Abstract
Root hairs are single cells specialized in the absorption of water and nutrients from the soil. Growing root hairs require intensive cell-wall changes to accommodate cell expansion at the apical end by a process known as tip or polarized growth. We have recently shown that cell wall glycoproteins such as extensins (EXTs) are essential components of the cell wall during polarized growth. Proline hydroxylation, an early posttranslational modification of cell wall EXTs that is catalyzed by prolyl 4-hydroxylases (P4Hs), defines the subsequent O-glycosylation sites in EXTs. Biochemical inhibition or genetic disruption of specific P4Hs resulted in the blockage of polarized growth in root hairs. Our results demonstrate that correct hydroxylation and also further O-glycosylation on EXTs are essential for cell-wall self-assembly and, hence, root hair elongation. The changes that O-glycosylated cell-wall proteins like EXTs undergo during cell growth represent a starting point to unravel the entire biochemical pathway involved in plant development.
Key words: cell wall, O-glycoproteins, extensins, proline hydroxylation, polarized growth, root hairs, P4H
P4Hs Defines the Potential O-Glycosylation Sites on HRGPs Targeted to Plant Cell Walls
Structural O-glycoproteins of plant cell walls grouped as HRGPs are defined by posttranslational covalent modifications that include the hydroxylation of proline residues into 4-hydroxyproline (Hyp) by prolyl 4-hydroxyproline (P4Hs). The conversion of Pro to Hyp in the secretory pathway affects protein conformation and provides reactive hydroxyl groups for further modification such as O-glycosylation. The sugars involved, short arabino-oligosaccharides in the case of extensins (EXTs) and larger arabinogalactan polysaccharides on arabinogalactan-proteins (AGPs),1 are incorporated by specific glycosyltransferases.2–4 The enzyme that generates peptidyl 4-hydroxyproline from proline residues is usually called prolyl 4-hydroxylase (P4H), although it is actually an oxygenase that introduces oxygen between the hydrogen and carbon atoms of peptidyl proline. This enzyme belongs to a family of 2-oxoglutarate-dependent dioxygenases that require 2-oxoglutarate and O2 as co-substrates, Fe2+ as a cofactor and ascorbate to prevent the rapid inactivation by self-oxidation.5 In our work we were able to inhibit plant proline hydroxylation of HRGPs by exposing roots to two different small molecule inhibitors (α,α-dipyridyl and ethyl-3,4-dihydroxybenzoate4). Remarkably, root hair elongation was also inhibited after this treatment, suggesting the existence of a link between proline hydroxylation and root hair growth. In addition, we identified T-DNA insertional mutants for each of three P4Hs (P4H2, P4H5 and P4H13), out of 13 P4Hs present in the Arabidopsis thaliana genome, with a clear short root hair phenotype (Fig. 1A and B) that corroborated the in planta biochemical inhibition.4 In all these mutants, root Hyp levels were drastically reduced, in agreement with the enzymatic blockage of P4Hs. Notably, overexpression of these hydroxylases displayed the opposite phenotype: extra-large root hairs (Fig. 1C). Based on that, it became clear that proline hydroxylation of HRGPs are absolutely required for cell expansion in root hairs. However, it remains unknown if inhibiting proline hydroxylation in other cell types or tissues would also affect cell expansion as it happened in root hairs.
Figure 1.
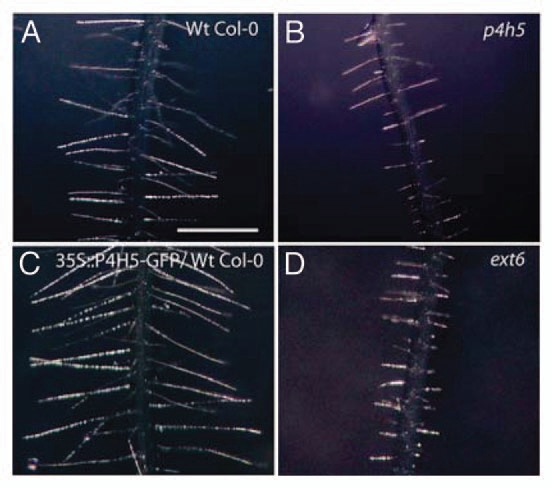
Root hair phenotypes in Arabidopsis thaliana. Root hairs in Wt Col-0 plants (A) and in the mutant p4h5 (B). Extra long root hairs in the 35S::P4H5-GFP overexpressing line in a Wt Col-0 background (C). Root hair phenotype in the putative P4H5-target ext6 mutant (D). Scale bar = 800 µm.
