Abstract
Radish (Raphanus sativus L.) was grown on four layers of paper towel moistened with distilled water with and without acetylcholine (ACh) for five days in the dark after sowing. ACh at 1 nM promoted the growth (emergence and elongation) of lateral roots of radish plants, but had no effect on the stems and main roots. Moreover, ACh enhanced the dry weight of roots [main (primary) + lateral roots]. Neostigmine, an inhibitor of acetylcholinesterase (AChE) also promoted the emergence and elongation of lateral roots, and atropine, a competitive inhibitor of ACh receptor, suppressed the emergence and elongation. ACh promoted the activities of glyceraldehyde-3-phosephate dehydrogenase (G-3-PD), nicotinamide adenine dinucleotide-specific isocitrate dehydrogenase (NAD-ICDH), succinate dehydrogenase (SDH) and cytochrome-c oxidase (Cyt-c OD) in seedlings. Moreover, ACh suppressed the activity of AChE and increased the amount of proteins and pyridine nucleotides (NAD and NADH) in the roots of the seedlings. It also increased the activities of NAD-forming enzymes [NAD synthetase and ATP-nicotinamide mononucleotide (ATP-NMN) adenyltransferase], and enhanced the amount of DNA in the roots of the seedlings. The relationship between ACh and the emergence and growth of lateral roots was discussed from a biochemical viewpoint.
Key words: acetylcholine, acetylcholine esterase, DNA, lateral root, NAD, NAD-forming enzyme, protein, Raphanus sativus
Introduction
Previously, we found that acetylcholine (ACh) and some other choline derivatives such as ACh, L-α-phosphatidylcholine and chlorocholinechloride [CCC; (2-chloroethyl) trimethyl ammoniumchloride] promote the elongation of pollen tubes in pistils of Lilium longiflorum cv. Hinomoto after self-incompatible pollination.1,2
Since Ewins3 provided evidence for the existence of ACh in ergot (Claviceps purpurea) as well as animals, the existence of Ach in higher plants was first reported in a nettle (Urtica urens) grown in the Himalaya Mountains by Emmelin and Feldberg.4 Hoshino5 confirmed the existence of Ach in Vigna seedlings by mass spectrometry. Hitherto, evidential data of the existence in plants were subsequently reported by many researchers.6–19 According to Kostir et al.20 and Tung and Raghavan,21 exogenous ACh influences germination and development at an early growth stage of plants. Bamel et al.22 described that exogenous ACh promotes adventitious root formation in leaf explants from in vitro raised tomato seedlings. Meanwhile, the detailed mechanism of the physiological response to ACh of plants has scarcely been clarified. In particular, no investigation has yet been reported on the effect of ACh on lateral root formation in intact seedlings of higher plants. The purpose of the present study is to clarify the action mechanism of ACh on emergence and elongation of lateral roots in plants from a physiological and biochemical viewpoint.
Results
The elongation of the total lateral roots per radish seedling during a 5-d culture after sowing was markedly promoted by exogenous acetylcholine (ACh), but the elongation of stems and main (primary) roots was not (Fig. 1). The optimum concentration of ACh for lateral root elongation was 1 nM (10−9 M). Therefore, respective data except Figure 1 were obtained using seedlings treated with 1 nM ACh. Moreover, ACh markedly promoted the emergence of lateral roots on a main root. Namely, numbers of the emergence per seedling were 14.1 ± 3.0 (n = 15) on seedlings with exogenous ACh and 5.1 ± 1.9 (n = 15) on seedlings without exogenous ACh (control). This tendency was also confirmed by a photographic evidence (Fig. 2). Moreover, respective lateral roots of seedlings with ACh were shown to be long length, as compared with those without ACh. In short, promotion of the length of total lateral roots per seedling by ACh was caused by both elongation and emergence. The dry weight of roots (main + lateral roots) per seedling was promoted approximately two times and the fresh weight approximately 10% by 1 nM ACh (Fig. 3). The activities of enzymes such as G-3-PD in glycolytic pathway in cytoplasmic matrix (cytosol), and NAD-ICDH and SDH in the citric acid cycle [the tricarboxylic acid (TCA) cycle] and Cyt-c OD in the electron transport system in mitochondria of radish seedlings were promoted by ACh (Table 1).
Figure 1.
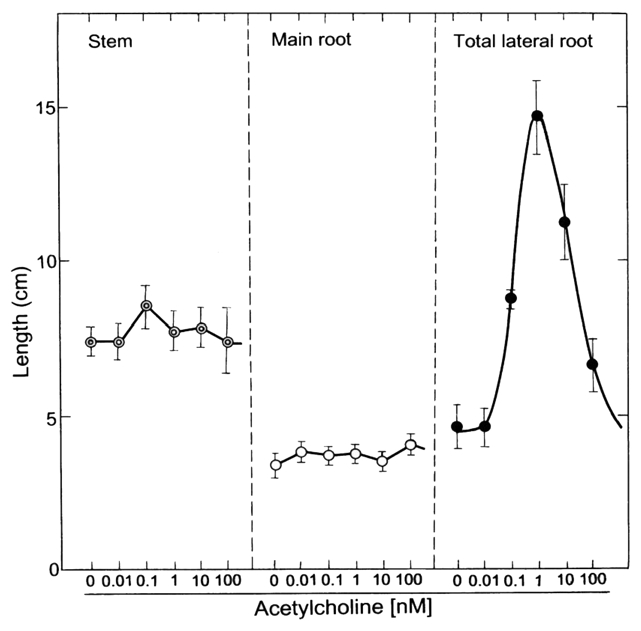
Effects of acetylcholine (ACh) on the elongation of stems and roots of radish seedlings. The seedlings were cultured in the presence and absence of acetylcholine (0, 0.01, 0.1, 1, 10 and 100 nM) for 5 d at 25°C in the dark. The lengths of the stem, the main (primary) root and total lateral roots per radish seedling at day 5 are shown. Vertical bars represent the means ± SD (n = 15).
Figure 2.
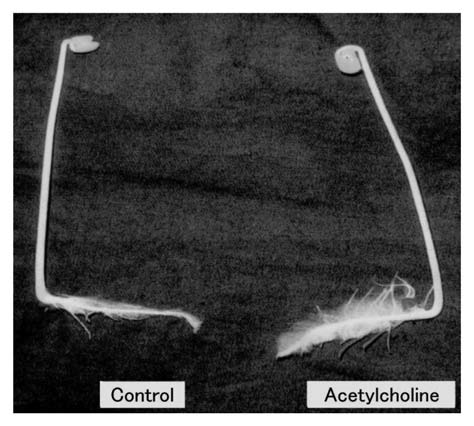
Photograph indicating effects of acetylcholine (ACh) on the root growth of radish seedlings. The seedlings were cultured in the presence and absence of acetylcholine (1 nM) for 5 d at 25°C in the dark. The number of lateral roots emerged on the main (primary) root of a seedling treated with ACh was shown to be abundant as compared with control. Control: without ACh, Acetylcholine: with ACh (1 nM).
