Abstract
In plants, the tight regulation of plasma membrane transporters is essential to maintain nutrient homeostasis. The mechanisms controlling the abundance of transporters, and other integral plasma membrane proteins, now come to light. Ubiquitination appears as a major signal initiating cargo endocytosis and sorting into multivesicular bodies prior to degradation in the vacuole. We have indeed demonstrated that the root iron transporter IRT1 is subjected to ubiquitin-dependent trafficking in root epidermal cells. This control is crucial to keep IRT1 levels at the cell surface low and to cope with the toxicity associated with other readily available metal substrates of IRT1. Our work combined with recent report on the BOR1 boron transporter establishes ubiquitination as a conserved mechanism of plasma membrane protein trafficking in plants and highlights its importance for plant nutrition.
Key words: Endocytosis, Iron, IRT1, BOR1, Degradation, monoubiquitin
Plasma membrane transporters play a pivotal role in all living organisms by providing the cell surface with a selective barrier for a wide range of nutrients, small molecules and ions. These transport proteins are very often under the control of sophisticated molecular mechanisms to adapt to changing environmental conditions and to ensure optimal absorption of their cognate substrates. Although the molecular events controlling the abundance of plasma membrane proteins at the cell surface are largely documented in non-plant model organisms, the situation in plants is much less understood. This is particularly true for the well-established ubiquitin-dependent endocytosis and sorting in late endosomes of plasma membrane proteins, for which evidence is still lacking in plants.
Recently, we showed that the root iron transporter IRT1 from the model plant Arabidopsis thaliana, which mediates Fe2+ and other divalent metal absorption from the soil,1 surprisingly localizes to the early endosomes/trans-Golgi network compartment (EE/TGN).2 Pharmacological interference with vesicular trafficking highlighted the dynamic behavior of IRT1 in root epidermal cells. IRT1, although localized to EE/TGN, rapidly cycles to the plasma membrane to perform iron uptake from the soil. We demonstrated that IRT1 is ubiquitinated in vivo and carries monoubiquitin moieties on several cytosol-exposed lysine residues, a process known as multiubiquitination. Reminiscent of the ubiquitin-dependent endocytosis and degradation of animal receptor tyrosine kinases and yeast nutrient permeases,3,4 we hypothesized that IRT1 ubiquitination may control its dynamics in the cell. Transgenic plants expressing a mutant version of IRT1 in which two lysine residues were replaced by arginine (IRT1K154K179R) indeed show accumulation of IRT1 protein at the plasma membrane. This clearly establishes the role of ubiquitination in driving IRT1 intracellular trafficking.
Whether the cell surface localization of IRT1K154K179R reflects a role of ubiquitination in the internalization from the plasma membrane, in the sorting at the multivesicular bodies, or both is an open question. Interestingly, IRT1K154K179R-expressing plants displayed dramatic growth reduction, likely due to uncontrolled uptake of metals. Altogether, our work demonstrates the existence of monoubiquitin-dependent endocytosis in plants and reveals its crucial role in keeping IRT1 level at the plasma membrane low to prevent metal toxicity. The uptake of Fe2+ by IRT1 indeed requires the prior reduction of Fe3+ by the FRO2 ferric chelate reductase, whose activity was shown to be limiting for iron uptake.5–7 The amount of Fe2+ produced by FRO2 is therefore low and favors the uptake of other substrates of IRT1 such as Zn2+, Mn2+, Co2+ and Cd2+. Limiting the pool of IRT1 at the plasma membrane by ubiquitin-dependent endocytosis appears as a protective mechanism to limit the absorption of readily available metals other than iron. This also highlights the necessity for a strict co-regulation of FRO2 and IRT1 to optimize iron uptake. The overexpression of IRT1 in transgenic Arabidopsis plants consequently leads to a strong overload of Zn and Mn in roots and aerial tissues, while iron only very mildly accumulates. This, combined with the existence of point mutants of IRT1 with altered selectivity,8 offers the long term perspective of engineering transgenic plants designed to phytoremediate polluted soil for a given element.
Only a handful of plasma membrane proteins were shown to be ubiquitinated in plants, including the PIN2 auxin efflux carrier,9 the FLS2 flagellin receptor10 and the PIP2;1 water channel.11 Yet, the role of ubiquitination for such proteins remained unclear until very recently. Along with our recent report of monoubiquitin-dependent trafficking of IRT1 came a similar study on the Arabidopsis BOR1 boron transporter. Like iron, boron is essential for plants but toxic when present in excess. BOR1 has been previously shown to be regulated by boron-inducible endocytosis and degradation in the vacuole.12 Kasai et al.13 now showed that high boron concentrations induced BOR1 mono- or diubiquitination and that mutation of a single lysine residue affected BOR1 trafficking to the vacuole.13 In yeast and mammals, ubiquitination has been shown to trigger the internalization of plasma membrane proteins from the cell surface and sorting in multivesicular bodies/late endosomes (MVBs/LEs) for targeting and degradation in the vacuole/lysosomes,3,4 although recent reports emphasize the role of ubiquitination in later stages of endocytosis.14 Similarly, BOR1 ubiquitination is not required for endocytosis from the plasma membrane, this step being probably mediated by tyrosine-based motives found on its cytosolic face,15 but is crucial for the sorting of BOR1 in multivesicular bodies for subsequent targeting and degradation in the vacuole.13
It will be interesting to pinpoint the exact role of IRT1 ubiquitination in either the control of its internalization from the plasma membrane, the targeting to the internal vesicles of multivesicular bodies for delivery to the vacuole, or both. Hence, ubiquitination constitutes a conserved process controlling the localization and the fate of plasma membrane proteins, and appears as a critical mechanism to maintain iron and boron homeostasis in plants (Fig. 1). The quest for the machinery driving the ubiquitination of plasma membrane proteins has now begun. Already, the PUB12 and PUB13 U-box E3 ubiquitin ligases have just been reported to polyubiquitinate the FLS2 flagellin receptor, driving its flagellin-dependent degradation and preventing excessive or prolonged activation of immune responses.16 Whether these E3 ubiquitin ligases, or members from the same family, act on other plasma membrane proteins will have to be determined in the future. This will provide breakthrough information on how the dynamics and the fate of integral plasma membrane proteins are driven, and will highlight the importance of ubiquitination in important biological processes such as nutrition, response to pathogens and hormone transport.