EXTs as Targets of P4Hs are Key Components for Cell Wall Network Self-Assembly in Root Hairs
To address the target specificity of P4Hs, we used a yeast two-hybrid approach using P4H5 as bait. Several polyproline type II repeat containing proteins such as LRX3 were identified as the main targets of P4H5 suggesting that EXTs might be one of its major targets.4 This result was also validated by homology modeling of P4H5 and related P4Hs (P4H2 and P4H13) on the minimal polyproline type-II peptide.4 All together, these results suggested that P4H5 and possibly P4H2/P4H13 preferentially hydroxylate proteins that contain polyproline repeats such as in Extensins and Proline-Rich Proteins (PRP). We cannot exclude from our analysis that these P4Hs also hydroxylate AGPs and other signaling and regulatory molecules that contain non-contiguous and contiguous proline repeats such as Argonaute 2,6 CLV3 and CLE2 glycopeptides7 and Hyp-systemins.8
To identify the putative P4Hs targets in root hairs, we took advantage of a co-expression analysis of the entire Arabidopsis network database (Aranet; aranet.mpimp-golm.mpg.de/aranet), which allowed us to recognize a subset of root hair extensins previously uncharacterized in this cell type. As queries, we chose cell wall genes known to be important for root hair growth, like expansins EXP7 and EXP18,9 proline-rich proteins PRP1 and PRP3,10 leucine-rich extensin protein LRX111,12 and the bLHL-type transcription factor RSL4, a master regulator of the expression of several root hair cell wall genes.13 In addition, we employed microarrays for functional genomics focused on root hair mutants,14–16 yielding the same group of EXTs.4 Based on these two approaches, we were able to identify several EXTs that seem to be important for root hair growth. This was later confirmed when T-DNA mutant lines for each of these EXTs exhibited drastically shorter root hairs (ext6 in Fig. 1D4). Regarding biochemical function of the O-glycosylated substrates, it has been previously shown that EXTs have the ability to form a tridimensional self-assembled network in the plant cell wall.17,18 In this context, the results obtained in our study teach us that there is almost no genetic redundancy for the root hair EXTs that build the network, as each of these EXTs seem to be crucial for the correct self-assembly of the wall. Analogously, when disrupting a single extensin gene (EXT3), a strong embryo lethal phenotype named root-, shoot-, hypocotyl-defective seedling (rsh), had been observed,17 consistent with the notion that EXTs are critical components of the EXTs-pectin network for further cell wall deposition. It is highly probable that such a network is also relevant to cell expansion in other plant cell types.
Impact of Proline Hydroxylation and O-Glycosylation on EXT Network Self-Assembly
To understand the biological meaning of the lack of hydroxylation/O-glycosylation on EXTs, we analyzed possible conformational changes induced by proline hydroxylation and O-arabynosylation of the small EXT repeat Ser(Pro)4 by performing molecular dynamics (MD) simulation. The modeling revealed that O-glycosylation stabilizes the helical conformation of the EXT short peptide whereas incomplete O-glycosylation increases its flexible conformation.4 Hence, we theorize that EXTs with incomplete hydroxylation/O-glycosylation have an enormous impact on the interaction of EXTs with the surrounding environment, including interaction with other EXTs in the network or with other modifying enzymes like EXT-specific peroxidases. It is important to take into account that a few peroxidases (such as PER13a, PER73, etc.) are highly co-expressed with several of the root hair EXTs. Thus, there is a good chance that at least some of them are responsible for the crosslinking of EXTs.4 It remains to be tested if crosslinking levels are abnormal and/or the EXT network is impaired in root hairs of the p4h mutants.
From an evolutionary perspective, it is interesting that proline hydroxylation was inhibited by RNAi in one P4H (Cr-P4H1) out of the 10 P4Hs present in the unicellular green algae Chlamydomonas reinhardtii, whose cell walls became drastically disrupted.19 This observation highlights a conserved function of proline hydroxylation/O-glycosylation of structural cell wall proteins throughout the evolution from ancient green algae to vascular plants.4,19 Furthermore, both studies suggest that there is a low level of genetic redundancy in P4Hs' functionality, although more studies are necessary to really understand their biological specificity and roles in plant cells.
We hypothesize that dynamic changes in the crosslinked EXT network in cell walls is sensed (directly or indirectly) by a putative receptor-like kinase (RLK) such as FERONIA (FER), recently shown to be involved in the RHO GTPases (RAC/ROP2) signaling pathway that controls ROS-Ca+2-mediated root hair development.20 Further experiments are needed to test whether FER (or other RLK)-RAC/ROP2-ROS-Ca+2 is actually the signaling pathway that links the root hair EXT network on the cell wall to the onset/stopping of root hair growth.