Figure 3.
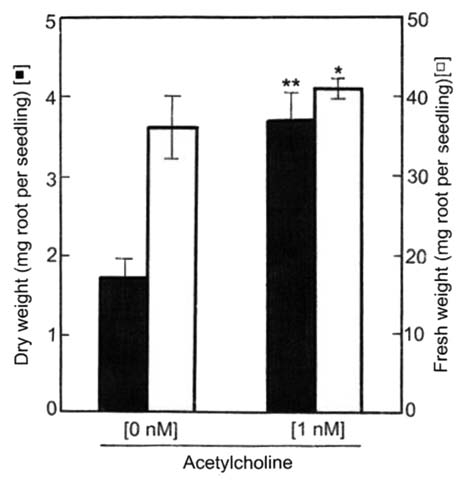
Effects of ACh on the dry and fresh weights of roots of radish seedlings. The seedlings were cultured in the presence and absence of acetylcholine (1 nM) for 5 d at 25°C in the dark. Dry and fresh weights of the roots (main root + lateral roots) per seedling are shown. Vertical bars represent the means ± SD (n = 15). The asterisk indicates a significant difference between in the absence and presence of ACh at **p < 0.01 and *p < 0.05 by the t-test (n = 15).
Table 1.
Effects of acetylcholine (Ach) on the activity of enzymes in the glyocolytic pathway in cytoplasmic matrix (cytosol) and in the TCA cycle and the electron transport system in mitochondria of radish seedlings
| Root | Stem | |||
| without ACh | with ACh | without ACh | with ACh | |
| [µmol(mg protein)−1 min−1] | ||||
| G3PD | 12.32 ± 0.72 | 14.36 ± 0.67* | 1.23 ± 0.03 | 2.05 ± 0.03** |
| NAD-ICDH | 16.32 ± 0.00 | 21.84 ± 0.69** | 4.14 ± 0.28 | 5.50 ± 0.14** |
| SDH | 2.68 ± 0.07 | 3.11 ± 0.00** | 2.10 ± 0.01 | 2.29 ± 0.00* |
| Cyt-c OD | 21.71 ± 0.94 | 24.75 ± 1.07* | 14.99 ± 0.84 | 22.18 ± 0.65** |
The seedlings were cultured in the presence and absence of acetylcholine (1 nM) for 5 d at 25°C in the dark. The asterisks indicate a significant difference between in the absence and presence of ACh at **p < 0.01 and *p < 0.05 by the t-test (n = 3). Root, the main (primary) root and total lateral roots per seedling; ACh, acetylcholine; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; SDH, succinate dehydrogenase; Cyt-c OD, cytochrome-c oxidase.
Since the exogenous ACh promoted the growth (emergence and elongation) of lateral roots, the effects of its modulators such as atropine and neostigmine on the growth of lateral roots were examined. As shown in Figure 4, atropine (10 µM), a competitive inhibitor of an ACh receptor, suppressed somewhat the elongation of roots (main + lateral roots) and stems. However, neostigmine (10 µM), an inhibitor of ACh esterase (AChE), promoted the elongation of the roots and had little effect on stem elongation. The promotion of root elongation by neostigmine seems to be caused by raising endogenous ACh level in roots treated by neostigmine, but quantification of the endogenous ACh level in roots is under investigation as the sequent experiment. In other words, atropine suppressed somewhat the respective lengths of both main roots and lateral roots, but neostigmine as well as ACh did not affected to the length of main roots.23 Taken together, neostigmine (10 µM) promoted the elongation of total lateral roots per seedling as well as ACh (1 nM). Exogenous ACh (1 nM) suppressed the activities of AChE in roots (main + lateral roots) and stems of radish seedlings by 35 and 20%, respectively (Fig. 5), but enhanced the protein content of roots (Fig. 6).
Figure 4.
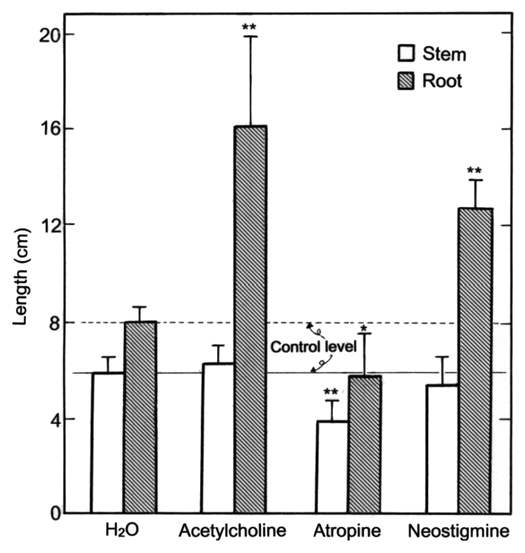
Effects of ACh and its modulators on the elongation of stems and roots (main + lateral roots) of radish seedlings. The seedlings were cultured in the presence and absence of ACh (1 nM), atropine (10 µM) or neostigmine (10 µM) for 5 d at 25°C in the dark. Vertical bars represent the means ± SD (n = 12). The asterisks indicate a significant difference between in the absence and presence of ACh, atropine or neostigmine at **p < 0.01 and *p < 0.05 by the t-test (n = 12).
Figure 5.
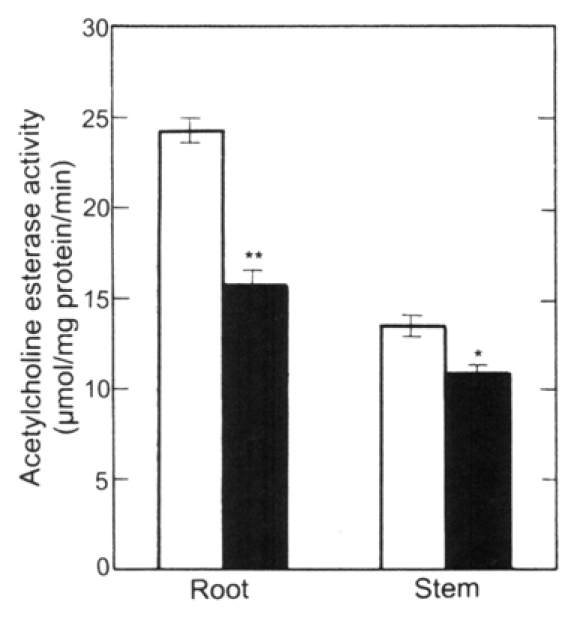
Effects of ACh on the activity of ACh esterase in the stems and roots (main + lateral roots) of radish seedlings. The seedlings were cultured in the presence and absence of ACh (1 nM) for 5 d at 25°C in the dark. Vertical bars represent the means ± SE of results from triplicate experiments. The asterisks indicate a significant difference between in the absence and presence of ACh at **p < 0.01 and *p < 0.05 by the t-test (n = 3). □, in the absence of ACh; ▪, in the presence of ACh.
Figure 6.
