Abstract
Filamentous fungi belonging to the genus Trichoderma have long been recognized as agents for the biocontrol of plant diseases. In this work, we investigated the mechanisms involved in the defense responses of Arabidopsis thaliana seedlings elicited by co-culture with Trichoderma virens and Trichoderma atroviride. Interaction of plant roots with fungal mycelium induced growth and defense responses, indicating that both processes are not inherently antagonist. Expression studies of the pathogenesis-related reporter markers pPr1a:uidA and pLox2:uidA in response to T. virens or T. atroviride provided evidence that the defense signaling pathway activated by these fungi involves salicylic acid (SA) and/or jasmonic acid (JA) depending on the amount of conidia inoculated. Moreover, we found that Arabidopsis seedlings colonized by Trichoderma accumulated hydrogen peroxide and camalexin in leaves. When grown under axenic conditions, T. virens produced indole-3-carboxaldehyde (ICAld) a tryptophan-derived compound with activity in plant development. In Arabidopsis seedlings whose roots are in contact with T. virens or T. atroviride, and challenged with Botrytis cinerea in leaves, disease severity was significantly reduced compared with axenically grown seedlings. Our results indicate that the defense responses elicited by Trichoderma in Arabidopsis are complex and involve the canonical defense hormones SA and JA as well as camalexin, which may be important factors in boosting plant immunity.
Key words: Arabidopsis, Trichoderma, phytostimulation, defense responses, jasmonic acid, salicylic acid, camalexin
Introduction
Plants are in continuous interaction with a plethora of microorganisms, including pathogens and symbionts. In the rhizosphere, extensive communication occurs between plants and their associated microbes by exchange of different classes of microbial and plant compounds. This molecular dialog will determine the final outcome of the relationship, usually through highly coordinated cellular processes.1
Filamentous fungi of the genus Trichoderma are common rhizosphere inhabitants. They have been widely studied due to their capacity to produce antibiotics, parasitize other fungi or compete with deleterious plant microorganisms.2 It has been known for many years that Trichoderma species enhance plant growth and productivity in axenic systems and in soil.3–5 In Arabidopsis thaliana, growth promotion by T. virens correlated with increased root biomass production and enhanced lateral root formation, which was attributed to the production of auxin signals by this fungus.6 Trichoderma also induces plant defense responses. Co-cultivation of cucumber plants with T. harzianum increased production of peroxidase and chitinase, while T. asperellum T34 conferred protection against Pseudomonas syringe pv. Lachrymans through regulation of proteins involved in adaptation to stress, isoprenoid and ethylene biosynthesis, photorespiration and carbohydrate metabolism.7,8 Nevertheless, the signaling mechanisms by which Trichoderma species activate plant immunity remain uncertain.
Plants possess various inducible defense mechanisms for protection against pathogen attack. An example of this is systemic acquired resistance (SAR), which is activated by a wide range of pathogens, especially (but not only) those that cause tissue necrosis.9 Similarly, colonization of plant roots by certain nonpathogenic rhizobacteria can induce systemic resistance (ISR) in the host plant.10 Both pathogen-induced SAR and rhizobacteria-mediated ISR are effective against different types of pathogens, and are typically characterized by a restriction of pathogen growth and a suppression of disease development compared with primary infected, non-induced plants. However, the signaling pathways controlling pathogen-induced SAR and rhizobacteria-mediated ISR differ. Whereas SAR requires endogenous accumulation of salicylic acid (SA), the signaling pathway controlling ISR functions independently of SA and requires intact responsiveness to the plant hormones jasmonic acid (JA) and ethylene.10–12 Additionally, it has been established that accumulation of phytoalexins and other low molecular weight antimicrobial metabolites is integral to plant protection. The chemical structures of phytoalexins vary among different plant families and include flavonoids, terpenoids and indoles.13
The antimicrobial properties of phytoalexins, which have been extensively investigated, suggest their potential function in the host defense machinery. However, only recently they have been found to make an important contribution to plant defense against particular pathogens.14 Mutations that cause reduced phytoalexin levels can lead to increased susceptibility of plants to pathogens such as Botrytis cinerea.15–17 The main phytoalexin detected in Arabidopsis is an indole derivative called camalexin (3-thiazol-2′-yl-indole).17–20 Camalexin accumulates in tissue exposed to infection by either avirulent or virulent strains of the bacterium Pseudomonas syringe and after inoculation with the fungus Cochliobolus carbonum and is able to inhibit bacterial and fungal growth.19,21,22
The indole ring of camalexin is produced from Trp through at least three CYP (cytochrome P450) steps. Trp is converted to indole-3-acetonitrile (IAN) by CYP79B2/CYP79B3 and CYP71A13. Conversion of Cys(IAN) to dihydrocamalexic acid and subsequently to camalexin is catalyzed by CYP71B15.23,24 Based on the pad3 mutant phenotype CYP71B15 has been involved in camalexin biosynthesis by converting cysteine-indole-3-acetonitrile to camalexin.24–26 Alternatively, camalexin biosynthesis may proceed through condensation of indole-3-carboxaldehyde (ICAld) with cysteine (Cys) followed by cyclization and decarboxylation.27
B. cinerea is the causal agent of gray mold and causes rot symptoms in more than 200 different plant species, including Arabidopsis. An intact ethylene/JA signaling pathway is thought to be necessary for resistance to necrotrophic pathogens, such as B. cinerea and E. carotovora, whereas the SA signaling pathway is believed to mediate resistance to biotrophic pathogens. However, Arabidopsis defense responses against B. cinerea may also involve camalexin induction.16 While SA or JA induce many defense responses, these signaling compounds are apparently not essential for camalexin production.17 It is possibly that several input signals are integrated to trigger camalexin biosynthesis. Based on this information, it is tempting to speculate that biotic interactions capable of regulating multiple defense responses may increase the fitness of plants when challenged with pathogens.
In this report, we used an in vitro Trichoderma-Arabidopsis interaction system,6 to explore some of the signaling networks involved in defense responses triggered by T. virens and T. atroviride in plants. Our data show that co-cultivation of plant roots with these fungi induces hydrogen peroxide, SA and JA accumulation, which correlates with induction of pathogenesis-related reporter markers pPr1a:uidA and pLox2:uidA. We also found that both T. virens and T. atroviride increased camalexin accumulation in plants and conferred resistance to B. cinerea. Taken together, our results show the simultaneous involvement of hormonal signaling and camalexin biosynthesis in Trichodermainduced plant immunity.