Figure 1.
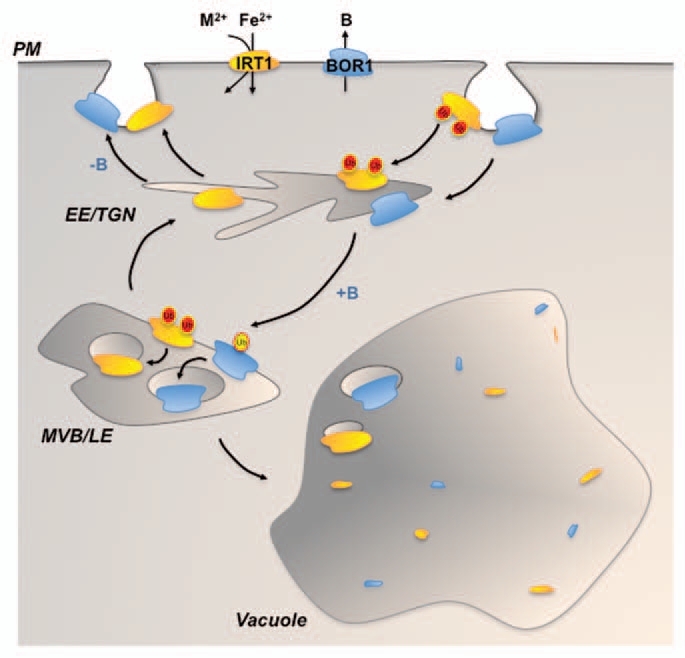
Ubiquitin-dependent trafficking of plasma membrane proteins in plants. Diagram illustrating the role of ubiquitination throughout the endocytic pathway of plant plasma membrane proteins with the example of IRT 1 (in orange) and BOR1 (in blue). IRT 1 multiubiquitination (red circles) controls either its internalization from the plasma membrane and/or its sorting in late endosomes required for vacuolar targeting, to maintain IRT 1 low at the plasma membrane independently of iron nutrition. BOR1 mono- or diubiquitination (yellow circle) is required when plants are exposed to high boron for BOR1 sorting in multivesicular bodies and subsequent targeting to the vacuole for degradation. The interplay of ubiquitin ligases and deubiquitinases thereby controls the dynamics of plasma membrane proteins. PM, plasma membrane; EE /TGN, Early endosome/Trans-Golgi network; MVB/LE , Multivesicular body/Late endosome compartment; B, Boron; M2+, Metals transported by IRT 1 in addition to Fe2+.
Acknowledgments
This work was supported by a Ph.D. fellowship from the French Ministry of National Education, Research and Technology (to M.B.), and Grant CDA0005/2007 from the Human Frontier Science Program Organization and Grant ANR-08-JCJC-0058 from the Agence Nationale de la Recherche (to G.V.).
References
- 1.Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, Friml J, et al. Monoubiquitin-dependent endocytosis of the IRON-REGULATED TRANSPORTER 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA. 2011;108:450–458. doi: 10.1073/pnas.1100659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: The network at work. Exp Cell Res. 2009;315:1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, Andre B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010;20:196–204. doi: 10.1016/j.tcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Yi Y, Guerinot ML. Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J. 1996;10:835–844. doi: 10.1046/j.1365-313x.1996.10050835.x. [DOI] [PubMed] [Google Scholar]
- 6.Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- 7.Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003;133:1102–1110. doi: 10.1104/pp.103.025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Pro. Natl Acad Sci USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 10.Goëhre V, Spallek T, Haeweker H, Mersmann S, Mentzel T, Boller T, et al. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol. 2008;18:1824–1832. doi: 10.1016/j.cub.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 11.Lee HK, Cho SK, Son O, Xu ZY, Hwang I, Kim WT. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell. 2009;21:622–641. doi: 10.1105/tpc.108.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takano J, Miwa K, Yuan L, von Wiren N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T. High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem. 2011;286:6175–6183. doi: 10.1074/jbc.M110.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, et al. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA. 2010;107:5220–5225. doi: 10.1073/pnas.0910744107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu DP, Lin WW, Gao XQ, Wu SJ, Cheng C, Avila J, et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]