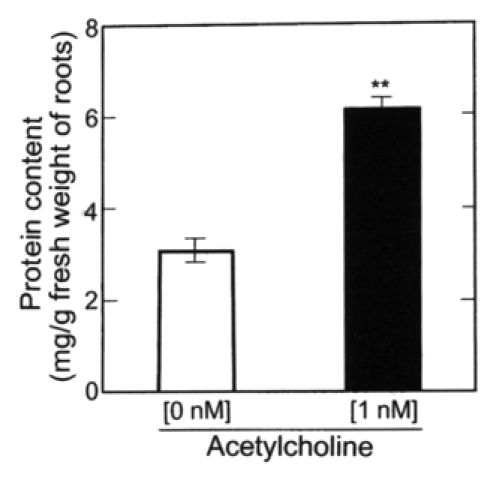
Effects of ACh on the amount of protein in roots (main + lateral roots) of radish seedlings. The seedlings were cultured in the presence and absence of ACh (1 nM) for 5 d at 25°C in the dark. Vertical bars represent the means ± SE of results from triplicate experiments. The asterisk indicates a significant difference between in the absence and presence of ACh at p < 0.01 by the t-test (n = 3).
ACh at 1 nM significantly enhanced the NAD and NADH contents in the roots (main + lateral roots) of radish seedlings (Fig. 7), but had little effect on the NADP and NADPH contents of the roots. The NAD and NADH contents were greater than NADP and NADPH contents.
Figure 7.
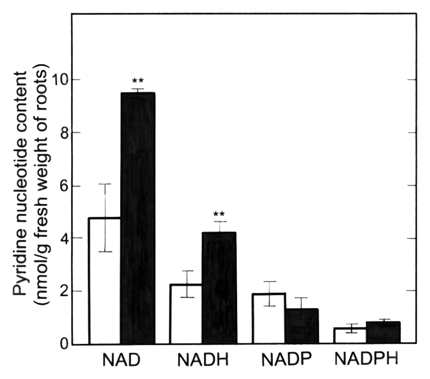
Effects of ACh on the amount of pyridine nucleotides in roots (main + lateral roots) of radish seedlings. The seedlings were cultured in the presence or absence of ACh (1 nM) for 5 d at 25°C in the dark. Vertical bars represent the means ± SE of results from triplicate experiments. The asterisks indicate a significant difference between in the absence and presence of ACh at p < 0.01 by the t-test (n = 3). □, in the absence of ACh; ▪, in the presence of ACh.
The activities of NAD synthetase and ATP-NMN adenyltransferase in the nuclear and cytosolic fractions of the roots (main + lateral roots) of radish seedlings were measured. Activities of both enzymes in both nuclear and cytosolic fractions were increased by 1 nM ACh, but the rate of the increase was higher in the nuclear fraction than in the cytosolic fraction (Fig. 8). In other words, in the nuclear fraction, the activities of the NAD synthetase and ATP-NMN adenyltrasferase were promoted by 70 and 40%, respectively, but in the cytosolic fraction, only 10 and 15%, respectively.
Figure 8.
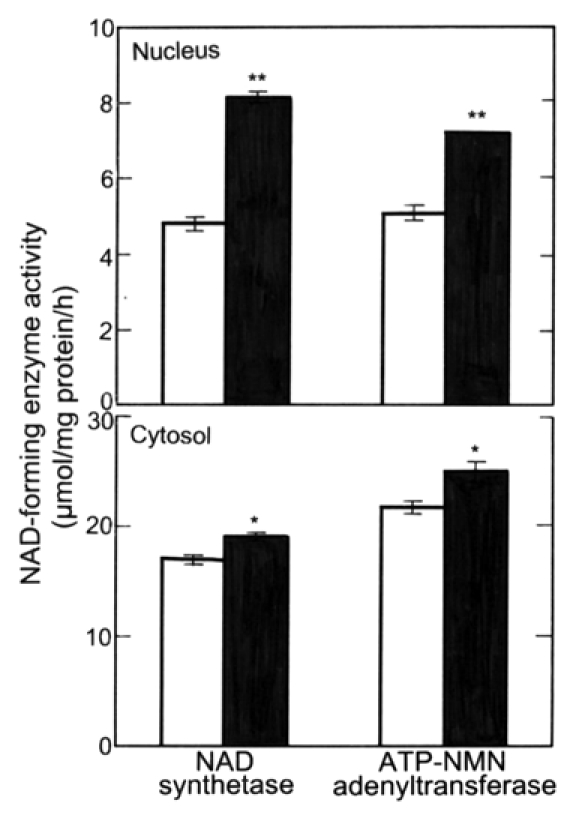
Effects of ACh on the activity of NAD-forming enzymes (NAD synthetase and ATP-NMN adenyltransferase) in nuclei and cytosol in roots (main + lateral roots) of radish seedlings. The seedlings were cultured in the presence and absence of ACh (1 nM) for 5 d at 25°C in the dark. Vertical bars represent the means ± SE of results from triplicate experiments. The asterisks indicate a significant difference between in the absence and presence of ACh at **p < 0.01 and *p < 0.05 by the t-test (n = 3). □, in the absence of ACh; ▪, in the presence of ACh.
The DNA content in the roots (main + lateral roots) of the seedlings treated with 1 nM ACh was 32% greater than control (Fig. 9A). This tendency was also confirmed by agarose gel electrophoresis. Namely, the DNA isolated from roots (main + lateral roots) of the seedlings treated with ACh showed a thick band, as compared with that without ACh (Fig. 9B).
Figure 9.
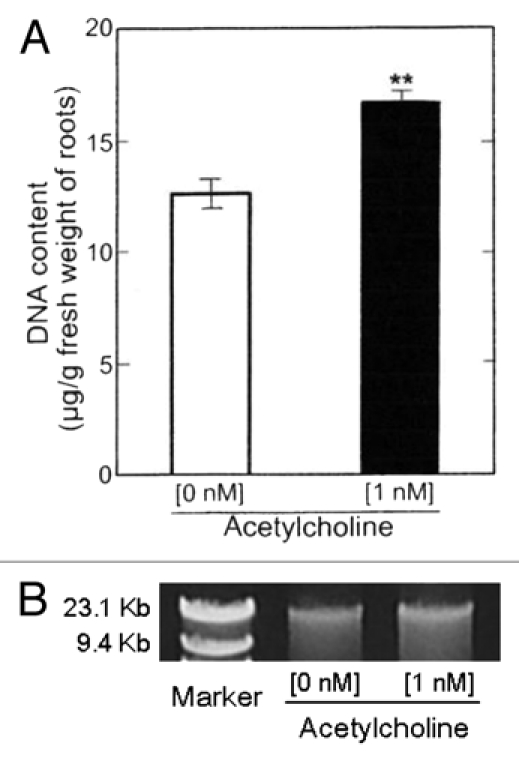
Effects of ACh on DNA content in roots (main + lateral roots) of radish seedlings. The seedlings were cultured in the presence and absence of ACh (1 nM) for 5 d at 25°C in the dark. (A) DNA fraction was obtained after the treatment with RNase. DNA content was estimated at A260. The ratio of A260/A280 in the roots of the seedlings cultured in the presence of ACh was 1.49 and that in the absence of ACh 1.51. Vertical bars represent the means ± SE of results from triplicate experiments. The asterisk indicates a significant difference between in the absence and presence of ACh at p < 0.01 by the t-test (n = 3). (B) Agarose gel eclectrophoresis image of the DNA content in DNA fractions. The electrophoresis was preformed using respective aliquots of the DNA fractions obtained in (A).