Results
Co-cultivation of Arabidopsis roots with Trichoderma stimulates lateral root development and plant biomass production.
To evaluate the growth response of plants during physical contact with Trichoderma, we performed bioassays in which WT Arabidopsis thaliana ecotype Columbia (Col-0) seedlings were germinated and grown for 4 d on Petri plates, then, a drop of distilled water (control treatment) or a spore suspension from T. virens or T. atroviride was deposited at the opposite side of the plates. Six days after inoculation (dai), physical contact between the mycelium and the primary root tips could be observed, and two days later the fungi covered roughly 30% of the root system (Fig. 1A–C). In order to detect any potential deleterious effect of Trichoderma, total plant biomass in control or inoculated seedlings was determined every two days. During the first two days, no significant differences were observed in biomass production between control plants and plants co-cultivated with T. virens or T. atroviride (Fig. 1D). However, from 4 to 8 dai, a 40% increase in total fresh weight was evident in Trichoderma co-cultivated plants. Enhanced lateral root proliferation was a typical response of Arabidopsis roots colonized by the mycelia of T. virens or T. atroviride, and no deleterious symptoms such as chlorosis or necrosis could be observed in leaves (Fig. 1A–C). Interestingly, co-cultivation with Trichoderma increased anthocyanin production in leaves (Figs. 1A–C and S1), which might occur as a con-sequence/parallel effect of defense induction.
Figure 1.
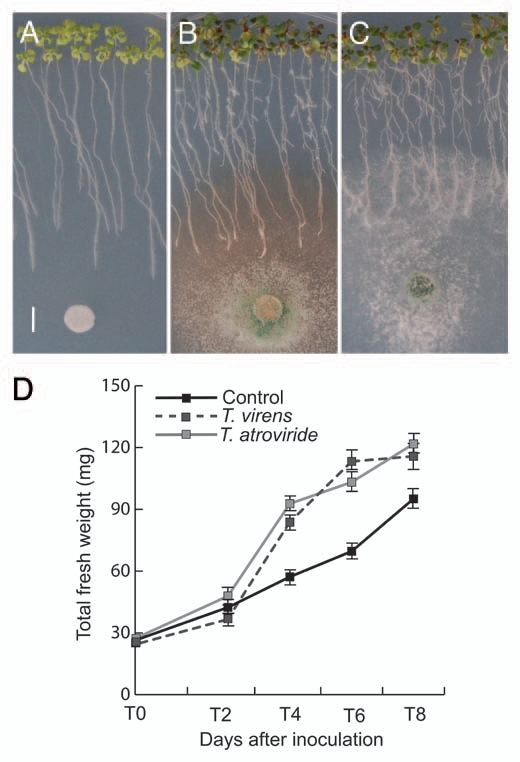
Effect of Trichoderma on Arabidopsis growth. Photographs of 10-d-old Arabidopsis (Col-0) seedlings grown on the surface of agar plates containing 0.2x MS medium. (A) Seedlings were treated with sterilized distilled water at day 4 and photographed 6 d later. Bar = 5 mm. (B) Representative photograph of Arabidopsis seedlings that were inoculated with T. virens at a distance of 5 cm from the root tip 4 d after germination and grown for a further 6 d period. (C) Photograph of Arabidopsis seedlings inoculated with T. atroviride at a distance of 5 cm from the root tip at 4 d after germination and grown for a further 6-d period. (D) Effects of Trichoderma on Arabidopsis biomass production. Mean ± SD values were plotted at the indicated days in the kinetic experiment (n = 30). The experiment was repeated twice with similar results.
Trichoderma induces hydrogen peroxide accumulation in Arabidopsis.
The production of reactive oxygen species (i.e., hydrogen peroxide, H2O2) is an early response in plant-pathogen or elicitor recognition.31,49 In Arabidopsis seedlings at 6 d of co-cultivation H2O2 production was detected by DAB staining. The appearance of a brownish-red precipitate in plant tissues indicates hydrogen peroxide accumulation via polymerization with 3,3′-diaminobenzidine (Fig. 2A–E). T. atroviride induced accumulation of H2O2 in leaves (Fig. 2B), in the primary root and mature lateral roots (Fig. 2D) and in meristems of young lateral roots (Fig. 2E) compared with the respective controls (Fig. 2A and C). H2O2 induction was much weaker in leaves than in roots (Fig. 2A–E).
Figure 2.
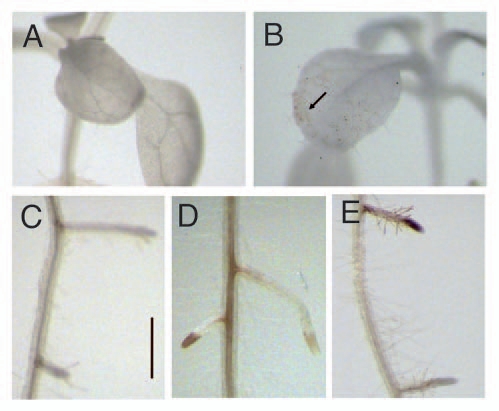
Effect of Trichoderma on H2O2 accumulation in Arabidopsis. Representative photographs of leaves (A) and roots (C) from axenicallygrown (control) seedlings or from seedlings co-cultivated with T. atroviride for 6 d (B, D and E). Arabidopsis seedlings were treated with a solution of 3,3′-diaminobenzidine (DAB). In the presence of H2O2, DAB polymerizes, forming a dark red-brown coloration in plant tissues. Microscopy was performed using a Leica MZ6 Stereomicroscope. Bar = 1 mm. Arrow shows the spots of accumulated H2O2. Photographs show representative individuals of at least 15 seedlings stained with DAB. The experiment was repeated twice with similar results.
Trichoderma induces hormone-dependent defense responses in Arabidopsis.
