 19 |
| Barley | P80/DHN5 | K9 | Cryoprotection of LDH | Increased tolerance to cold stress | 81 |
| Peach | PCA 60 | Y2K9 | Cryoprotection of LDH | Increased tolerance to freeze | 26, 82 |
| Wheat | DHN5 | YSK2 | Enhanced thermostability of bglG and GOD/POD | Increased tolerance to drought and salt tress | 28, 62 |
| Citrus | CuCOR19 | SK3 | Cryoprotection of Catalase and LDH | Increased tolerance to cold stress | 18, 59 |
| Citrus | CuCOR19 | SK3 | Reduction of lipid peroxidation by scavenging peroxyl and hydroxyl radicals | Increased tolerance to cold stress | 83, 84 |
| Citrus | CuCOR15 | SK2 | Binding to Cu2+, Fe3+, Co2+, Ni2+, Zn2+ but not to Mg2+, Ca2+ and Mn2+ | Increased tolerance to cold stress | 31 |
ADH, alcohol dehydrogenase; bglG, fungal β-glucosidase; GOD, glucose oxidase; LDH, lactate dehydrogenase; PA, phosphatidic acid; PG, phosphatidyglycerol; PS, phosphatidyl-Ser; POD, peroxidase.
On the other hand, Xu et al.66 found out that expression of BjDHN2 and BjDHN3 proteins from Brassica juncea in transgenic tobacco plants resulted in higher tolerance to heavy-metal (Cd2+ and Zn2+) stress. The transgenic plants showed lower electrolyte leakage and malondialdehyde production, suggesting that BjDHN2 and BjDHN3 could enhance tolerance to heavy metals by attenuating lipid peroxidation and protecting cellular membranes.
It is worth mentioning that plants overexpressing DHNs are more tolerant to abiotic stress, is not a general rule. Several LEA proteins including dehydrins from desiccation tolerant resurrection plant Craterostigma plantagineum failed to increase drought tolerance in tobacco.67 Similarly, the overexpression of RAB18 in Arabidopsis did not improve freeze and drought tolerance.68 Whereas, the co-expression of the same gene together with another DHN (Cor47) led to improved freeze tolerance but not drought tolerance.69 These authors found also that Arabidopsis transgenics for both DHNs Lti29 and Lti30, stand better freezing conditions than lines overexpressing single DHN genes. These findings suggest that in some circumstances some DHN can act in synergistic way to improve freeze tolerance.
Dehydrins Responsive to Biotic Stresses
The role of DHNs in abiotic stress tolerance was largely documented. In contrast, whether these proteins are involved in biotic stress response remains an open question. Nonetheless, wounding was reported to induce the expression of specific DHNs such as BcDh2 of Boea crassifolia.70 It is worth to note that plant wounding which is a common biotic stress exerted by insects or herbivores is also regarded as a dehydration stress because it is associated with cellular damage that leads to water loss. Interestingly, as wounding activates jasmonate and salicylic acid stress signaling pathways, the wound-induced expression of BcDh2 is mediated by these hormonal signals. The induction by jasmonic acid and methyl jasmonate of CpDHN1 (Y2K dehydrin) from Cicer pinnatifidum and of PgDHN1 (S8K4 dehydrin) from white spruce Picea glauca was reported in references 71 and 72. Rouse et al.73 performed a promoter analysis of A. thaliana cold-inducible Kn-type dehydrin gene Lti30 (Xero 2) using GUS reporter gene and they concluded that the Lti30 promoter also displayed wounding-induction among other treatments. Sun et al.74 have observed a positive effect of low concentrations of exogenous salicylic acid (up to 0.25 mM) on the expression of drought-inducible dehydrins in barley seedlings subjected to drought stress. In contrast, higher concentrations of salicylic acid (0.25–0.50 mM) have led to the decrease of dehydrin expression under the same growth conditions (water stress).
On the other hand, Turco et al.75 have reported the expression of several dehydrin-like proteins in drought-tolerant oak species Quercus ilex in response to infection with Phytophthora cinnamomi.