References
- 1.Lamport DTA, Miller DH. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1972;48:454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egelund J, Obel N, Ulvskov P, Geshi N, Pauly M, Bacic A, Larsen Petersen B. Molecular characterization of two Arabidopsis thaliana glycosyltransferase mutants, rra1 and rra2, which have a reduced residual arabinose content in a polymer tightly associated with the cellulosic wall residue. Plant Mol Biol. 2007;64:439–449. doi: 10.1007/s11103-007-9162-y. [DOI] [PubMed] [Google Scholar]
- 3.Gille S, Hänsel U, Ziemann M, Pauly M. Identification of plant cell wall mutants by means of a forward chemical genetic approach using hydrolases. Proc Natl Acad Sci USA. 2009;106:14699–14704. doi: 10.1073/pnas.0905434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velasquez SM, Ricardi MM, Gloazzo Dorosz J, Fernandez PV, Nadra AD, Pol-Fachin L, et al. O-glycosylated cell wall extensins are essential in root hair growth. Science. 2011;33:1401–1403. doi: 10.1126/science.1206657. [DOI] [PubMed] [Google Scholar]
- 5.Tiainen P, Myllyharju J, Koivunen P. Characterization of a second Arabidopsis thaliana prolyl 4-hydroxylase with distinct substrate specificity. J Biol Chem. 2005;280:1142–1148. doi: 10.1074/jbc.M411109200. [DOI] [PubMed] [Google Scholar]
- 6.Hon WC, Wilson MI, Harlos K, Claridge TDW, Schofield CJ, Pugh CW, et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 7.Ohyama K, Shinohara H, Ogawa-Ohnishi M, Matsubayashi Y. A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nature Chem Biol. 2009;5:578–580. doi: 10.1038/nchembio.182. [DOI] [PubMed] [Google Scholar]
- 8.Ryan CA, Pearce G. Systemic signaling in tomato plants for defense against herbivores: Isolation and characterization of three novel defense-signaling glycopeptides hormones coded in a single precursor gene. Proc Natl Acad Sci USA. 2003;100:14573–14577. doi: 10.1074/jbc.M304159200. [DOI] [PubMed] [Google Scholar]
- 9.Won SK, Lee YJ, Yeon Lee H, Kyung Heo Y, Cho M, Hyung-Taeg Cho. Cis-element- and transcriptome-based screening of root hair-specific genes and their functional characterization in Arabidopsis. Plant Physiol. 2009;150:1459–1473. doi: 10.1104/pp.109.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernhardt C, Tierney ML. Expression of AtPRP3, a proline-rich structural cell wall protein from Arabidopsis is regulated by cell-type-specific developmental pathways involved in root hair formation. Plant Physiol. 2000;122:705–714. doi: 10.1104/pp.122.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, et al. Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol. 2003;131:1313–1326. doi: 10.1104/pp.102.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumberger N, Steiner M, Ryser U, Keller B, Ringli C. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003;35:71–81. doi: 10.1046/j.1365-313x.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 13.Yi K, Menand B, Bell E, Dolan L. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet. 2010;42:264–267. doi: 10.1038/ng.529. [DOI] [PubMed] [Google Scholar]
- 14.Diet A, Link B, Seifert GJ, Schellenberg B, Wagner U, Pauly M, et al. The Arabidopsis root hair cell wall formation mutant lrx1 is suppressed by mutations in the RHM1 gene encoding a UDP-L-rhamnose synthase. Plant Cell. 2006;18:1630–1641. doi: 10.1105/tpc.105.038653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MA, Raymond MJ, Smirnoff N. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J. 2006;45:83–100. doi: 10.1111/j.1365-313X.2005.02609.x. [DOI] [PubMed] [Google Scholar]
- 16.Deal RB, Henikoff S. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev Cell. 2010;18:1030–1040. doi: 10.1016/j.devcel.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, et al. Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA. 2008;105:2226–2231. doi: 10.1073/pnas.0711980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valentin R, Cerclier C, Geneix N, Aguié-Béhin V, Gaillard C, Ralet MC, Cathala B. Elaboration of extensin-pectin thin film model of primary plant cell wall. Langmuir. 2010;26:9891–9898. doi: 10.1021/la100265d. [DOI] [PubMed] [Google Scholar]
- 19.Keskiaho K, Hieta R, Sormunen R, Myllyharju J. Chlamydomonas reinhardtii has multiple prolyl 4-hydroxylases, one of which is essential for proper cell wall assembly. Plant Cell. 2007;19:256–269. doi: 10.1105/tpc.106.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duang Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]