Infection of plants with pathogens or colonization of roots with certain beneficial microbes causes the induction of defense responses, which may depend on plant production of the alarm signals SA, JA and/or ET.12 However, the defense signaling pathways that are activated in Arabidopsis upon exposure to Trichoderma remain unknown. To examine whether root colonization by Trichoderma involved altered SA or JA responses, we monitored expression of selected marker genes that are upregulated by these hormones. We used transgenic Arabidopsis lines expressing β-glucuronidase (uidA, GUS) fusions to the Pr1a promoter, which is activated by SA,28 and the Lox2 promoter, activated by JA.29 Arabidopsis transgenic seedlings expressing each of these markers were co-cultivated with either T. virens or T. atroviride and the roots allowed to be colonized by the fungi. Axenically-grown seedlings did not show pPr1a:uidA expression in shoots at different time points in a time-course experiment (Fig. 3A–E). Interestingly, during pre-contact or physical contact between the roots and mycelium pPr1a:uidA expression was activated in shoots of plants co-cultivated with both T. virens and T. atroviride (Fig. 3F–O). The time required for the activation of the Pr1 promoter depended on the fungal species and amount of conidia inoculated. pPr1a:uidA expression in plants co-cultivated with 106 conidia of T. virens significantly increased at 4-dai and continued to increase gradually to a maximum at 8 d. Changes in GUS activity by T. atroviride were evident at 6-dai and reached a maximum at 8 dai. In both cases, this response was apparently delayed when less conidia (103) were inoculated and was detectable only at 8 dai in cotyledons and older leaves (Fig. 3J and O). SA clearly induced changes in pPr1a:uidA expression (Fig. 3P). In contrast, the JA-activated marker pLox2:uidA was better induced by low density of Trichoderma at 8-dai (Fig. 4A–P). These data suggest that SA and JA are likely involved in Arabidopsis responses to Trichoderma inoculation.
Figure 3.

Effect of T. virens and T. atroviride on expression of the pathogen-related response gene marker pPr1a:uidA. In a time-course plant-fungus co-cultivation experiment GUS expression in seedlings was determined every two days after inoculation. SA was used as a positive control and dimethyl sulfoxide served as negative control. Bar = 1 mm. Photographs show representative individuals of at least 20 stained seedlings. The experiment was repeated twice with similar results.
Figure 4.
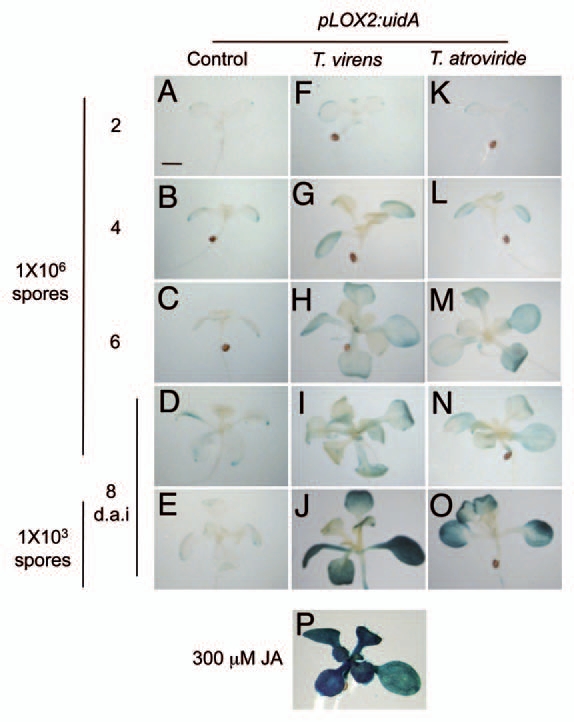
Effects of T. virens and T. atroviride on expression of the JA responsive gene marker pLox2:uidA. GUS expression in seedlings was determined every 2 d after inoculation in a time-course plant-fungus co-cultivation experiment. JA was used as a positive control and dimethyl sulfoxide served as negative control. Bar = 1 mm. Photographs show representative individuals of at least 20 stained seedlings. The experiment was repeated twice with similar results.
Interaction between Arabidopsis roots and Trichoderma induces SA and JA accumulation in leaves.
To determine whether the changes in the expression of pPr1a:uidA and pLox2:uidA markers were associated with changes in endogenous SA and/or JA content in plants co-cultivated with Trichoderma, free SA and JA were quantified in WT Arabidopsis (Col-0) plants at 8 dai. A 3- to 4-fold increase in the accumulation of SA was observed in plants inoculated with Trichoderma (106 spores) when compared with axenically-grown seedlings, with slightly higher levels upon treatment with T. atroviride (Fig. 5A). The levels of JA dramatically increased in plants co-cultivated with T. virens and to a lesser extent with T. atroviride (Fig. 5B). These data indicate that T. virens and T. atroviride can induce both SA and JA accumulation.
Figure 5.
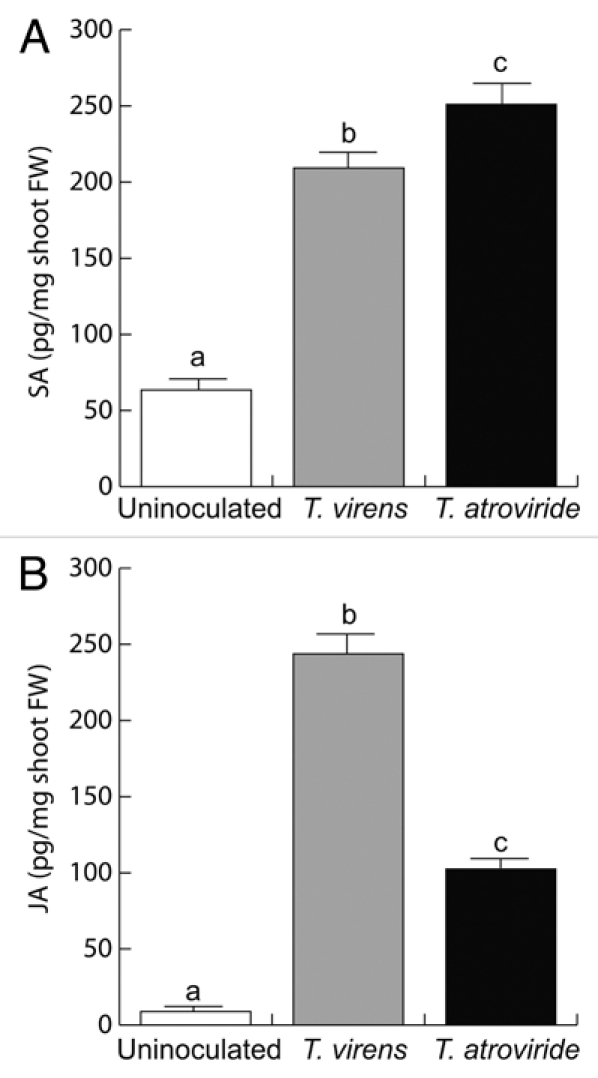
Effect of Trichoderma co-cultivation on SA and JA accumulation in Arabidopsis leaves. (A) Free SA or (B) JA at 8 d of co-cultivation with either T. virens or T. atroviride. Error bars represent the SE. Different letters are used to indicate means that differ significantly (p < 0.05). The experiment was repeated three times with similar results.
