Abstract
In a large group of fungi, mating results in a dikaryon, a cell in which the two nuclei—one from each parent cell—share a single cytoplasm for a period of time without undergoing nuclear fusion. The dikaryon stage is typical in the life cycles of many fungal species primarily in the Basidiomycota, a large group that includes mushrooms, bracket fungi and many phytopathogenic fungi, such as the corn pathogen Ustilago maydis. Recently, we described that in U. maydis two conserved DNA-damage checkpoint kinases, Chk1 and Atr1, work together to control the dikaryon formation. However, how this pathway is activated during the dikaryon formation and how its activation/deactivation is coordinated with the different cell cycle phases is unknown. Here we propose and discuss several hypothesis to address these questions.
Key words: DNA damage response, phytopathogenic fungus, cell cycle, corn smut, Ustilago maydis, virulence
In the phytopathogenic fungus Ustilago maydis, virulence and sexual development are intricately interconnected.1 A prerequisite for generating the infectious stage is the mating of two compatible budding haploid cells to generate, after cell fusion, an infective dikaryotic filament. Once the fungus enters the plant tissue, the dikaryotic state dominates the period of growth occurring during the infectious phase. Dikaryons are cells in which two nuclei, one from each parent cell, share a single cytoplasm for a period of time without undergoing nuclear fusion.2 Maintenance of the dikaryotic state requires an elaborated cell cycle that relies on a synchronized nuclear division and the development of specialized projections (known as clamp connections) formed close to the position of the future septum formation. One nucleus enters and divides in the developing clamp cell, whereas the other divides in the main hypha, with the result that mitosis occurs in two distinct cell compartments.3 These processes take place during G2 phase, which has to be properly enlarged for this purpose.
The establishment and maintenance of dikaryotic growth is controlled by a heterodimeric homeodomain transcription factor, the b-complex, which subunits (bW and bE) are provided by each compatible mating partner.4 For a long time, a connection between the b heterodimer and the cell cycle control was predicted, although the details behind these connections were largely unknown.5 Recently, we described that Chk1 and Atr1, two DNA-damage checkpoint kinases, were activated in response to the formation of b-heterodimer and that this activation resulted in a transient G2 cell cycle arrest, most likely providing the time window required for appropriated dikaryon cell division.6,7 The absence of either Chk1 or Atr1 kinases resulted in defects in the ability of the dikaryotic cells to divide properly and therefore proliferation was affected.
Chk1 and Atr1 kinases are part of a signaling cascade devoted to cope with DNA damage, which role is conserved in a large number of eukaryotic organisms including U. maydis.8 The described new role of Atr1 and Chk1 during pathogenic development in U. maydis fits in the emerging view that elements from the DNA damage response cascade can be utilized to modulate developmental processes in virtue to their ability to interact with cell cycle machinery elements.9 In U. maydis there are two major questions concerning these connections that remain to be uncovered, and our current view and ideas about these questions are discussed below.
How a DNA Damage Response Pathway is Activated by a Transcriptional Factor during Dikaryon Formation?