Discussion
In our preliminary experiments, ACh promoted the emergence and elongation of lateral roots in Vigna sesquipedalis, Avena sativa and Raphanus sativus grown in the dark. In particular, the effect of ACh was clear in radish (Raphanus sativus) seedlings.23 Consequently, in the present study, we used radish seedlings grown in the dark to analyze the physiological and biochemical actions of ACh. According to Jaffe,24,25 ACh action can be mediated by phytochrome. In our case, ACh action may be connected with the action of red light absorbing form phytochrome (Pr form phytochrome), because etiolated seedlings grown in the dark are generally known to keep Pr form phytochrome. According to reports in the late 1990s,26,27 etiolated seedlings contain phytochrome A (Phy A), but green plants contain phytochrome B (Phy B). Therefore, ACh24,25 and Phy A26,27 may cooperate to promote the emergence and elongation of lateral roots of etiolated seedlings.
As shown in Figure 1, the effective ACh concentrations in the present experiments were within the physiological range from 0.1 to 100 nM. The half-maximal dose of ACh was 0.1 nM (Fig. 1), indicating that the response may occur in planta. It is noteworthy that ACh (0.1–100 nM) promoted the growth such as the emergence and elongation of lateral roots and the optimum concentration for the growth was 1 nM. ACh over the optimum concentration decreased the length of total lateral roots in proportion to the concentration. This phenomenon may depend on suppression of metabolic turnover (biochemical reaction) affected by activation and/or induction of enzymes, plant growth regulator, and so on. For instance, such suppression as above is generally known as phenomena caused by plant growth substances such as plant hormones as shown in many text books regarding plant physiology. On the other hand, the growth of main roots and stems was not affected by ACh (Fig. 1). The emergence and elongation of the lateral roots of radish seedlings might be markedly sensitive to ACh as compared with other physiological responses in many plant species. For instance, the growth of the plant other than its roots has been reported to be affected by ACh at higher concentrations such as µM level28 and 10 mM level.29 Furthermore, the elongation of pollen tubes in pistils of Lilium longiflorum cv. Hinomoto after self-incompatible pollination was promoted by ACh at 100 µM (not nM).1 According to Bamel et al.22 the formation of adventitious roots and secondary roots in excised leaves of Lycopersicon esculentum plants cultured in vitro on Murashige and Skoog's medium30 is promoted by ACh at higher concentrations (µM∼mM level). In short, the action (sensitivity) of ACh seems to be different between the lateral root formation of intact seedlings and adventitious root formation in excised plant organs. Moreover, other physiological responses to ACh in many plant species may depend on the respective concentrations of ACh in plant organs.
ACh at 1 nM had small effect (about 10%) on the fresh weight of the roots (main + lateral roots) of radish seedlings, but it greatly increased (about two times) the dry weight of the roots (Fig. 3). Probably, ACh promoted the translocation of carbohydrates such as sugars and starch from cotyledons to roots and thus promoted the emergence and elongation of lateral roots. In other words, ACh seems to participate in carbon metabolic activities and the translocation of carbohydrates in both organs of cotyledons and roots. In fact, the activities of enzymes of the glycolytic pathway in cytoplasmic matrix (cytosol) and the TCA cycle and the electron transport system in mitochondria were also promoted by ACh as shown in Table 1. Namely, the metabolic activities in nuclei, mitochondria, cytosol, etc., in cells of both organs of cotyledons and roots of seedlings with ACh are probably enhanced to progress metabolic turnover of sugars, organic acids, pyridine nucleotides, nucleic acids, amino acids and proteins, as compared with those without ACh. In short, the progress of metabolic turnover seems to enhance the emergence and elongation of lateral roots.
We examined the effect of atropine and neostigmine on the growth of roots. Atropine suppressed and neostigmine promoted the root growth as expected (Fig. 4). These results suggest that atropine suppressed the action of the receptor of ACh, and neostigmine enhanced the level of ACh in the seedlings. In addition, atropine suppressed not only the root elongation but also the stems elongation though slightly (Fig. 4). This suggests that a decrease in the physiological action of ACh results in suppression of the growth such as cell division and cell elongation not only in roots but also in other plant organs.
ACh lowered the activity of AChE in the stems and roots of radish seedlings (Fig. 5), indicating that ACh suppressed induction and/or activation of AChE. Furthermore, in the seedlings treated with ACh, the activity of AChE did not change significantly during the storage at −80°C for 55 d, but in the seedlings without ACh treatment, it was inactivated approximately 55% during this period.23 This suggests that exogenous ACh stabilizes AChE during storage at −80°C.
The protein content in the roots of radish seedlings treated with ACh was higher than that without ACh treatment (Fig. 6). This may be closely related to the promotion of root elongation by ACh (Fig. 1). The translocation of carbohydrates and other metabolites from cotyledons to stems and roots may be promoted by ACh (compare Fig. 3), resulting in an increase in protein content as active metabolites for physiological actions.
The quantity of pyridine nucleotides such as NAD and NADH was increased by ACh as shown in Figure 7. This may also be related to the promotion of the growth of roots through metabolic activation by ACh. According to Hayaishi,31 the final step of NAD-formation includes two pathways mediated by NAD synthetase and ATP-NMN adenyltransferase as NAD-forming enzymes, which are located in nuclei in animal cells. In the present experiment using plants, both enzymes were also localized in the cytosol and nuclei. Namely, NAD and NADH formed by these enzymes in cytosol probably play a main role as coenzymes. NAD formed in nuclei probably plays a role to enhance the stability and level of DNA after conversion into poly(ADP-ribose) [for details, see Hayaishi31]. The localization and function of NAD synthetase and ATP-NMN adenyltransferase in nuclei in higher plants were confirmed in the present experiment using radish roots (Fig. 8). Soybean roots32 and rice plants33 also possessed the function of NAD synthetase and ATP-NMN adenyltransferase. Furthermore, ACh enhanced the activities of NAD synthetase and ATP-NMN adenyltransferase in the nuclear fraction by 70% and 40%, respectively and those in the cytosol fraction by 10% and 15%, respectively, by ACh (Fig. 8). ACh seems to stimulate NAD formation more strongly in nuclei than in the cytosol. Namely, the stimulation of NAD formation by ACh is probably associated with enhancement of the emergence and elongation of lateral roots of radish seedlings as shown in Figure 1.