Trichoderma induces camalexin accumulation in Arabidopsis and produces indole-3-carboxaldehyde.
The main phytoalexin that accumulates in Arabidopsis after infection by fungi or bacteria is 3-thiazol-2′-yl-indole (camalexin). To investigate whether Trichoderma inoculation could increase camalexin production, we determined camalexin levels in axenically-grown seedlings, seedlings treated with AgNO3, a well-known inducer of camalexin, or in seedlings at 8-dai with T. virens or T. atroviride. Gas chromatography-mass spectrometry (GC-MS) analysis revealed that seedlings co-cultivated with T. atroviride as well as plants treated with AgNO3 dramatically increased (15-fold) camalexin accumulation when compared with control seedlings. Co-cultivation with T. virens also resulted in strongly increased camalexin levels (Fig. 6). Previously, we determined the production of several indolic compounds by Trichoderma, including indole-3-acetic acid, indole-3-acetaldehyde and indole-3-ethanol.6 Further analysis of the metabolites produced by Trichoderma by GC-MS revealed the presence of indole-3-carboxaldehyde (ICAld, retention time 10.79 min, m/z 144) (Fig. 7A–C), a compound previously reported by Zook and Hammerschmidt (1997), as a camalexin reactant.27 When A. thaliana was grown in the presence of ICAld, the production of camalexin increased significantly (Fig. 7D), indicating that this compound is likely involved in camalexin biosynthesis. Supply of L-Trp to T. virens culture medium increased ICAld accumulation in culture supernatants (Fig. S2).
Figure 6.
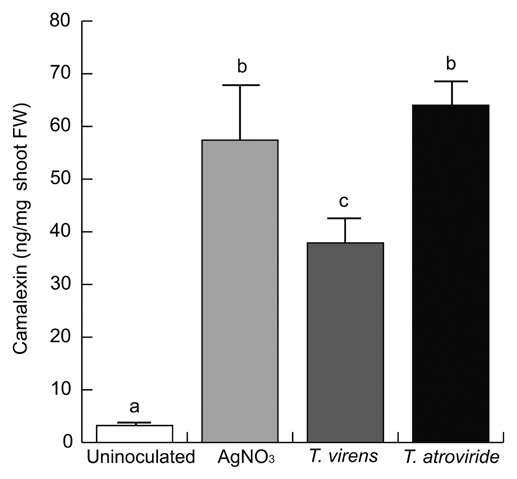
Effect of Trichoderma co-cultivation on camalexin accumulation in Arabidopsis. Camalexin levels were determined in leaves of axenically-grown WT Arabidopsis seedlings, in seedlings treated with 5 mM AgNO3, or co-cultivated 8 d with T. virens or T. atroviride. Bars show the mean ± SD of five independent biological replicates. Each replicate included 20 seedlings. Different letters are used to indicate means that treatments differ significantly (p < 0.05). The experiment was repeated twice with similar results.
Figure 7.
Identification of indole-3-carboxaldehyde from underivatized samples from T. virens growth medium by GC-MS. (A) chromatogram from neutral ethyl acetate extract obtained from 1 l of culture medium of T. virens, arrows indicate the presence of different indolic compounds, indole-3-acetaldehyde (IAAld), indole-3-ethanol (IEt) and indole-3-carboxaldehyde (ICAld). (B) ICAld standard. (C) the 70-eV electron-impact full-scan mass spectra from m/z 50 to 500 of ICAld standard. (D) Indole-3-carboxaldehyde increases camalexin biosynthesis. Induction of camalexin in shoots of Arabidopsis after treatment with 300 µM ICAld for 72 h. Bars shown in (D) represent the mean ± SD of three independent replicates. Each replicate included 20 seedlings. Different letters are used to indicate means that differ significantly (p < 0.05). The experiment was repeated twice with similar results.
Trichoderma confers resistance to Botrytis cinerea.
Camalexin is essential for resistance to B. cinerea in Arabidopsis.16,17 The results from SA and JA accumulation, pPr1a:uidA and pLox2:uidA expression, and camalexin accumulation suggest that Trichoderma may act as potential defense-inducing fungus. To study whether Trichoderma could effectively activate defense mechanisms that lead to pathogen resistance, we tested the responses of 12-d-old Arabidopsis plants grown axenically or co-cultivated with T. virens or T. atroviride and further inoculated with the necrotrophic pathogen Botrytis cinerea, which causes spreading necrotic lesions on leaves. In these experiments, B. cinerea conidia were inoculated over the leaf surfaces and disease symptoms evaluated 3 d after inoculation with the pathogen. In control plants B. cinerea was found to induce necrotic lesions in over 82% of inoculated leaves (Fig. 8A). In contrast, in plants colonized by T. virens or T. atroviride, only 22% and 25% of leaves, respectively, presented necrotic lesions caused by B. cinerea infection (Fig. 8A). Five days after pathogen inoculation, it was found that B. cinerea caused death in about 70% of control plants; whereas only 10% of Trichoderma colonized plants were dead at this stage (Fig. 8B).
Figure 8.
