Abstract
Plants possess versatile strategies that permit efficient use of limited nutrient resources during senescing process. This metabolic adjustment is critical for prevention of diverse cellular damage and thus for reproductive success and offspring production, particularly under environmental stress conditions. However, it is largely unknown how age-dependent resistance to cellular damages is established and how it is influenced by environmental stress signals during senescing process. We found that the VNI2 (VND-INTERACTING 2) transcription factor, which belongs to the NAC (NAM/ATAF1, 2/CUC2) transcription factor family, plays a role in the age-dependent induction of stress resistance. The VNI2 transcription factor is transcriptionally induced during senescing process and regulates COR/RD genes by binding directly to their promoters. The COR/RD proteins play a role in the protection from diverse cellular damages during senescing process. Notably, the transcriptional activation activity of VNI2 is further elevated under high salinity. These results indicate that plants increase environmental stress resistance by inducing the VNI2 gene to assure their reproductive success, supporting signaling crosstalk between stress resistance response and senescing process.
Key words: abscisic acid, arabidopsis, COR/RD, salt stress, senescence, VNI2
Leaf Senescence and Environmental Stress Resistance
Leaf senescence, which is the final developmental step of plant life cycle, is orderly and active process.1 The tightly controlled developmental process is particularly important for nutrient recycling and energy redistribution, assuring reproductive success.1–3 Since plants are vulnerable to environmental changes as they age, protection strategies to complete their life cycle and produce offspring are necessary during senescing process,1 especially under environmental stress conditions.
It has been well-known that environmental stress responses and aging process is intimately interconnected.1,3 Increased environmental stress resistance is frequently accompanied by prolonged longevity.4–6 Conversely, mutants that exhibit expanded life span are also resistant to environmental stress. The close relationship between aging process and environmental stress response have been documented extensively in many living organisms such as Drosophila melanogaster, Saccharomyces cerevisiae and Mus musculus.6–10 In Arabidopsis, numerous senescence-associated genes (SAGs) have been identified,1 and some of them have been shown to be responsive to various environmental stresses, suggesting that environmental stress resistance and leaf senescence are interrelated. However, the underlying molecular mechanisms linking environmental stress regulation and leaf senescence are largely unknown.
The Arabidopsis VNI2 gene encoding a member of NAC transcription factor is induced by salt stress and senescing process,11 suggesting a role of VNI2 as a signaling web between salt stress resistance and leaf senescence. Whereas transgenic plants overexpressing the VNI2 gene (35S:VNI2) exhibit prolonged leaf longevity with enhanced resistance to high salinity, the VNI2-deficient vni2-1 mutant shows accelerated leaf senescence. It is also susceptible to high salinity. Extensive gene expressional analysis revealed that expression of stress-responsive genes is greatly altered but that of SAGs is uninfluenced in the 35S:VNI2 transgenic and vni2-1 mutant plants. In particular, the VNI2 transcription factor regulates COR15A/B and RD29A/B genes by binding directly to their promoters.
The COR15A/B and RD29A/B genes are well-known marker genes that are involved in abiotic stress responses.12,13 The promoters of the COR/RD genes have multiple cis-acting elements that are responsive to diverse stress signals, such as osmotic stress, drought and high salinity. Despite their importance in environmental stress responses, the COR/RD genes have not been explored in leaf senescence. Expression of the COR/RD genes gradually increases as plants senesce.11 Notably, transgenic plants overexpressing either the COR15A/B or RD29A/B genes also exhibit prolonged leaf longevity, indicating that the COR/RD genes play a role in leaf senescence. In nature, plants encounter diverse environmental stress conditions during senescing process. It is well known that environmental resistance correlates with senescing periods.4–6 Whereas leaf longevity is prolonged in stress-resistant plants,14–16 premature senescence occurs in stress-susceptible plants.17 Since longer senescing period contributes to reproductive success, it seems that plants have evolved a physiological mechanism that induces stress resistance as they grow old (Fig. 1).
Figure 1.
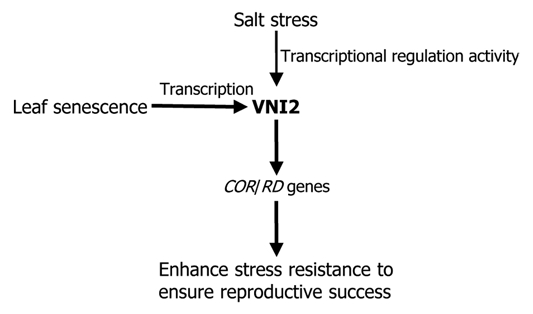
Proposed working model of VNI2 in the regulation of leaf senescence under high salinity. The VNI2 gene is transcriptionally induced during leaf senescing process. The VNI2 transcription factor regulates the COR/RD genes by binding to their gene promoters. The COR/RD proteins play a role in the protection of leaf cells from diverse cellular damages, establishing age-dependent stress resistance. The transcriptional activation activity of VNI2 is also modulated by salt stress, linking plant salt stress resistance to leaf senescence.