Since the growth such as the cell division and cell elongation of roots precede the emergence and elongation of lateral roots, the increase in DNA is considered to precede the growth of lateral roots. According to Hayaishi,31 much of the NAD formed in nuclei is converted to poly (ADP-ribose) which plays an important role in the promotion of DNA-ligase activity, the repair of DNA lesion, cell division, etc., and the other NAD formed in nuclei is transferred to cytoplasm to play a role as a coenzyme. Perhaps the nuclear DNA content in roots of radish seedlings treated with ACh is high, because the amount of NAD in roots of radish seedlings treated with ACh was high as shown in Figure 7. As expected, the amount of DNA was increased more than 30% by ACh (Fig. 9). In the experiment shown in Figure 9, the mitochondrial DNA was included in the DNA content, but chloroplast DNA was not, because etiolated roots cultured in the dark were used for DNA quantification. In the present study, nuclei and mitochondria were not examined separately. Studies on the effect of ACh on the amount of DNA in nuclei and mitochondria are now in progress. However, it is clear that the increase in NAD level by ACh (Fig. 7) is closely associated with the increase in DNA level (Fig. 9A and B) by ACh.
In conclusion, ACh, which is known as a typical neurotransmitter in animals, promoted the growth of young plants, such as the emergence and elongation of lateral roots in radish seedlings at a concentration as low as 1 nM. This seems to depend on the enhancement of translocation of metabolic substances from cotyledons to stems and roots by exogenous ACh. Some metabolic systems, such as the glycolytic pathway in cytoplasm and the TCA cycle and the electron transport system in mitochondria, were also activated by ACh (Table 1), as well as metabolic systems participating in the increase in quantity of NAD, DNA and protein. Figure 10 shows a schematic representation of a putative model of regulation of lateral root growth of radish seedlings by ACh. Although we do not fully understand the mechanism by which ACh promotes the emergence and total length of lateral roots of radish seedlings, further studies on the root growth mechanism associated with ACh may help to elucidate the mechanism of signal transduction in this system. These issues are currently being investigated from viewpoints of biochemistry and molecular biology.
Figure 10.
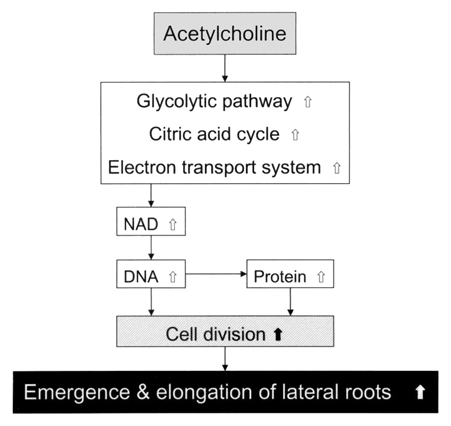
Schematic representation of the mechanism of the action of ACh on the emergence and elongation of lateral roots of radish (Raphanus sativus) seedlings. White arrows represent the enhancement due to physiological and biochemical activation involving metabolic alterations and the formation of NAD, DNA and protein in the stems and roots [main (primary)/lateral], and black (thick) arrow represents expected enhancement, on the basis of data.
Materials and Methods
Plant materials.
Radish (Raphanus sativus L. cv. Sofutori) plants were grown on four layers of wet paper towel (Crecia Comfort; Comfort service 100, Crecia Corporation, Tokyo) in plastic boxes (14.5 × 9.2 × 8.2 cm) containing 40 ml of distilled water with or without ACh. Fifteen seeds were sown in each box in the dark at 25°C. Five days after sowing, morphological and biochemical analyses of some organs of radish seedlings were performed. In all experiments except for Figure 1, in which seedlings treated with 0, 0.01, 0.1, 1, 10 and 100 nM ACh, seedlings treated without and with 1 nM ACh, respectively were used for biochemical analysis.
Morphological analyses.
Seedlings 5 d after sowing were separated into cotyledons, stems and main (primary) roots with lateral roots. After the respective lengths of stem, main root and lateral roots per seedling were measured, the so-called roots including both main and lateral roots were dried at 80°C for 3 d so as to measure the dry weight. The total elongation of all lateral roots per seedling was estimated by measuring the length of respective lateral roots emerged on the main root.
Application of ACh modulators.
Seeds were sown on four layers of wet paper towel (Kimwipe; S-200, Jujo-Kimbery, Tokyo) in glass-test tubes (Φ2.5 cm × 10 cm) containing atropine (10 µM atropine sulfate monohydrate), neostigmine (10 µM stigmine bromide), ACh (1 nM), or distilled water (control). Four seeds per test tube for above drug application were used in triplicate. After seeds were sown, test tubes were sealed with Parafilm (American National Can, Menasha, WI USA), covered with aluminum foil and incubated in the dark at 25°C. Five days after sowing, the length of main root and total length of lateral roots per seedling were measured.
Quantification of proteins.
The protein contents of the roots (main + lateral roots) of seedlings were measured by the method of Shiozaki and Tezuka34 and Kodama and Tezuka.35 After determining the fresh weight of the roots of the seedlings at 5 d after sowing, the roots (0.5 g fresh weight) were homogenized in a glass homogenizer with 1.5 ml of 5% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000 g for 2 min. The pellet was suspended in 1.5 ml of 5% (w/v) TCA and again centrifuged at 10,000 g for 2 min. The pellet was suspended in 1.5 ml of 0.1 N NaOH, incubated shaking at 25°C for 2 h, and centrifuged at 10,000 g for 2 min. The supernatant was used for protein quantification. Protein was quantified by the method of Lowry et al.36 with bovine serum albumin as the standard.
Measurements of G-3-PDH, SDH, NAD-ICDH and Cyt-c OD activities.
Enzyme preparation was performed at a temperature kept below 4°C. The roots and stems (respective 1 g fresh weights) of seedlings with and without acetylcholine 5 d after sowing were homogenized separately in a mortar with 3 ml of 50 mM potassium phosphate (K-PO4) (pH 7.4) containing 0.1 g polyvinylpolypyrrolidone (PVPP; Polyclar AT) and centrifuged at 20,000 g for 5 min. The supernatant was filtered through a layer of Miracloth (Calbiochem-Novabiochem, San Diego, CA USA). The respective filtrates (enzyme samples) were used to assay the activities of G-3PDH, SDH, NAD-ICDH and Cyt-c OD participating the pathways from glucose to ATP. The protein in the enzyme samples was quantified by the method of Lowry et al.36 with bovine serum albumin (BSA) as the standard.