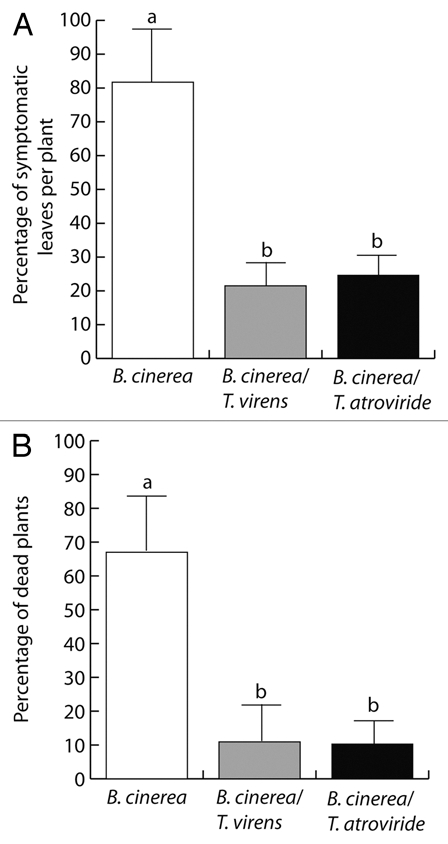
Trichoderma confers protection against Botrytis cinerea in Arabidopsis seedlings. Twelve-day-old axenically-grown A. thaliana seedlings or seedlings co-cultivated 3-d with T. virens or T. atroviride in vitro were placed in Petri dishes containing 0.2x MS medium and mock (water) treated or inoculated with 5 µl of 1 × 106 Botrytis cinerea spores per ml solution/per leaf, depositing the inoculum on leaf surfaces. The number of symptomatic leaves per plant (A) and dead plants (B) are shown. Bars show the mean ± SD of 30 Arabidopsis seedlings. Different letters are used to indicate means that treatments differ significantly (p < 0.05). The experiment was repeated three times with similar results.
Discussion
Fungi are an enormous resource of structurally diverse metabolites, which may affect interactions with plants and other organisms. Deciphering the signaling processes that mediate these interactions represents a continuous challenge. Although not directly economically important, the model plant Arabidopsis thaliana offers a number of experimental advantages over crop species, including its small size, short life cycle, and the suitability to be grown under axenic conditions. The adoption of an Arabidopsis-Trichoderma model has increased our knowledge about the molecular and physiological mechanisms by which Trichoderma modulate plant growth and development.6
Here we show that colonization of Arabidopsis roots by T. virens or T. atroviride sustained increased plant biomass production at 8 dai (Fig. 1). Root colonization by these fungi was associated to induction of lateral root development and anthocyanin accumulation in leaves (Figs. 1 and S1). Anthocyanin accumulation was highly dependent on the amount of conidia of Trichoderma applied. Importantly, we did not observe chlorosis or necrosis symptoms in leaves of seedlings co-cultivated with either T. virens or T. atroviride indicating that high fungal colonization in roots is not detrimental to plant growth and development.
We found that Arabidopsis roots-colonized by Trichoderma accumulated H2O2 (Fig. 2), indicating that Trichoderma triggers plant defense responses through reactive oxygen species (ROS). Interactions between plants and microbes involve recognition and signaling events that are distinct for different microbial elicitors. These microbial elicitors may belong to pathogen-or microbe-associated molecular patterns (PAMPs or MAMPs).30 At the cellular level, the result of pathogen recognition often becomes apparent as a necrosis localized at the site of attack, called the hypersensitive response, which is accompanied by cellular changes such as an oxidative burst leading to the release of ROS, a rise in salicylic acid levels, and the induction of defense genes such as those coding for pathogenesis-related (PR) proteins.31–33 The contribution of H2O2 in plant protection by Trichoderma and its relationship with SA and JA pathways remains to be investigated.
Certain Trichoderma species have been previously found to elicit physical or chemical changes related to plant defense by activating ISR.34,35 ISR elicited by Trichoderma may suppress plant diseases caused by a range of phytopathogens in both greenhouse and field conditions. In this study, we used transgenic Arabidopsis lines expressing β-glucuronidase (GUS) fusions to elucidate the signal transduction pathway by which T. virens and T. atroviride elicit plant defense responses. In our experiments, pre-contact or direct physical contact between the Arabidopsis root system and Trichoderma stimulated pPr1a:uidA expression and SA accumulation in plants (Figs. 3 and 5A). The concerted action of PR proteins, some of which display antimicrobial activity may act restricting pathogen growth. Low fungal concentration was also found to induce pLox2:uidA and JA accumulation (Figs. 4 and 5B). Both SA and JA changes have been previously described in cucumber plants inoculated with Trichoderma asperellum strain T34,36 indicating that hormonal defense protection of plants is widespread among Trichoderma species. These results are in agreement with previous reports that T. virens induces the expression of PAL in maize through a JA-dependent response.34,35 Moreover, our data suggest that SA and JA are involved in the signal transduction events mediating Trichoderma induced defense responses and are even consistent with the SA/JA antagonism. Similar defense activation patterns have been previously identified in Arabidopsis seedlings inoculated with plant growth promoting rhizobacteria.37–39
Antimicrobial compounds can be produced during normal plant growth and are usually stored in specialized organs or tissues such as trichomes, oil glands or epidermal cell layers.40 Arabidopsis is a member of the Brassicaceae family, which is known for the production of various indolic constituents, including several indolic glucosinolates and closely related indolyl thiazoles, including camalexin and 6-methoxycamalexin.40–42 Camalexin production can be elicited by bacterial and fungal phytopathogens and possess antimicrobial activity. In this work, we showed that Arabidopsis seedlings colonized with T. virens or T. atroviride accumulate greater levels of camalexin than axenically-grown plants (Fig. 6). Interestingly, we found that T. virens also produces ICAld, a compound related to indole-3-acetic acid metabolism likely involved in camalexin biosynthesis.43 ICAld concentration in T. virens liquid extracts increased when Trp was supplied in the culture medium (Fig. S2), suggesting that Trp might be a precursor for ICAld. A number of aldehydes possess the ability to react with Cys to form the corresponding thiazolidinecarboxylic acid.27 It has been reported that the synthesis of camalexin may proceed through the condensation of indole-3-carboxaldehyde with cysteine followed by a two-step oxidation and decarboxylation.44 We found that application of ICAld increased camalexin accumulation in Arabidopsis seedlings (Fig. 7). These results indicate that Trichoderma species can induce both SA- and JA-mediated defense responses and camalexin accumulation. Hormone-dependent signaling and camalexin accumulation are considered independent or complimentary mechanisms involved in plant immunity. The results that T. virens produces ICAld suggest that plants might use this compound for camalexin production by either direct or indirect means. The activity of ICAld on plant signaling was evidenced monitoring primary root growth and adventitious root formation, which were clearly altered by ICAld supply to the medium (Fig. S3). The combination of such complex defense and/or plant growth regulating mechanisms may account for better performance of plants inoculated with Trichoderma.