A main question to be answered concerns how the b heterodimer, a transcriptional factor, activates the Atr1-Chk1 cascade, which in normal conditions responds to DNA damage. Attempts to correlate activation of Atr1-Chk1 cascade during b-induction with massive DNA damage—using the formation of Rad51 foci as reporter for active DNA repair—were unsuccessful.7 One possibility could be that the putative DNA damage is different from double strand break damage, so alternative DNA repair pathways such as base excision repair (BER) are recruited, and therefore no need for Rad51. In other eukaryotic systems, BER-mediated signaling is independent on Atr1-Chk1, but perhaps in U. maydis is more simplified than in higher eukaryotes for instance, and involves Atr1-Chk1 pathway.10 Another possibility is that a limited DNA damage (for instance, a single double strand break, not detectable using the Rad51-GFP reporter) induced by gene products regulated by b heterodimer, was responsible of the developmental activation of the DNA damage cascade during the induction of the virulence program in U. maydis. This explanation was inspired in the role of HO endonuclease during mating-type switching in Saccharomyces cerevisiae.11 In opposition to these explanations suggesting coupling between DNA damage and b induction is worth to say that no defect in the ability to arrest cell cycle or to infect plants was apparent in cells lacking Brh2, a BRCA2-like protein that is required for DNA repair.12 An alternative to DNA damage is that b induction may alter the kinetics of progression through S phase (perhaps via depletion of nucleotide pools or delaying the firing of late origins). In fact, genes involved in DNA replication such as those encoding putative subunits of polymerase (pol2, um01008; pol1, um04529), DNA replication licensing factor (um06402) or ribonucleoside reductase (rrn1, um11750) are downregulated after b-induction.13 However, it has not been addressed whether such a gene transcription downregulation is the cause of cell cycle arrest or whether the transcription of these genes is downregulated because the cell cycle arrest (in other words, is not clear whether they are cause or consequence). One relatively simple way to test this idea, the use of FACS analysis to determine whether b induction prolongs S phase, indicated no obvious defect in S-phase progression in cells producing the b heterodimer.7 One appealing hypothesis is that a few replication origins could be more sensitive and that these origins could be responsible of the cell cycle arrest. Currently we are characterizing a wide collection of replication origins in U. maydis and carrying out bi-dimensional analysis of replication intermediates trying to detect differences. Finally a third alternative to explain how a transcription factor triggers a DNA-damage cascade is based in two recent reports showed that activation of DNA damage response cascade can be triggered in the absence of DNA damage by stable association of elements of the cascade with chromatin.14,15 Whether a similar mechanism could explain our observations in U. maydis will need additional research.
How to Alternate Activation/Deactivation Cycles of Atr1-Chk1 Cascade Coupled to Cell Cycle Transitions in the Dikaryon?
The second main question to be addressed is how the cell cycle arrest is intermittently released during biotrophic development. The current idea is that Atr1-Chk1 cascade has to be activated by the b heterodimer every cell cycle, to provide the extended G2 phase, but that once nuclei are separated in different cell compartments (one in the clamp cell, the other in the main hypha) this signal cascade has to be shut off, to allow the G2/M transition. The predicted downregulation must occur at least at two different levels: the transcriptional activity of b heterodimer has to be inhibited and the Chk1-Atr1 signaling has to be stopped. Downregulation of b heterodimer occurs most likely through the activity of the Clp1 (Clampless1) protein. This factor was first described in the mushroom Coprinopsis cinerea as needed for clamp formation as well as for the distribution of nuclei during cell division of the dikaryon, via its ability to interact with the A-complex (the ortholog of b heterodimer in this mushroom).16 In U. maydis, Clp1 interaction with bW blocks b-dependent functions, such as the b-dependent G2 cell cycle arrest: for instance, the forced expression of clp1 in strains where filamentation is induced by an active bE/bW heterodimer suppresses the cell cycle block. Moreover, clp1 mutants are unable to release the bE/bW-triggered cell cycle arrest.17 Interestingly clp1 transcription is indirectly activated by the b heterodimer, via the induction of the transcriptional activator Rbf1, so the downregulation of b heterodimer is the result of a negative feedback by end product.
How the second level of downregulation, the attenuation of Atr1-Chk1 signal, occurs is unknown. Recently it has been described that in mammalian cells the Akt/PKB kinase is able to override DNA damage-induced G2 arrest, via phosphorylation of Chk1.18 This way, the cell becomes refractory to Atr1-Chk1 activation late in G2 before the onset of mitosis. Interestingly, in U. maydis, the transcriptional levels of ukb1, encoding the putative ortholog of PKB, are increased during b-induction, via Rbf1, in a similar way as clp1 is upregulated.13 Moreover, U. maydis cells defective in ukb1 are affected in proliferation in planta, producing plant symptoms that remind those obtained when plants were infected with chk1 or atr1 mutants (small tumors and absence of teliospore production).19 We entertained the hypothesis that as it happens in mammalian cells, in U. maydis Ukb1 could downregulate the Atr1/Chk1 pathway through direct phosphorylation of Chk1.