The activity of G-3-PDH was measured by the method of Sugiyama.37 The activity was estimated by following the increase in A340 at 25°C in a reaction mixture (1 ml) that contained 0.7 ml of the mixture of 38 mM Na4P2O7, 50 mM Na2HAsO4 and 0.3 mM disodium-ethylene-diaminetetraacetic acid (Na2-EDTA), 0.035 ml of 4.9 mM NAD(P), 0.1 ml enzyme sample, 0.095 ml distilled water and 0.07 ml of DL-glyceraldehyde-3-phosphate (G-3-P). The activity of SDH was measured by the method of Singer et al.38 The activity was estimated by following the increase in A600 at 25°C in a reaction mixture (1 ml) that contained 0.2 ml of 0.2 M K-PO4 (pH 7.5), 0.1 ml of 10 mM KCN, 0.003 ml of 30 mM N-ethylmaleimide, 0.03 ml of 0.05% phenazine methosulfate (PMS), 0.1 ml of 0.2 M succinate, 0.467 ml of distilled water and 0.1 ml of enzyme sample. The activity of NAD-ICDH was measured by the method of Tezuka and Laties.39 The activity was estimated by following the increase in A340 at 25°C in a reaction mixture (1 ml) that contained 0.17 ml of 0.2 M K-PO4 (pH 7.6), 0.1 ml of 0.1 M MgCl2, 0.08 ml of 20 mM isocitrate, 0.03 ml of 5 mM KCN, 0.07 ml of 0.2 M nicotinamide, 0.5 ml of distilled water and 0.02 ml of enzyme sample. The activity of Cyt-c OD was estimated by following the decrease in A550 at 25°C in a reaction mixture (0.3 ml) that contained 0.02 ml of 0.5 M K-PO4 (pH 7.5), 0.01 ml of 2% (w/v) digitonin, 0.15 ml of distilled water, 0.02 ml of enzyme sample and 0.1 ml of 0.1% cytochrome c.
Mesurement of acetylcholine esterase activity.
AChE was also prepared below 4°C. The roots (0.3 g in fresh weight) of seedlings 5 d after sowing were homogenized in a mortar with 1.5 ml of 10 mM potassium phosphate (K-PO4) (pH 8.0) containing 10 mM L-cysteine and centrifuged at 15,000 g for 10 min. The resulting pellet was suspended in 1 ml of 10 mM K-PO4 (pH 8.0) containing 10 mM L-cysteine and 0.25% (w/v) digitonin and allowed to stand for 30 min in an ice bath. The suspended pellet was centrifuged at 15,000 g for 10 min, and the supernatant was filtered through a layer of Miracloth. The filtrate (ca. 1 ml) was loaded on a column (Φ1 cm × 10 cm) of Sephadex G-25 (coarse powder) equilibrated with 10 mM K-PO4 (pH 8.0) containing 10 mM L-cysteine and eluted to remove low molecules with the same eluent used for equilibration. After discarding 2.5 ml of the eluate, the protein-rich (enzyme) fraction (2 ml) was collected to assay the activity of AChE. The activity of AChE was measured by a modified version1 of the method of Fluck and Jaffe.9 The activity was estimated by following the increase in A412 at 25°C in a reaction mixture (1 ml) that contained 160 µl of 0.2 M K-PO4 (pH 8.0), 10 µl of 1 mM 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), 30 µl of 20 mM acetylthiocholine, H2O (770 µl) and 30 µl of enzyme fraction. The AChE activity was estimated as ΔsampleA412 − ΔcontrolA412 which indicates a difference between ΔA412 of sample per min and ΔA412 of control per min. The protein in the enzyme sample was quantified by the method of Lowry et al.36 with bovine serum albumin (BSA) as the standard.
Extraction and quantification of pyridine nucleotides.
Pyridine nucleotides were extracted from roots (main + lateral roots) of respective seedlings with and without ACh by the method of Tezuka et al.40 The roots with and without ACh, respectively, were weighed and divided into two rations of equal weight, one for the extraction of the oxidized (NAD and NADP) form of pyridine nucleotides, and the other for the extraction of the reduced (NADH and NADPH) form of pyridine nucleotides. One half of the root sample (1.5 g) was homogenized at 90–95°C for 2 min with 0.1 N HCl (2 ml) to extract oxidized coenzymes (NAD and NADP), and the remaining half (1.5 g) with 0.1 N NaOH to extract reduced coenzymes (NADH and NADPH). Each homogenate was then rapidly chilled in an ice bath and the extracts for oxidized and reduced pyridine nucleotide fractions were adjusted to pH 6.5 with NaOH and pH 7.5 with HCl, respectively, and then 0.2 M glycylglycine buffer (0.5 ml) at pH 6.5 and pH 7.5, respectively, were added. The volume of each fraction was measured. Each fraction was centrifuged at 10,000 g for 20 min at 4°C. Supernatants were stored at −80°C prior to the assay of pyridine nucleotides. To estimate possible losses of pyridine nucleotides during extraction, we added authentic standards [NAD(P)H at 2 nmol, NAD(P) at 10 nmol] to the alkaline and acidic extracts before homogenization. Recovery of NAD(P) and NAD(P)H was 96–101 and 75–90%, respectively.
Pyridine nucleotides were quantified by a modified version40 of the method of Nisselbaum and Green.41 For the measurements of the levels of NAD and NADH, 0.15 ml of supernatant was mixed in a cuvette with 0.24 ml of 0.1 M glycylglycine buffer (pH 7.4), 0.06 ml of 0.2 M nicotinamide, 0.05 ml of 0.01 M phenazine methosulfate (PMS), 0.04 ml of 0.012 M thiazolyl blue (MTT) and 0.02 ml of the solution of alcohol dehydrogenase (1 mg ml−1) (from yeast). The cuvette with the mixture was placed in a spectrophotometer and the wavelength was set at 570 nm. At zero time, 0.04 ml of 80% ethyl alcohol was added. The reaction was followed at 25°C for 2 min. After the reaction, 0.002 ml of 0.2 mM authentic NAD was added and the change in absorbance was followed for a further 2 min.
For the measurement of the levels of NADP and NADPH, 0.14 ml of the supernatant was mixed in a cuvette with 0.225 ml of 0.1 M glycylglycine buffer (pH 7.4), 0.06 ml of 0.2 M nicotinamide, 0.05 ml of 0.01 M of PMS, 0.04 ml of 0.012 M MTT and 0.08 ml of 0.01 M glucose-6-phosphate. The cuvette was placed in a spectrophotometer set at 570 nm. At zero time, 0.005 ml of a solution of glucose-6-phosphate dehydrogenase (100 µg ml−1) was added. The reaction was followed at 25°C for 2 min. After the reaction, 0.002 ml of 0.2 mM authentic NADP was added and the change in absorbance was followed for a further 2 min.
Isolation of nuclei.
After measuring the fresh weight of the roots (main + lateral roots) of seedlings, the root sample was homogenized in a mortar with 20 ml of a grinding medium containing 10 mM 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris)/HCl (pH 7.4) and 0.4 M sucrose. The homogenate was filtered through a layer of Miracloth and centrifuged at 100 g for 10 min. The supernatant was used for the assay of the activity of NAD-forming enzymes in the cytosolic fraction without nuclei. The pellet was suspended in 10 ml of 2.2 M sucrose containing 3 mM MgCl2 and centrifuged at 40,000 g for 35 min. The pellet was suspended in 2 ml of 5 mM Tris/HCl (pH 7.4), and used for the assay of the activity of NAD-forming enzymes in the nuclear fraction.
Assay of the activities of NAD-forming enzymes.