In accordance with its involvement in activating defense-signaling pathways, T. virens and T. atroviride were found to be effective against the fungal necrotizing pathogen B. cinerea, a gray mold that causes rot symptoms in more than 200 different plant species. Our results of disease susceptibility showed that colonization of Arabidopsis roots by T. virens or T. atroviride reduced disease symptoms and plant death caused by this pathogen in leaves (Fig. 8). The correlation found between defense gene expression, H2O2 induction, SA and JA accumulation, camalexin production and reduced disease symptoms in Arabidopsis colonized by Trichoderma, suggests that the combined activation of these defense pathways might be important to confer plant immunity against a fungal necrotizing pathogen. The characterization of two ISR elicitors secreted by T. virens was recently described. Peptides with antimicrobial activity termed peptaibols, which are produced by T. virens were shown to have ISR elicitor effects, and they systemically induced defense in cucumber leaves.45 The second ISR elicitor of T. virens is an extracellular small protein (Sm1), which is overproduced in the presence of cotton plants.34,46 In maize, the metabolic pathways that lead to the establishment of Sm1-mediated ISR involves the signaling networks associated with salicylic acid, green leaf volatiles and jasmonic acid metabolism.34,35,46 Our results are in agreement with these findings by showing that Trichoderma species can confer protection in Arabidopsis seedlings against B. cinerea, possibly by producing diffusible signals prior or during direct contact with roots.
We conclude that Trichoderma species are capable of regulating multiple defense responses. In addition to the defense gene induction and hormone biosynthesis, we now provide chemical evidence supporting a role for Trichoderma in phytoalexin induction, an important defense response less characterized. The combined effect of these responses may ultimately determine the role played by particular Trichoderma isolates in conferring disease resistance to crops.
Materials and Methods
Plant material and growth conditions.
All mutant and transgenic lines were derived from parental Arabidopsis ecotype Columbia-0 (Col-0). Arabidopsis transgenic lines used in this work expressed a JA-inducible lipoxygenase2 (At3g45140) or the pathogenesis-related1 (At2g14610) gene promoters fused to the uidA reporter gene, which are referred as pLox2:uidA29 and pPr1a:uidA,28 respectively. Seeds were surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) household bleach (6% NaOCl) for 7 min. After five washes in distilled water, seeds were germinated and grown on agar plates containing 0.2x MS medium (Murashige and Skoog basal salts mixture, Cat M5524: Sigma, St. Louis). Plates were placed vertically at a 65 degrees angle to allow root growth along the agar surface and unimpeded aerial growth of the hypocotyls. Plants were grown at 24°C in a growth chamber with a 16 h light (200 µmolm2s−1)/8 h darkness photoperiod.
Fungal growth and plant co-cultivation experiments.
The following fungal strains were used in this work: Trichoderma virens Gv.29-8 and Trichoderma atroviride (Formerly Trichoderma harzianum) IMI 206040. T. virens and T. atroviride were evaluated in vitro for their ability to elicit defense responses in Arabidopsis. Fungal spore densities of ∼1 × 106 or 1 × 103 spores were inoculated by placing the spores at 5 cm in the opposite ends of agar plates containing 4-d-old germinated Arabidopsis seedlings (10 seedlings per plate). Plates were arranged in a completely randomized design. The seedlings were cultured for different time periods in a Percival AR95L growth chamber. The percentages of primary roots colonized by Trichoderma were determined with a ruler by measuring the primary root length and the surface covered by fungal hyphae.
Histochemical analysis.
For histochemical analysis of GUS activity, Arabidopsis seedlings were incubated 12 to 14 h at 37°C in a GUS reaction buffer (0.5 mg/ml of 5-bromo-4-chloro-3indolyl-β-D-glucuronide in 100 mM sodium phosphate, pH 7). The stained seedlings were cleared using the method of Malamy and Benfey (1997).47 For each marker line and for each treatment, at least 15 transgenic plants were analyzed. A representative plant was chosen and photographed, using a Leica MZ6 stereomicroscope.
Determination of H2O2 production.
he production of H2O2 in Arabidopsis seedlings co-cultivated with Trichoderma was determined at 6 dai. The seedlings were included in 1 mg/ml solution of 3,3′-diaminobenzidine (DAB; Sigma), incubated 2 h, fixed and cleared in alcoholic solution. In presence of H2O2, DAB polymerizes, forming a dark red-brown precipitate staining. After these procedures the seedlings were examined for the production of H2O2 by microscopy. For each treatment at least 15 plants were analyzed. A representative plant was chosen and photographed, using a Leica MZ6 stereomicroscope.
Anthocyanin determination.
Anthocyanin content was determined in WT Arabidopsis (Col-0) seedlings 6 d after Trichoderma inoculation. 100 mg leaf samples were placed in an Eppendorf tube containing 1 ml of 0.1% HCl in methanol for 48 h at 4°C. After this period, the methanol extracts were analyzed in a spectrophotometer at 530 nm. The amount of anthocyanin in the extracts was reported as described by Pirie and Mullins (1976).48
SA and JA extraction and measurements.
SA and JA extraction and determinations were performed in Arabidopsis thaliana (ecotype Col-0) shoots at 8 d after Trichoderma inoculation. For sample preparation, plants were sectioned at the root/shoot interface. Plant tissues were frozen and ground in liquid N2. Three hundred milligrams ground tissue was placed in an eppendorf tube, homogenized with 500 µl isopropanol/H2O/concentrated HCl (2:1:0.002, v/v), and 200 ng orto-anisic acid (OA; Sigma) added to serve as internal standard for SA and shaken for 30 sec. Samples were centrifuged at 11,500 rpm for 3 min, supernatants were collected and subjected to SA extraction with 200 µl of dichloromethane. SA and JA were derivatized with acetyl chloride in methanol (1 ml/250 µl), sonicated for 15 min and heated for 1 h at 75°C. After cooling, the derivatized sample was evaporated and resuspended in 25 µl of ethyl acetate for GC-MS analysis. Gas chromatography-selected ion monitoring mass spectrometry (GC-SIM-MS) and retention time were established for SA-ME (2.3 min, m/z 152), OA-ME (3.2 min, m/z 166) and (7.5 min, m/z 224) respectively. JA was quantified by comparison with a standard curve obtained by using purified Me-JA (Sigma).
Camalexin determination.