Modulation of Plant Defense Responses by Dehydrins
Recently, it has been reported that in addition to its contribution in enhancing osmotic stress tolerance, the wheat DHN-5 seems to have a pleiotropic effect on stress responses in Arabidopsis.76 Transcriptome profiling revealed that DHN-5 overexpression affects the expression of not only genes involved in abiotic stress tolerance (i.e., LEA, RD29B, RAB18 and LTI30), but also those related to defense responses: several genes coding for PR (pathogenesis related) proteins were transcriptionally activated by DHN-5. Most interestingly, the authors found that DHN-5 interferes with jasmonate (JA) signaling. Overexpression of DHN-5 resulted in downregulation of genes encoding 3 members of JAZ (jasmonate-ZIM domain) proteins, which are negative regulators of JA signaling.77 However, DHN-5 transgenic plants were more resistant to JA than wild type. Moreover, as in the case of a JA insensitive mutant jai3-1, these transgenic lines showed compromised expression of a subset of JA- and wound-responsive genes (which are regulated by the MYC2 transcription factor),78 but activation of the genes responsive to pathogen responses (Fig. 1). Considering the role of JA as a key signaling molecule in defense mechanisms against pathogens, it would be therefore interesting to explore whether DHN-5 can influence (via the alteration of MYC2-dependent JA responsive genes) the level of plant resistance to pathogen attacks. It remains however unclear whether DHNs confer plant resistance to pathogens. Nevertheless, such an assumption should be well thought-out especially since few DHNs were reported to have antibacterial activities. The overexpression of an SK3-type dehydrin ERD10 of Arabidopsis in E. coli leads to a bacterial growth inhibition which seems to be linked to the K-segments.79 These observations were reinforced this year by similar findings with RR46, another SK3-type from rice which beside E. coli can inhibit the growth of a number of Gram+ bacteria.80 Interestingly, these authors showed that synthetic K-segments (i.e., KKK KGL KEK IKE KLP GHK) are still also able to exert inhibitory effects but limited to some Gram+ bacteria. They claim that amino acids in K-segments can in some cases adopt a transmembrane structure, similar to that found in other antimicrobial peptides which use this property to interact with bacterial cell membranes, and hence causing bacterial growth inhibition. Based on these DHN related antibacterial activities, it is therefore attractive to speculate that some DHNs can somehow inhibit the growth of pathogens hence improving plant resistance against them. Knowing their association to abiotic stresses, some DHNs can then act as a connection node in the cross talk between biotic and abiotic stress signaling pathways. It is generally thought that abiotic stress response is prioritized by the plant over the biotic stress response. In this respect, perhaps DHNs may be selected along evolution as part of a defense mechanism in plants against opportunistic bacterial infections usually present during water scarcity periods. Alternatively, some DHNs can via their antibacterial activity alleviate the biotic stress response even when common biotic stress signaling pathways are ineffective and therefore might serve as the last intracellular fort once the pathogen invades the plant cell.
Figure 1.
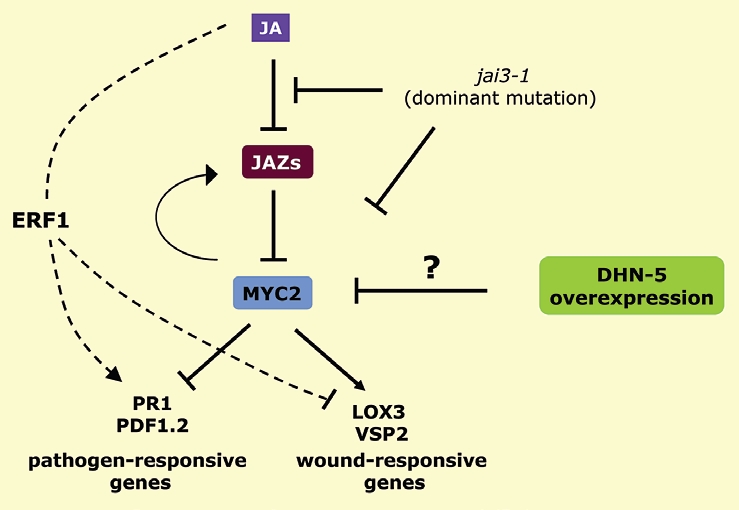
DHN-5 interferes with JA signaling pathway. Overexpression of DHN-5 causes decreased sensitivity to jasmonate (JA), and affects a subset of JA-responsive genes, downregulation of wound-induced genes and upregulation of pathogen-responsive genes in Arabidopsis thaliana. JAZ (jasmonate ZIM-domain) proteins are negative regulators of JA-signaling. JAI3 encodes a member of JAZ protein, JAZ3. Myc2 (JAI1) is a bHLH-type transcription factor. ERF1 is a transcription factor, which mediates responses to ethylene and JA, antagonistic to MYC2 function. How DHN-5 affects JA signaling is unknown. For details, see references 76–78.