NAD synthetase was assayed at 30°C for 30 min in a reaction mixture (1 ml) that contained 0.1 ml of 0.25 M N-Tris (hydroxymethyl) methyl-2-aminoethanefulfonic acid (TES)/KOH (pH 9.0), 0.2 ml of 5 mM deamide NAD, 0.1 ml of 0.2 M L-glutamine, 0.08 ml of 50 mM ATP, 0.1 ml of 1.64 M nicotinamide, 0.01 ml of 5 mM MgCl2, an enzyme sample (0.1 ml or 0.2 ml) from the nuclear or cytosolic fraction and distilled water (0.31 ml or 0.21 ml, respectively) by a modified version of the method of Tezuka and Murayama.32 Immediately after the assay, 1 N HCl (0.1 ml) was added to the mixture, which was then heated at 95°C for 2 min and cooled in an ice bath. After cooling, 1 N NaOH (0.1 ml) and TES/KOH (0.25 M, 0.1 ml, pH 7.6) were added to the mixture.
ATP-nicotinamide mononucleotide (ATP-NMN) adenyltransferase was assayed by a modified version of the method of Tezuka and Murayama.32 The respective enzyme samples (0.2 ml each) from the nuclear and cytosolic fraction and distilled water (0.21 ml) were added to a reaction mixture (1 ml) that contained 0.4 ml of 0.25 M TES/KOH (pH 7.0), 0.05 ml of 0.1 M MgCl2, 0.05 ml of 0.05 M ATP, 0.04 ml of 1.64 M nicotinamide, 0.05 ml of 0.04 M nicotinamide mononucleotide. Then, 0.42 ml of 0.18 N HCl was added to the mixture, and then the mixture was heated at 95°C for 2 min and cooled in an ice bath. Then 0.05 ml of 1.5 N NaOH and 0.2 ml of TES/KOH (pH 7.6) were added to the mixture. Finally, NAD formed by NAD synthetase and ATP-NMN adenyltransferase was measured by the method of Tezuka et al.40 Protein was determined according to Lowry et al.36 using BSA as the standard.
Extraction and quantification of DNA.
DNA was extracted by a modified version of the method of Liu et al.42 After measuring the fresh weight of roots (main + lateral roots), the roots were homogenized in a mortar with 100 µl of grinding medium containing 0.05 M Tris/HCl (pH 7.5), 0.3 M NaCl, 20 mM disodium-ethylenediaminetetraacetate (Na2-EDTA) (pH 8.0) and 0.5% (w/v) sodium dodecyl sulfate (SDS), 5 M urea, 10 mM mercaptoethanol and 5% (v/v) phenol. The mixture (3∼4 v/w) containing the grinding medium as above: [phenol:chloroform:isoamylalcohol (25:24:1, v:v:v)] = 1:1 (v:v) was added to the homogenate. The resulting homogenate was transferred in a plastic tube and capped. After the tube was horizontally shaken for 20 min, the tube was centrifuged at 15,000 g for 5 min. The resulting supernatant was transferred in a new tube to add 2 volumes of ethanol and shaken. After allowed to stand for 1 min at room temperature, the tube was centrifuged at 15,000 g for 5 min. The resulting supernatant was discarded using a micropipette. The pellet was washed with 0.5 ml of 70% (v/v) ethanol and centrifuged at 15,000 g for 3 min. The resulting supernatant was completely discarded and pellet was somewhat dried. The dried pellet was dissolved with 0.65 ml of a mixture containing TE [10 mM TRIS-HCl (pH 8.0) and 1 mM Na2-EDTA]. The dissolved pellet was allowed to stand for 40 min at 37°C after adding 1 µl of 10 mg ml−1 RNase. Then, 0.38 ml of 20% (w/v) polyethylene glycol (PEG) 8,000 in 2.5 M NaCl was added to the dissolved pellet. The resulting pellet with PEG was allowed to stand for 30 min in an ice bath and centrifuged at 20,000 g for 15 min. The resulting pellet was again centrifuged at 20,000 g for 10 min after adding 1 ml of 70% (v/v) ethanol. After the supernatant was discarded, the pellet was allowed to stand for 10 min at 70°C after adding 0.1 ml of TE as above. Then, the pellet was centrifuged at 20,000 g for 10 min after adding 0.1 ml of phenol, 0.2 ml of ethanol and 10 ml of 3 M sodium acetate. The resulting pellet was centrifuged at 20,000 g for 10 min after adding 1 ml of 70% (v/v) ethanol and dried up. The dried pellet was dissolved with 50 µl of TE for quantification of DNA. The quantity of DNA was estimated using a spectrophotometer set at 260 nm and besides confirmed to show different level between samples with and without ACh due to agarose gel electrophoresis by the method of Liu et al.42
All experiments in the present study were repeated three times with similar results and representative results are shown.
Acknowledgments
We thank Prof. Setsuyuki Aoki (Graduate School of Information Science, Nagoya University) for his helpful advice about the isolation and the quantification of DNA and critical discussion. We also thank Prof. Shozo Hiroki (Graduate School of Information Science, Nagoya University) for the interest and encouragement in the experiment.