Camalexin was extracted from leaves of WT Arabidopsis seedlings 8 d after T. virens or T. atroviride inoculation. As a positive control for camalexin induction, 12-d-old plants were treated with 5 mM AgNO3 for 12 h, or 300 µM indole-3-carboxaldehyde (Sigma) for 72 h. Camalexin levels were determined as described by Glazebrook and Ausubel (1994),21 and GC-MS analysis performed. For GC-MS analysis 100 mg per sample of shoot material were submerged in 800 µl of methanol and kept at 80°C for 20 min. The supernatant was transferred to a vial, evaporated under a stream of nitrogen and redissolved in 10 µl of methanol, and injected 2 µl for GC-SIM-MS analysis. The ions with m/z 58, 142 and 200 were monitored. Camalexin (Rt 18.0 min) was quantified by comparison with a standard curve obtained by using purified camalexin kindly provided by Prof. J. Glazebrook (University of Minnesota) and dissolved in methanol for chemical analysis.
Identification and quantification of indole-3-carboxaldehyde.
For ICAld determination, an inoculum of 1 × 106 conidia of T. virens was added to 1 l potato dextrose broth (Sigma), and grown for 3 d at 28°C with shaking at 200 rpm. To evaluate the effect of Trp supply on ICAld accumulation, the medium was supplemented with L-Trp (Merck) at a concentration of 100 mg/l. For ICAld determinations, the fungal culture was filtered and the pH of the supernatant adjusted to 7 using 2 N NaOH. Indolic compounds in supernatant filtrate were extracted three times with 1 l of ethyl acetate. The extracts were combined and evaporated to dryness under a stream of nitrogen, taken up and diluted 1:10 (v/v) without L-Trp in the medium and 1:100 (v/v) with L-Trp before GC-MS analysis. ICAld was identified by comparison with mass spectra from the library (NIST/EPA/NIH, “Chem Station” Agilent Technologies Rev. D.04.00 2002). The identity of ICAld was further confirmed by comparison of retention time in the fungal extract with samples of the pure ICAld (Sigma). The molecular ion was monitored after electron impact ionization (70 eV). ICAld, m/z 144. To estimate the amount of ICAld produced by T. virens, we constructed a standard curve.
Mass spectrometry analysis.
Identification and determination of all compounds was performed using a gas chromatography-mass spectrometry system. Samples were injected in an Agilent 6850 Series II gas chromatograph equipped with an Agilent MS detector model 5973 and 30-mm × 0.2 mm × 0.25 mm, 5% phenyl methyl silicone capillary column (HP-5 MS). The operating conditions used were: 1 ml min−1 helium as carrier gas, 300°C detector temperature and 250°C injector temperature. The column was held for 5 min at 150°C and programmed at 5°C min−1 to a 278°C final temperature for 5 min.
Bioassays for Trichoderma-induced resistance against B. cinerea.
To test plant protection conferred by T. virens and T. atroviride against B. cinerea, Arabidopsis seedlings were inoculated with a fungal density of ∼1 × 106 spores by placing the spores at 1 cm at the opposite ends of agar plates containing 9-d-old germinated Arabidopsis seedlings (10 seedlings per plate). Trichoderma was allowed to grow for 3-d to elicit defense responses by the physical contact of the mycelium with the root system. B. cinerea was grown on PDA medium for 12 d, at this time, the conidia were collected and resuspended in sterilized distilled water. Arabidopsis shoots were then inoculated with ∼1 × 106 conidia of B. cinerea and leaves exhibiting soft rot symptoms were determined by visual inspection 3 d later. Numbers of symptomatic leaves per seedling were counted as a measure of disease severity. The percentage of dead plants was determined 5 d after pathogen inoculation for a total of 30 plants. Plants were grown at 24°C in a chamber with a 16 h light (200 µmol m2 s−1)/8 h darkness photoperiod.
Data analysis.
Experiments were statistically analyzed in the SPSS 10 program (SPSS, Chicago). Univariate and multivariate analyzes with a Tukey's post hoc test were used for testing differences in the biochemical analysis for SA, JA, ICAld, camalexin and anthocyanin measurements, number of lesions and percentage of dead plants in WT Arabidopsis. Different letters are used to indicate means that differ significantly (p ≤ 0.05).
Acknowledgments
We thank Drs. Frederick M. Ausubel and Detlef Weigel for kindly providing us with Arabidopsis transgenic seeds and Dr. Jane Glazebrook for kindly sharing camalexin standard. This work was supported by grants from the Consejo Nacional de Ciencia y Tecnología (CONACYT, México, grant no. 43978) and the Consejo de la Investigación Científica (UMSNH, México, grant no. CIC 2.26).