Modulation of Transcriptional Regulation Activity of VNI2 by High Salinity
Transcription factor activity is regulated at multiple steps: transcription, chromatin modification, posttranscriptional RNA metabolism, modulation of subcellular localization, dynamic dimer formation and posttranslational chemical modification.18
Modulation of transcriptional regulation activity is also an intriguing mechanism regulating transcription factor activity.19,20 The VNI2 transcription factor has been previously reported as a transcriptional repressor.21 We also found that VNI2 acts as a transcriptional repressor under normal growth conditions. The VNI2 transcription factor possesses a transcriptional activation domain in the C-terminal region. Notably, the transcriptional activation activity is modulated by environmental stresses, such as high salinity. It has been demonstrated that the VNI2 transcription factor behaves as a transcription activator under high salinity, providing a new layer of transcriptional regulation. It is apparent that the transcriptional regulation activity of VNI2 is also modulated through interactions with cis-acting elements. Transient expression assays using Arabidopsis protoplasts revealed that the VNI2 transcription factor binds directly to the promoters of the COR and RD genes and activates their transcription even under normal growth conditions. Consistently, the COR and RD genes are upregulated in the 35S:VNI2 transgenic plants. These observations indicate that the transcriptional regulation activity of VNI2 is influenced by environmental stress as well as by specific interactions with cis-acting elements in the promoters of target genes.
The VNI2 gene is also regulated developmentally through senescing process. Therefore, the regulation of the COR/RD genes by VNI2 is age-dependent. When environmental stress is applied to senescing plants, the VNI2 activity is further elevated by increasing the transcriptional activation activity. Regulation of the transcriptional regulation activity is certainly a direct and rapid way of responding efficiently to incoming environmental fluctuations, ensuring reproductive success under environmental stress conditions without premature death.
Acknowledgments
This work was supported by the Leaping Research Program (20100014373) provided by the National Research Foundation of Korea and by grants from the Plant Signaling Network Research Center (20100001457), the National Research Foundation of Korea (20100028147), and from the Next-Generation BioGreen 21 program (Plant Molecular Breeding Center No. PJ008103), Rural Development Administration, Republic of Korea.
References
- 1.Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, et al. The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnol J. 2003;1:3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- 3.Lim PO, Kim HJ, Nam HG. Leaf senescence. Annu Rev Plant Biol. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- 4.Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S, Tedesco P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol. 2001;36:1609–1617. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TE, Lithgow GJ, Murakami S. Hypothesis: interventions that increase the response to stress offer the potential for effective life prolongation and increased health. J Gerontol A Biol Sci Med Sci. 1996;51:392–395. doi: 10.1093/gerona/51a.6.b392. [DOI] [PubMed] [Google Scholar]
- 8.Murakami S, Johnson TE. Life extension and stress resistance in Caenorhabditis elegans modulated by the tkr-1 gene. Curr Biol. 1998;8:1091–1094. doi: 10.1016/s0960-9822(98)70448-8. [DOI] [PubMed] [Google Scholar]
- 9.Longo VD. Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies and mammalian neuronal cells. Neurobiol Aging. 1999;20:479–486. doi: 10.1016/s0197-4580(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 10.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 11.Yang SD, Seo PJ, Yoon HK, Park CM. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell. 2011;23:2155–2168. doi: 10.1105/tpc.111.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Wang WQ, Zhang GL, Kaminek M, Dobrev P, Xu J, Gruissem W. Senescence-inducible expression of isopentenyl transferase extends leaf life, increases drought stress resistance and alters cytokinin metabolism in cassava. J Integr Plant Biol. 2010;52:653–669. doi: 10.1111/j.1744-7909.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharabi-Schwager M, Lers A, Samach A, Guy CL, Porat R. Overexpression of the CBF2 transcriptional activator in Arabidopsis delays leaf senescence and extends plant longevity. J Exp Bot. 2010;61:261–273. doi: 10.1093/jxb/erp300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, et al. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA. 2007;104:19631–19636. doi: 10.1073/pnas.0709453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo PJ, Park JM, Kang SK, Kim SG, Park CM. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta. 2011;233:189–200. doi: 10.1007/s00425-010-1293-8. [DOI] [PubMed] [Google Scholar]
- 18.Yun J, Kim SG, Hong S, Park CM. Small interfering peptides as a novel way of transcriptional control. Plant Signal Behav. 2008;3:615–617. doi: 10.4161/psb.3.9.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell. 2009;21:3493–3505. doi: 10.1105/tpc.109.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao YJ, Song QX, Chen HW, Zou HF, Wei W, Kang XS, et al. Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta. 2010;232:1033–1043. doi: 10.1007/s00425-010-1238-2. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi M, Ohtani M, Mitsuda N, Kubo M, Ohme-Takagi M, Fukuda H, et al. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell. 2010;22:1249–1263. doi: 10.1105/tpc.108.064048. [DOI] [PMC free article] [PubMed] [Google Scholar]