Conclusions and Perspectives
With increasing data from diverse research fields, DHNs appear to be an amazingly versatile group of LEA proteins presumably due to their intrinsically unstructured character. They exhibit myriads of functions (e.g., chaperone, cryoprotective, antifreeze, radical-scavenging, ion-binding functions) when exposed to various stress factors, including drought, high-salinity stress, low temperature stress, heavy-metal stress, and perhaps also to biotic stresses. Despite the relevant progress made toward understanding the role of DHNs, the molecular mechanisms through which they can enhance stress tolerance remain unknown. Nevertheless, the recent report of Brini et al.76 provides insights into this complex question. It is plausible that some DHNs can have a regulatory function in stress responses. In the case of DHN-5, the regulatory role may be attributed to its potential capacity to shuttle between the cytoplasm and the nucleus,47 perhaps according to its phosphorylation status as was previously reported on the maize counterpart RAB17.45 Finally, these findings provide for the first time that a DHN might contribute to understanding the mechanism that regulates the plant defense responses. Future work should broadly examine other DHNs to learn whether DHN-dependent regulatory mechanisms modulate pathogen responses.
References
- 1.Galau GA, Hughes DW, Dure LIII. Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol Biol. 1986;7:155–170. doi: 10.1007/BF00021327. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JK, DeSimone N, Lingle W, Dure L. Cellular concentrations and unifomity of cell-type accumulation of two LEA proteins in cotton embryos. Plant Cell. 1993;5:769–780. doi: 10.1105/tpc.5.7.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray EA. Molecular responses to water deficit. Plant Physiol. 1993;103:1035–1040. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- 5.Dure LIII, Crouch M, Harada JJ, Ho T, Mundy J, Quatrano RS, et al. Common. amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol. 1989;12:475–486. doi: 10.1007/BF00036962. [DOI] [PubMed] [Google Scholar]
- 6.Close TJ. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant. 1996;97:795–803. doi: 10.1111/j.1399-3054.1996.tb00546.x. [DOI] [Google Scholar]
- 7.Svensson J, Ismail AM, Palva ET, Close TJ. Dehydrins. In: Storey KB, Storey JM, editors. Sensing, Signalling and Cell Adaptation. Amsterdam, the Netherlands: Elsevier Science; 2002. pp. 155–171. [Google Scholar]
- 8.Close TJ. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol Plant. 1997;100:291–296. doi: 10.1111/j.1399-3054.1997.tb04785.x. [DOI] [Google Scholar]
- 9.Ouellet F, Houde M, Sarhan F. Purification, characterization and cDNA cloning of the 200 kDa protein induced by cold acclimation in wheat. Plant Cell Physiol. 1993;34:59–65. [PubMed] [Google Scholar]
- 10.Takahashi R, Joshee N, Kitagawa Y. Induction of chilling resistance by water stress, and cDNA sequence analysis and expression of water stress-regulated genes in rice. Plant Mol Biol. 1994;26:339–352. doi: 10.1007/BF00039544. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148:6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell SA, Close TJ. Dehydrins: Genes, proteins, and associations with phenotypic traits. New Phytol. 1997;137:61–74. doi: 10.1046/j.1469-8137.1997.00831.x. [DOI] [Google Scholar]
- 13.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/S0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 14.Tompa P, Szász C, Buday L. Structural disorder throws new light on moonlighting. Trends Biochem Sci. 2005;30:484–489. doi: 10.1016/j.tibs.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Lisse T, Bartels D, Kalbitzer HR, Jaenicke R. The recombinant dehydrin-like desiccation stress protein from the resurrection plant Craterstigma plantagineum displays no defined three-dimensional structure in its native state. J Biol Chem. 1996;377:555–561. doi: 10.1515/bchm3.1996.377.9.555. [DOI] [PubMed] [Google Scholar]