Abbreviations
- WT
wild type
- dai
days after inoculation
- JA
jasmonic acid
- SA
salicylic acid
- ET
ethylene
- ROS
reactive oxygen species
- SAR
systemic acquired resistance
- ISR
induced systemic resistance
- PR
pathogenesis-related proteins
- PAL
phenylalanine ammonia-lyase
- ICAld
indole-3-carboxaldehyde
- Cys
cysteine
- GUS
β-glucuronidase
- GC-MS
gas chromatography-mass spectrometry
Supplementary Material
References
- 1.Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J. The role of microbial signals in plant growth and development. Plant Signal Behav. 2009;4:701–712. doi: 10.4161/psb.4.8.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species: opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 3.Chang YC, Backer R, Klefield O, Chet I. Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis. 1986;70:145–148. doi: 10.1094/PD-70-145. [DOI] [Google Scholar]
- 4.Yedidia I, Shivasta AK, Kalpulnik Y, Chet I. Effect of Trichoderma harzianum on microelement concentration and increased growth of cucumber plants. Plant Soil. 2001;235:235–242. doi: 10.1023/A:1011990013955. [DOI] [Google Scholar]
- 5.Adams P, De-Leij FA, Lynch JM. Trichoderma harzianum Rifai 1295-22 mediates growth promotion of Crack willow (Salix fragilis) saplings in both clean and metal-contaminated soil. Microb Ecol. 2007;54:306–313. doi: 10.1007/s00248-0069203-0. [DOI] [PubMed] [Google Scholar]
- 6.Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J. Trichoderma virens,a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009;149:1579–1592. doi: 10.1104/ pp.108.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yedidia I, Benhamou N, Chet I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol. 1999;65:1061–1070. doi: 10.1128/aem.65.3.1061-1070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segarra G, Van-der-Ent S, Trillas I, Pieterse CMJ. MYB72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial microbe. Plant Biol. 2009;11:90–96. doi: 10.1111/j.1438-8677.2008.00162.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryals JA, Urs HN, Williams MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 11.Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 12.Koornneef A, Pieterse CM. Crosstalk in defense signaling. Plant Physiol. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darvill AG, Albersheim P. Phytoalexins and their elicitors a defense against microbial infection in plants. Annu Rev Plant Physiol. 1984;35:243–275. doi: 10.1146/annurev.pp.35.060184.001331. [DOI] [Google Scholar]
- 14.Bednarek P, Osbourn A. Plant-microbe interactions: chemical diversity in plant defense. Science. 2009;324:746–748. doi: 10.1126/science.1171661. [DOI] [PubMed] [Google Scholar]
- 15.Thomma BPHJ, Nelissen I, Eggeront K, Broekaert WF. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999;19:163–171. doi: 10.1046/j.1365313X.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari S, Plotnikova JM, De-Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2 but not SID2, EDS5 or PAD5. Plant J. 2003;35:193–205. doi: 10.1046/j.1365313X.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Galletti R, Denoux C, De-Lorenzo G, Ausubel FM, Dewdney J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007;144:367–379. doi: 10.1104/ pp.107.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jejelowo OA, Conn KL, Tewari JP. Relationship between conidial concentration, germling growth and phytoalexin production by Camelina sativa leaves inoculated with Alternaria brassicae. Mycol Res. 1991;95:928–934. doi: 10.1016/S0953-7562(09)80089-0. [DOI] [Google Scholar]
- 19.Tsuji J, Jackson E, Gage DA, Hammerschmidt R, Sommerville SC. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv. syringae. Plant Physiol. 1992;98:1304–1309. doi: 10.1104/ pp.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers EE, Glazebrook J, Ausubel FM. Mode of action of the Arabidopsis thaliana phytoalexin camalexin and its role in Arabidopsis-pathogen interactions. Mol Plant Microbe Interact. 1996;9:748–757. doi: 10.1094/MPMI-9-0748. [DOI] [PubMed] [Google Scholar]
- 21.Glazebrook J, Ausubel FM. Isolation of phytoalexindeficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, et al. Phytoalexin deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glawischnig E, Hansen BG, Olsen CE, Halkier BA. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism. Proc Natl Acad Sci USA. 2004;101:8245–8250. doi: 10.1073/pnas.0305876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottcher C, Westphal L, Schmotz C, Prade E, Scheel D, Glawischnig E. The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell. 2009;21:1830–1845. doi: 10.1105/tpc.109.066670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou N, Tootle TL, Glazebrook J. Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell. 1999;11:2419–2428. doi: 10.1105/tpc.11.12.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatoš A, et al. CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol. 2006;141:1248–1254. doi: 10.1104/pp.106.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zook M, Hammerschmidt R. Origin of the thiazole ring of camalexin, a phytoalexin from Arabidopsis thaliana. Plant Physiol. 1997;113:463–468. doi: 10.1104/pp.113.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah J, Tsui F, Klessing DF. Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact. 1997;10:69–78. doi: 10.1094/MPMI.1997.10.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Schommer C, Palatnik J, Aggarwal P, Chetelat A, Cubas P, Farmer E, et al. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008;6:230. doi: 10.1371/journal.pbio.0060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boller T, Yang-He S. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamb C, Dixon R. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 32.Asselbergh B, Curvers K, Franca SC, Audenaert K, Vuylsteke M, Van-Breusegem F, et al. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007;144:1863–1877. doi: 10.1104/pp.107.099226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penninckx IA, Thomma BP, Buchala A, Métraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djonović S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM. Sm1, a proteinaceous elicitor by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant Microbe Interact. 2006;19:838–853. doi: 10.1094/MPMI-19-0838. [DOI] [PubMed] [Google Scholar]
- 35.Djonović S, Vargas WA, Kolomiets MV, Horndeski M, Wiest A, Kenerley CM. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007;145:875–889. doi: 10.1104/pp.107.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segarra G, Casanova E, Bellido D, Odena MA, Oliveira E, Trillas I. Proteome, salicylic acid and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics. 2007;7:3943–3952. doi: 10.1002/pmic.200700173. [DOI] [PubMed] [Google Scholar]
- 37.Verhagen BWM, Glazebrook J, Tong-Zhu T, Chang HS, van-Loon LC, Pieterse CMJ. The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact. 2004;17:895–908. doi: 10.1094/MPMI.2004.17.8.895. [DOI] [PubMed] [Google Scholar]
- 38.van Loon LC. Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol. 2007;119:243–254. doi: 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- 39.Eulgem T. Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci. 2005;10:71–78. doi: 10.1016/j.tplants.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Bednarek P, Bednarek M, Svatoš A, Scheneider B, Doubsky J, Mansurova M, et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- 41.Frey M, Chomet P, Glawischning E, Stettner C, Grün S, Winklmair A, et al. Analysis of a chemical plant defense mechanism in grasses. Science. 1997;277:696–699. doi: 10.1126/science.277.5326.696. [DOI] [PubMed] [Google Scholar]
- 42.Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. Glucosinolate required for an Arabidopsis innate immune response. Science. 2009;323:95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devys M, Barbier M. Indole-3-carboxaldehyde in the cabbage Brassica oleracea: a systematic determination. Phytochemistry. 1991;30:389–391. doi: 10.1016/00319422(91)83690-M. [DOI] [Google Scholar]
- 44.Dzurilla M, Kutschy P, Zaletova J, Ruzinsky M, Kovacik V. Synthesis of camalexin. Molecules. 2001;6:716–720. doi: 10.3390/60900716. [DOI] [Google Scholar]
- 45.Viterbo A, Wiest A, Brotman A, Chet I, Kenerley C. The 18mer peptaibols form Trichoderma virens elicit plant defense responses. Mol Plant Pathol. 2007;8:737–746. doi: 10.1111/j.13643703.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 46.Vargas WA, Djonovic S, Sukno SA, Kenerley CM. Dimerization controls the activity of fungal elicitors that trigger systemic resistance in plants. J Biol Chem. 2008;283:19804–19815. doi: 10.1074/jbc.M802724200. [DOI] [PubMed] [Google Scholar]
- 47.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopisis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- 48.Pirie A, Mullins MG. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate and abscisic acid. Plant Physiol. 1976;58:468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