On basis of these ideas, our hypothesis proposes that attenuation of G2 arrest occurs at two levels: an immediate shut off of the signaling through the Atr1/Chk1 pathway mediated by the inhibition of Chk1 by the PKB-like kinase Ukb1; and a posterior second level of transcriptional shut-off of b heterodimer by the Clp1 protein (Fig. 1). Both negative regulators, Ukb1 and Clp1 are end-products of a transcriptional cascade formed by b heterodimer and its downstream regulator Rbf1. Clp1 inhibits b heterodimer, so we propose that a transcription network defined by sequential waves of expression of transcription factors might function independently of any extrinsic control. This transcriptional network may oscillate independently of the Cdk-cyclin oscillator (responsible of cell cycle transitions) although they are coupled in a manner that it provides the proper timing to ensure the cell cycle events. A transcriptional network model in which transcription factors expressed in one step bind to the promoters of genes encoding transcription factors that function in a subsequent step and inhibits the previous one were recently proposed as an emergent property of the transcription factor network that functions as a cell cycle oscillator independently of, and in tandem with, the CDK oscillator, during cell cycle transitions.20 From our hypothesis a few predictions could be made, and one of these is that altering the transcriptional rate of the promoters responsible of intermediate regulatory factors (e.g., clp1 or ukb1) might affect the length of G2 phase and thereby the ability to properly form the dikaryon. These predictions are being tested currently in our laboratory.
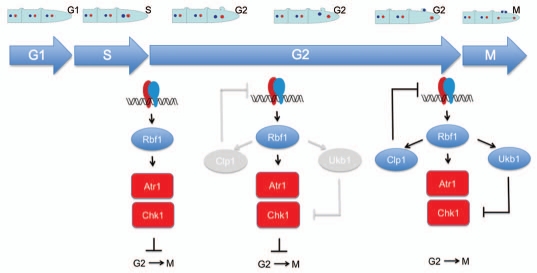
Figure 1.
Hypothetical model of b-dependent G2 enlargement during dikaryon cell cycle. The b heterodimer activates a transcriptional cascade, which includes the Rbf1 regulator, resulting in the activation of the Atr1-Chk1 cascade. Chk1 inhibits the Cdc25 phosphatase resulting in a delay in G2/M transition, providing the expected enlargement of G2 phase required for the formation of clamp-like structures as well as the associated nuclear movements. During this enlarged G2 phase, the transcriptional activity of Rbf1 also generates the accumulation of two regulators: the Clp1 protein, which inhibits the b heterodimer; and the Ukb1 kinase, that is proposed to inhibit Chk1. Once these two regulators reach a threshold, the pathway is downregulated at transcriptional level (by Clp1-mediated inhibition of b heterodimer) as well as at the level of the signal cascade (by the Ukb1-mediated inhibition of Chk1), enabling the transition from G2 to M phase.