- 16.Danyluk J, Perron A, Houde M, Limin A, Fowler B, Benhamou N, et al. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell. 1998;10:623–638. doi: 10.1105/tpc.10.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail AM, Hall AE, Close TJ. Purification and partial characterization of a dehydrin involved in chilling tolerance during seedling emergence of cowpea. Plant Physiol. 1999;120:237–244. doi: 10.1104/pp.120.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara M, Terashima S, Kuboi T. Characterization and cryoprotective activity of cold-responsive dehydrin from Citrus unshiu. J Plant Physiol. 2001;158:1333–1339. doi: 10.1078/0176-1617-00600. [DOI] [Google Scholar]
- 19.Kovacs D, Kalmar E, Torok Z, Tompa P. Chaperone activity of ERD10 and ERD14, two disordered stressrelated plant proteins. Plant Physiol. 2008;147:381–390. doi: 10.1104/pp.108.118208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouillon JM, Eriksson SK, Harryson P. Mimicking the plant cell interior under water stress by macromolecular crowding: Disordered dehydrin proteins are highly resistant to structural collapse. Plant Physiol. 2008;148:1925–1937. doi: 10.1104/pp.108.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rorat T. Plant dehydrins—Tissue location, structure and function. Cell Mol Biol Lett. 2006;11:536–556. doi: 10.2478/s11658-006-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saavedra L, Svensson J, Carballo V, Izmendi D, Welin B, Vidal S. A dehydrin gene in Physcomitrella patens is required for salt and osmotic stress tolerance. Plant J. 2006;45:237–249. doi: 10.1111/j.1365-313X.2005.02603.x. [DOI] [PubMed] [Google Scholar]
- 23.Koag MCh, Fenton RD, Wilkens S, Close TJ. The binding of maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant Physiol. 2003;131:309–316. doi: 10.1104/pp.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazuoka T, Oeda K. Purification and characterization of COR85-oligomeric complex from cold acclimated spinach. Plant Cell Physiol. 1994;35:601–611. [Google Scholar]
- 25.Houde M, Daniel C, Lachapelle M, Allard F, Laliberté S, Sarhan F. Immunolocalization of freezing-tolerance associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. Plant J. 1995;8:583–593. doi: 10.1046/j.1365-313X.1995.8040583.x. [DOI] [PubMed] [Google Scholar]
- 26.Wisniewski M, Webb R, Balsamo R, Close TJ, Yu XM, Griffith M. Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: A dehydrin from peach (Prunus persica) Physiol Plant. 1999;105:600–608. doi: 10.1034/j.1399-3054.1999.105402.x. [DOI] [Google Scholar]
- 27.Reyes JL, Campos F, Wei H, Arora R, Yang Y, Karlson DT, et al. Functional dissection of hydrophilins during in vitro freeze protection. Plant Cell Environ. 2008;31:1781–1790. doi: 10.1111/j.1365-3040.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 28.Brini F, Saibi W, Hanin M, Amara I, Gargouri A, Masmoudi K. The wheat dehydrin DHN-5 exerts a heat-protective effect on β-glucosidase and glucose oxidase activities. Biosci Biotechnol Biochem. 2010;74:1050–1054. doi: 10.1271/bbb.90949. [DOI] [PubMed] [Google Scholar]
- 29.Ellis RJ. From chloroplasts to chaperones: how one thing led to another. Photosynth Res. 2004;80:333–343. doi: 10.1023/B:PRES.0000030439.62331.d0. [DOI] [PubMed] [Google Scholar]
- 30.Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- 31.Hara M, Fujinaga M, Kuboi T. Metal binding by citrus dehydrin with histidine-rich domains. J Exp Bot. 2005;56:2695–2703. doi: 10.1093/jxb/eri262. [DOI] [PubMed] [Google Scholar]
- 32.Heyen BJ, Alsheikh MK, Smith EA, Torvik CF, Seals DF, Randall SK. The calcium-binding activity of a vacuole-associated, dehydrin-like protein is regulated by phosphorylation. Plant Physiol. 2002;130:675–687. doi: 10.1104/pp.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krüger C, Berkowitz O, Stephan UW, Hell R. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J Biol Chem. 2002;277:25062–25069. doi: 10.1074/jbc.M201896200. [DOI] [PubMed] [Google Scholar]