References
- 1.Tezuka T, Akita I, Yoshino N, Suzuki Y. Regulation of self-incompatibilty mechanism in Lilium longiflorum by acetylcholine and cAMP. J Plant Physiol. 2007;164:878–885. doi: 10.1016/j.jplph.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Tezuka T, Akita I, Yoshino N. Self-incompatibility involved in the level of acetylcholine and cAMP. Plant Signal Behav. 2007;2:475–476. doi: 10.4161/psb.2.6.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ewins AJ. Acetylcholine, a new active principle of ergot. Biochem J. 1914;8:44–49. doi: 10.1042/bj0080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emmelin N, Feldberg W. The mechanism of the sting of the common nettle (Urtica urens) J Physiol. 1947;106:440–455. doi: 10.1113/jphysiol.1947.sp004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino T. Identification of acetylcholine as a natural constituent of Vigna seedlings. Plant Cell Physiol. 1983;24:829–834. [Google Scholar]
- 6.Hartmann E. Uber den Nachweis eines Neurohormanes bein Laumooskallus und seine Beeinflussung durch das Phytochrom. Planta. 1971;101:159–165. doi: 10.1007/BF00387626. [DOI] [PubMed] [Google Scholar]
- 7.Satter RL, Applewhite PB, Galston AW. Phytochrome-controlled nyctinasty in Albizia julibrissin. Plant Physiol. 1972;50:523–525. doi: 10.1104/pp.50.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans ML. Promotion of cell elongation in Avena coleoptiles by acetylcholine. Plant Physiol. 1972;50:414–416. doi: 10.1104/pp.50.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fluck RA, Jaffe MJ. Cholinesterases from plant tissues. III. Distribution and subcellular localization in Phaseolus aureus Roxh. Plant Physiol. 1974;53:752–758. doi: 10.1104/pp.53.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann E, Kilbinger K. Lliquid-chromatographic determination of light-dependent acetylcholine concentration in moss callus. Biochem J. 1974;137:24952. doi: 10.1042/bj1370249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann E, Kilbinger K. Occurrence of light-dependent acetylcholine concentrations in higher plants. Experientia. 1974;30:1387–1388. doi: 10.1007/BF01919649. [DOI] [PubMed] [Google Scholar]
- 12.Verbeek M, Vendrig JC. Are acetylcholine-like cotyledon-factors involved in the growth of the cucumber hypocotyls? Z Pflanzcnphysiol. 1977;83:335–340. [Google Scholar]
- 13.Hoshino T, Oota Y. The occurrence of acetylcholine in Lemna gibba G3. Plant Cell Physiol. 1978;19:769–776. [Google Scholar]
- 14.Lees GL, Lahue R, Thompson JE. Changes in acetylcholine titer of sensing cotyledons. J Exp Bot. 1978;29:1117–1124. doi: 10.1093/jxb/29.5.1117. [DOI] [Google Scholar]
- 15.Momonoki YS, Momonoki T. Changes in acetylcholine levels following leaf wilting and leaf recovery by heat stress in plant cultivars. Jpn J Crop Sci. 1991;60:283–290. [Google Scholar]
- 16.Tretyn A, Kendrick RE. Acetylcholine in plants: presence, metabolism and mechanism of action. Bot Rev. 1991;57:33–72. doi: 10.1007/BF02858764. [DOI] [Google Scholar]
- 17.Wessler I, Kibinger H, Bittinger F, Kirkpatrick CJ. The biological role of non-neuronal acetylcholine in plants and humans. Jpn J Pharmacol. 2001;85:2–10. doi: 10.1254/jjp.85.2. [DOI] [PubMed] [Google Scholar]
- 18.Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, et al. Evolutional study on acetylcholine expression. Life Sci. 2003;72:1745–1756. doi: 10.1016/S0024-3205(02)02478-5. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima K, Misawa H, Moriwaki Y, Fujii YX, Fujii T, Horiuchi Y, et al. Ubiquitous expression of acetylcholine and its biological function in life forms without nervous systems. Life Sci. 2007;80:2206–2209. doi: 10.1016/j.lfs.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 20.Kostir J, Klenha J, Jiracek V. The effect of acetylcholine on seed germination in agricultural plants. Rostlinna Vyroba. 1965;12:1239–1279. [Google Scholar]
- 21.Tung HF, Raghavan V. Effects of growth retardants on the growth of excised roots of Dolichos lablab L. in culture. Ann Bot (Lond) 1968;32:509–519. [Google Scholar]
- 22.Bamel K, Gupta SC, Gupta R. Acetylcholine causes rooting in leaf explants of in vitro raised tomato (Lycopersicon esculentum Miller) seedlings. Life Sci. 2007;80:2393–2396. doi: 10.1016/j.lfs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama K. Studies on promotion of the growth of plant roots by acetylcholine. Nagoya University; 2003. Master's Thesis. (in Japanese) [Google Scholar]
- 24.Jaffe MJ. Phytochrome-mediated bioelectric potentials in mung bean seedlings. Science. 1968;162:1016–1017. doi: 10.1126/science.162.3857.1016. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe MJ. Evidence for the regulation of phytochrome-mediated process in bean roots by the neurohumor, acetylcholine. Plant Physiol. 1970;46:768–777. doi: 10.1104/pp.46.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuya M, Schafer M. Photoperception and signaling of induction reaction by different phytochromes. Trends Plant Sci. 1996;11:301–307. [Google Scholar]
- 27.Khurana JP, Kochhar A, Tyagi AK. Photosensory perception and signal transduction in higher plants: molecular genetic analysis. Crit Rev Plant Sci. 1998;17:465–539. [Google Scholar]
- 28.Tretyn A, Kendrick RE. Induction of leaf unrolling by phytochrome and acetylcholine in etiolated wheat seedlings. Photochem Photobiol. 1990;52:123–129. doi: 10.1111/j.1751-097.1990.tb01765.x. [DOI] [Google Scholar]
- 29.Dekhuijzen HM. The effect of acetylcholine on growth and on growth inhibition by CCC in wheat seedlings. Planta. 1973;111:149–156. doi: 10.1007/BF00386275. [DOI] [PubMed] [Google Scholar]
- 30.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–477. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 31.Hayaishi O. Poly(ADP-ribose) and ADP-ribosylation of proteins—New function of NAD in molecular biology. In: Egami N, Kuraishi S, Sato S, Hidaka T, editors. Oxygen and Life. 2nd edition. Vol. 55. Tokyo: Publishing Division of University of Tokyo; 1984. pp. 93–143. (UP BIOLOGY series). [Google Scholar]
- 32.Tezuka T, Murayama Y. Formation of pyridine nucleotides under symbiotic and non-symbiotic conditions between soybean nodules and free-living rhizobia. Phytochemistry. 2002;61:637–644. doi: 10.1016/S0031-9422(02)00364-3. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, Takahashi H, Tamura K, Huang J, Yu LH, Kawai-Yamada M, et al. Enhanced dihydroflavonol-4-reductase activity and NAD homoeostasis leading to cell death tolerance in transgenic rice. Proc Natl Acad Sci USA. 2005;102:7020–7025. doi: 10.1073/pnas.0502556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiozaki N, Tezuka T. Enhancement of the protective system against oxidative stress in eggplants by solar UV radiation. Environ Sci. 1999;7:231–239. [Google Scholar]
- 35.Kodama Y, Tezuka T. Compulsory winding in the opposite direction of climbing plants promotes yield. Plant Physiol Biochem. 2004;42:349–354. doi: 10.1016/j.plaphy.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 37.Sugiyama T. Methods of the extraction, purification and measurement of enzyme activity connected with photosynthesis. In: Kato S, Miyachi S, Murata Y, editors. Method for photosynthetic research. Tokyo, Japan: Kyoritsu Publishing Co.; 1981. pp. 224–228. [Google Scholar]
- 38.Singer TP, Oestreicher G, Hogue P, Contreiras J, Brando I. Regulation of succinate dehydorgenase in higher plants. Plant Physiol. 1973;52:616–621. doi: 10.1104/pp.52.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tezuka T, Laties GG. Isolation and characterization of inner membrance-associated and matrix NAD-specific isocitrate dehydrogenase in potato mitochondria. Plant Physiol. 1983;72:959–963. doi: 10.1104/pp.72.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tezuka T, Yamaguchi F, Ando Y. Physiological activation in radish plants by UV-A radiation. J Photochem Photobiol B. 1994;24:33–40. doi: 10.1016/1011-1344(94)07006-7. [DOI] [Google Scholar]
- 41.Nisselbaum JS, Green S. A simple ultramicro method for determination of pyridine nucleotides in tissues. Anal Biochem. 1969;27:212–217. doi: 10.1016/0003-2697(69)90025-6. [DOI] [PubMed] [Google Scholar]
- 42.Liu YG, Mitsukawa N, Oosumi T, Whittier RF. Efficient isolation and mapping of Arabidopisi thailiana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463. doi: 10.1046/j.1365-313X.1995.08030457.x. [DOI] [PubMed] [Google Scholar]