References
- 1.Steinberg G, Perez-Martin J. Ustilago maydis, a new fungal model system for cell biology. Trends Cell Biol. 2008;18:61–67. doi: 10.1016/j.tcb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Brown AJ, Casselton LA. Mating in mushrooms: increasing the chances but prolonging the affair. Trends Genet. 2001;17:393–400. doi: 10.1016/s0168-9525(01)02343-5. [DOI] [PubMed] [Google Scholar]
- 3.Gladfelter A, Berman J. Dancing genomes: fungal nuclear positioning. Nat Rev Microbiol. 2009;7:875–886. doi: 10.1038/nrmicro2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruzel EK, Hull CM. Establishing an unusual cell type: how to make a dikaryon. Curr Opin Microbiol. 2010;13:706–711. doi: 10.1016/j.mib.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Martin J, Castillo-Lluva S, Sgarlata C, Flor-Parra I, Mielnichuk N, Torreblanca J, et al. Pathocycles: Ustilago maydis as a model to study the relationships between cell cycle and virulence in pathogenic fungi. Mol Genet Genomics. 2006;276:211–229. doi: 10.1007/s00438-006-0152-6. [DOI] [PubMed] [Google Scholar]
- 6.de Sena-Tomas C, Fernandez-Alvarez A, Holloman WK, Perez-Martin J. The DNA damage response signaling cascade regulates proliferation of the phytopathogenic fungus Ustilago maydis in planta. Plant Cell. 2011;23:1654–1665. doi: 10.1105/tpc.110.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielnichuk N, Sgarlata C, Perez-Martin J. A role for the DNA-damage checkpoint kinase Chk1 in the virulence program of the fungus Ustilago maydis. J Cell Sci. 2009;122:4130–4140. doi: 10.1242/jcs.052233. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Martin J. DNA-damage response in the basidiomycete fungus Ustilago maydis relies in a sole Chk1-like kinase. DNA Repair (Amst) 2009;8:720–731. doi: 10.1016/j.dnarep.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Sherman MH, Bassing CH, Teitell MA. Regulation of cell differentiation by the DNA damage response. Trends Cell Biol. 2011;21:312–319. doi: 10.1016/j.tcb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holloman WK, Schirawski J, Holliday R. The homologous recombination system of Ustilago maydis. Fungal Genet Biol. 2008;45:31–39. doi: 10.1016/j.fgb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasmyth K. Regulating the HO endonuclease in yeast. Curr Opin Genet Dev. 1993;3:286–294. doi: 10.1016/0959-437x(93)90036-o. [DOI] [PubMed] [Google Scholar]
- 12.Kojic M, Kostrub CF, Buchman AR, Holloman WK. BRCA2 homolog required for proficiency in DNA repair, recombination and genome stability in Ustilago maydis. Mol Cell. 2002;10:683–691. doi: 10.1016/s1097-2765(02)00632-9. [DOI] [PubMed] [Google Scholar]
- 13.Heimel K, Scherer M, Vranes M, Wahl R, Pothiratana C, Schuler D, et al. The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog. 2010:6. doi: 10.1371/journal.ppat.1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilla CY, Melo JA, Toczyski DP. Colocalization of sensors is sufficient to activate the DNA damage checkpoint in the absence of damage. Mol Cell. 2008;30:267–276. doi: 10.1016/j.molcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soutoglou E, Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320:1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inada K, Morimoto Y, Arima T, Murata Y, Kamada T. The clp1 gene of the mushroom Coprinus cinereus is essential for A-regulated sexual development. Genetics. 2001;157:133–140. doi: 10.1093/genetics/157.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimel K, Scherer M, Schuler D, Kamper J. The Ustilago maydis Clp1 protein orchestrates pheromone and b-dependent signaling pathways to coordinate the cell cycle and pathogenic development. Plant Cell. 2010;22:2908–2922. doi: 10.1105/tpc.110.076265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu N, Hegarat N, Black EJ, Scott MT, Hochegger H, Gillespie DA. Akt/PKB suppresses DNA damage processing and checkpoint activation in late G2. J Cell Biol. 2010;190:297–305. doi: 10.1083/jcb.201003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramovitch RB, Yang G, Kronstad JW. The ukb1 gene encodes a putative protein kinase required for bud site selection and pathogenicity in Ustilago maydis. Fungal Genet Biol. 2002;37:98–108. doi: 10.1016/s1087-1845(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 20.Orlando DA, Lin CY, Bernard A, Wang JY, Socolar JE, Iversen ES, et al. Global control of cell cycle transcription by coupled CDK and network oscillators. Nature. 2008;453:944–947. doi: 10.1038/nature06955. [DOI] [PMC free article] [PubMed] [Google Scholar]