- 34.Nylander M, Svensson J, Palva ET, Welin BV. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol. 2001;45:263–279. doi: 10.1023/A:1006469128280. [DOI] [PubMed] [Google Scholar]
- 35.Rorat T, Grygorowicz WJ, Irzykowski W, Rey P. Expression of KS-type dehydrins is primarily regulated by factors related to organ type and leaf developmental stage during vegetative growth. Planta. 2004;218:878–885. doi: 10.1007/s00425-003-1171-8. [DOI] [PubMed] [Google Scholar]
- 36.Skiver K, Mundy J. Gene expression in response to abscisic acid osmotic stress. Plant Cell. 1990;2:503–212. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bray EA. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. doi: 10.1016/S1360-1385(97)82562-9. [DOI] [Google Scholar]
- 38.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinnusamy V, Schumaker K, Zhu JK. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot. 2004;55:225–236. doi: 10.1093/jxb/erh005. [DOI] [PubMed] [Google Scholar]
- 40.Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol. 2003;6:410–417. doi: 10.1016/S1369-5266(03)00092-X. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi-Shinozaki K, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10:88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 43.Goday A, Jensen AB, Culiáñez-Macià FA, Albà MM, Figueras M, Serratosa J, et al. The maize abscisic acidresponsive protein Rab17 is located in the nucleus and interacts with nuclear-localization signals. Plant Cell. 1994;6:351–360. doi: 10.1105/tpc.6.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen AB, Goday A, Figueras M, Jessop AC, Pagès M. Phosphorylation mediates the nuclear targeting of the maize Rab17 protein. Plant J. 1998;13:691–697. doi: 10.1046/j.1365-313X.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 45.Riera M, Figueras M, Lopez C, Goday A, Pagès M. Protein kinase CK2 modulates developmental functions of the abscisic acid responsive protein Rab17 from maize. Proc Natl Acad Sci USA. 2004;101:9879–9884. doi: 10.1073/pnas.0306154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godoy JA, Lunar R, Torres-Schumann S, Moreno J, Rodrigo RM, Pintor-Toro JA. Expression, tissue distribution and subcellular localization of dehydrin TAS14 in salt-stressed tomato plants. Plant Mol Biol. 1994;26:1921–1934. doi: 10.1007/BF00019503. [DOI] [PubMed] [Google Scholar]
- 47.Brini F, Hanin M, Lumbreras V, Irar S, Pagès M, Masmoudi K. Functional characterization of DHN5, a dehydrin showing a differential phosphorylation pattern in two Tunisian durum wheat (Triticum durum Desf.) varieties with marked differences in salt and drought tolerance. Plant Sci. 2007;172:20–28. doi: 10.1016/j.plantsci.2006.07.011. [DOI] [Google Scholar]
- 48.Alsheikh MK, Heyen BJ, Randall SK. Ion binding properties of the dehydrin ERD14 are dependent upon phosphorylation. J Biol Chem. 2003;278:40882–40889. doi: 10.1074/jbc.M307151200. [DOI] [PubMed] [Google Scholar]
- 49.Alsheikh MK, Svensson JT, Randall SK. Phosphorylation regulated ion-binding is a property shared by the acidic subclass dehydrins. Plant Cell Environ. 2005;28:1114–1122. doi: 10.1111/j.1365-3040.2005.01348.x. [DOI] [Google Scholar]
- 50.Prášil IT, Prášilová P, Marík P. Comparative study of direct and indirect evaluations of frost tolerance in barley. Field Crops Res. 2007;102:1–8. doi: 10.1016/j.fcr.2006.12.012. [DOI] [Google Scholar]
- 51.Ismail AM, Hall AE, Close TJ. Allelic variation of a dehydrin gene cosegregates with chilling tolerance during seedling emergence. Proc Natl Acad Sci USA. 1999;96:13566–13570. doi: 10.1073/pnas.96.23.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houde M, Dhindsa RS, Sarhan F. A molecular marker to select for freezing tolerance in Gramineae. Mol Gen Genet. 1992;234:43–48. doi: 10.1007/BF00272343. [DOI] [PubMed] [Google Scholar]
- 53.Vítámvás P, Kosová K, Prášilová P, Prášil IT. Accumulation of WCS120 protein in wheat cultivars grown at 9°C or 17°C in relation to their winter survival. Plant Breed. 2010;129:611–616. doi: 10.1111/j.1439-0523.2010.01783.x. [DOI] [Google Scholar]
- 54.Pelah D, Wang W, Altman A, Shoseyov O, Bartels D. Differential accumulation of water stressrelated proteins, sucrose synthase and soluble sugars in Populus species that differ in their water stress response. Physiol Plant. 1997;99:153–159. doi: 10.1111/j.1399-3054.1997.tb03443.x. [DOI] [Google Scholar]
- 55.Park SY, Noh KJ, Yoo JH, Yu JW, Lee BW, Kim JG, et al. Rapid upregulation of dehydrin3 and dehydrin4 in response to dehydration is a characteristic of drought-tolerant genotypes in barley. J Plant Biol. 2006;49:455–462. doi: 10.1007/BF03031126. [DOI] [Google Scholar]
- 56.Labhilili M, Joudrier P, Gautier MF. Characterization of cDNAs encoding Triticum durum dehydrins and their expression patterns in cultivars that differ in drought tolerance. Plant Sci. 1995;112:219–230. doi: 10.1016/0168-9452(95)04267-9. [DOI] [Google Scholar]
- 57.Peng Y, Reyes JL, Wei H, Yang Y, Karlson D, Covarrubias AA, et al. RcDhn5, a cold acclimationresponsive dehydrin from Rhododendron catawbiense rescues enzyme activity from dehydration effects in vitro and enhances freezing tolerance in RcDhn5-overexpressing Arabidopsis plants. Physiol Plant. 2008;134:583–597. doi: 10.1111/j.1399-3054.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 58.Yin Z, Rorat T, Szabala BM, Ziółkowska A, Malepszy S. Expression of a Solanum sogarandinum SK3-type dehydrin enhances cold tolerance in transgenic cucumber seedlings. Plant Sci. 2006;170:1164–1172. doi: 10.1016/j.plantsci.2006.02.002. [DOI] [Google Scholar]
- 59.Hara M, Terashima S, Fukaya T, Kuboi T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta. 2003;217:290–298. doi: 10.1007/s00425-003-0986-7. [DOI] [PubMed] [Google Scholar]
- 60.Houde M, Dallaire S, N'Dong D, Sarhan F. Overexpression of the acidic dehydrin WCOR410 improves freezing tolerance in transgenic strawberry leaves. Plant Biotechnol J. 2004;2:381–387. doi: 10.1111/j.1467-7652.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- 61.Kaye C, Neven L, Hofig A, Li QB, Haskell D, Guy C. Characterization of a gene for spinach CAP160 and expression of two spinach cold-acclimation proteins in tobacco. Plant Physiol. 1998;116:1367–1377. doi: 10.1104/pp.116.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brini F, Hanin M, Lumbreras V, Amara I, Khoudi H, Hassairi A, et al. Overexpression of wheat dehydrin DHN5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Rep. 2007;26:2017–2026. doi: 10.1007/s00299-007-0412-x. [DOI] [PubMed] [Google Scholar]
- 63.RoyChoudhury A, Sengupta DN. Transgenic tobacco plants overexpressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep. 2007;26:1839–1859. doi: 10.1007/s00299-007-0371-2. [DOI] [PubMed] [Google Scholar]
- 64.Cheng Z, Targolli J, Huang X, Wu R. Wheat LEA genes, PMA80 and PMA1959, enhance dehydration tolerance of transgenic rice (Oryza sativa L.) Mol Breed. 2002;10:71–82. doi: 10.1023/A:1020329401191. [DOI] [Google Scholar]
- 65.Figueras M, Pujal J, Saleh A, Save R, Pagès M, Goday A. Maize Rab17 overexpression in Arabidopsis plants promotes osmotic stress tolerance. Ann Appl Biol. 2004;144:251–257. doi: 10.1111/j.1744-7348.2004.tb00341.x. [DOI] [Google Scholar]
- 66.Xu J, Zhang YX, Wei W, Han L, Guan ZQ, Wang Z, et al. BjDHNs confer heavy-metal tolerance in plants. Mol Biotechnol. 2008;38:91–98. doi: 10.1007/s12033-007-9005-8. [DOI] [PubMed] [Google Scholar]
- 67.Iturriaga G, Schneider K, Salamini F, Bartels D. Expression of desiccation-related proteins from the resurrection plant Craterostigma plantagineum in transgenic tobacco. Plant Mol Biol. 1992;20:555–558. doi: 10.1007/BF00040614. [DOI] [PubMed] [Google Scholar]
- 68.Lång V, Palva ET. The expression of a rab-related gene, rab18, is induced by abscisic acid during the coldacclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1992;20:951–962. doi: 10.1007/BF00027165. [DOI] [PubMed] [Google Scholar]
- 69.Puhakainen T, Hess MV, Mäkelä P, Svensson J, Heino P, Palva ET. Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. Plant Mol Biol. 2004;54:743–753. doi: 10.1023/B:PLAN.0000040903.66496.a4. [DOI] [PubMed] [Google Scholar]
- 70.Shen Y, Tang MJ, Hu YL, Lin ZP. Isolation and characterization of a dehydrin-like gene from drought-tolerant Boea crassifolia. Plant Sci. 2004;166:1167–1175. doi: 10.1016/j.plantsci.2003.12.025. [DOI] [Google Scholar]
- 71.Bhattarai T, Fettig S. Isolation and characterization of a dehydrin gene from Cicer pinnatifidum, a drought-resistant wild relative of chickpea. Physiol Plant. 2005;123:452–458. doi: 10.1111/j.1399-3054.2005.00478.x. [DOI] [Google Scholar]
- 72.Richard S, Morency MJ, Drevet C, Jouanin L, Séguin A. Isolation and characterization of a dehydrin gene from white spruce induced upon wounding, drought and cold stresses. Plant Mol Biol. 2000;43:1–10. doi: 10.1023/A:1006453811911. [DOI] [PubMed] [Google Scholar]
- 73.Rouse DT, Marotta R, Parish RW. Promoter and expression studies on an Arabidopsis thaliana dehydrin gene. FEBS Lett. 1996;381:252–256. doi: 10.1016/0014-5793(96)00051-8. [DOI] [PubMed] [Google Scholar]
- 74.Sun X, Xi DH, Feng H, Du JB, Lei T, Liang HG, et al. The dual effects of salicylic acid on dehydrin accumulation in water-stressed barley seedlings. Russ J Plant Physiol. 2009;56:348–354. doi: 10.1134/S1021443709030078. [DOI] [Google Scholar]
- 75.Turco E, Close TJ, Fenton RD, Ragazzi A. Synthesis of dehydrin-like proteins in Quercus ilex L. and Quercus cerris L. seedlings subjected to water stress and infection with Phytophthora cinnamomi. Physiol Mol Plant Pathol. 2004;65:137–144. doi: 10.1016/j.pmpp.2004.11.010. [DOI] [Google Scholar]
- 76.Brini F, Yamamoto A, Jlaiel L, Takeda S, Hobo T, Dinh HQ, et al. Pleiotropic effects of the wheat dehydrin DHN-5 on stress responses in Arabidopsis. Plant Cell Physiol. 2011;52:676–688. doi: 10.1093/pcp/pcr030. [DOI] [PubMed] [Google Scholar]
- 77.Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 78.Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campos F, Zamudio F, Covarrubias AA. Two different late embryogenesis abundant proteins from Arabidopsis thaliana contain specific domains that inhibit Escherichia coli growth. Biochem Biophys Res Commun. 2006;342:406–413. doi: 10.1016/j.bbrc.2006.01.151. [DOI] [PubMed] [Google Scholar]
- 80.Zhai C, Lan J, Wang H, Li L, Cheng X, Liu G. Rice dehydrin K-segment have in vitro antibacterial activity. Biochemistry (Moscow) 2011;76:645–650. doi: 10.1134/S0006297911060046. [DOI] [PubMed] [Google Scholar]
- 81.Bravo LA, Gallardo J, Navarrete A, Olave N, Martinez J, Alberdi M, et al. Cryoprotective activity of a cold-induced dehydrin purified from barley. Physiol Plant. 2003;118:262–269. doi: 10.1034/j.1399-3054.2003.00060.x. [DOI] [Google Scholar]
- 82.Artlip TS, Callahan AM, Basset CL, Wisniewski ME. Seasonal expression of a dehydrin gene in sibling deciduous and evergreen genotypes of peach (Prunus persica [L.] Batsch.) Plant Mol Biol. 1997;33:61–70. doi: 10.1023/A:1005787909506. [DOI] [PubMed] [Google Scholar]
- 83.Hara M, Terashima S, Fukaya T, Kuboi T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta. 2003;217:290–298. doi: 10.1007/s00425-003-0986-7. [DOI] [PubMed] [Google Scholar]
- 84.Hara M, Fujinaga M, Kuboi T. Radical scavenging activity and oxidative modification of citrus dehydrin. Plant Physiol Biochem. 2004;42:657–662. doi: 10.1016/j.plaphy.2004.06.004. [DOI] [PubMed] [Google Scholar]


