Abstract
Iron (Fe) is an essential micronutrient for plants whose deficiency presents a major worldwide agricultural problem. Moreover, Fe is not easily available in neutral to alkaline soils, rendering plants deficient in Fe despite its abundance. Plants secrete phenolics, such as protocatechuic acid (PCA) and caffeic acid (CA), to take up and utilize apoplasmic precipitated Fe, but despite the rapid progress in understanding cellular and subcellular Fe transport, the molecular mechanisms of phenolics synthesis and secretion are not clear. Recently, we isolated and characterized a phenolics efflux transporter in rice by characterizing a mutant in which the amount of PCA and CA in the xylem sap was dramatically reduced, which we hence named phenolics efflux zero 1 (pez1). PEZ1 is a plasma membrane protein that transports PCA when expressed in Xenopus laevis oocytes, and characterization of PEZ1 knockdown and overexpressing plants revealed that it plays an essential role in solubilizing precipitated apoplasmic Fe. The identification of PEZ1 will increase our understanding of apoplasmic Fe solubilization as well as promote research on phenolics efflux mechanisms in different organisms.
Key words: iron, Oryza sativa, phenolics transport, protocatechuic acid, xylem sap
Although mineral soils contain over 6% iron (Fe),1 it predominantly exists as Fe(III) chelates, and plants ultimately cannot absorb Fe under various physiological conditions such as high soil pH in alkaline soils.2 Thus, plants growing in high-pH soils are not very efficient in developing and stabilizing chlorophyll, resulting in the yellowing of leaves, poor growth and reduced yield. Plants, however, have developed sophisticated mechanisms to take up the small amount of soluble Fe. Non-graminaceous plants release protons, secrete phenolics, reduce Fe(III), and finally, take up Fe2+.3–5 Once Fe is solubilized, Fe(III) is reduced to Fe2+ by a membrane-bound Fe(III) reductase oxidase.6 Then Fe2+ is transported into the root by an iron-regulated transporter (IRT1). In contrast, graminaceous plants rely on an Fe(III) chelation system through the secretion of mugineic acid (MA) family phytosiderophores.3,7,8 The MAs are secreted to the rhizosphere through TOM1 9 and then they chelate Fe(III); the resulting Fe-MA complex is transported by the Yellow Stripe family transporters (OsYSL15 in the case of rice10). Rice plants also have the ability to take up Fe2+ through the OsIRT1 transporter.11
In plants, Fe uptake from the apoplasm is well documented at the molecular level, with the exception of phenolics synthesis and efflux. Phenolics, such as protocatechuic acid (PCA), are reported to chelate Fe(III) solubilization and reduce it to Fe2+ in vitro.12 Moreover, removing the secreted phenolics in hydroponic culture solution triggers Fe deficiency responses in roots by inhibiting the solubilization and utilization of apoplasmic Fe.13 In this manner, phenolics play a major role in Fe solubilization, besides which PCA and other phenolics play a diverse role in biological systems, such as acting as antioxidants and free radical scavengers, and in nitric oxide synthase.14–17 Phenolics are also converted to lignin and suberin through the action of peroxidases.2 The activity of peroxidases, as well as the formation of lignin, decreases under Fe deficient conditions.2,18 As suberin plays an important role in controlling the movement of solutes,19 the role of phenolics in controlling water and mineral transport cannot be overlooked. Thus, understanding the molecular mechanism of phenolics efflux transport is crucial for developing strategies to mitigate widespread Fe deficiency.
PEZ1 was isolated in an effort to characterize T-DNA mutants for genes regulated by cadmium (Cd). PEZ1 belongs to the multidrug and toxic compound extrusion transporter family whose members transport small organic compounds.20 The substrates of PEZ1 were identified by analyzing liquid chromatography/mass spectrometry data profiles of the xylem sap of pez1-1 and pez1-2 mutants. The data indicated that PEZ1 transports PCA and caffeic acid (CA). Furthermore, PEZ1 transported radiolabeled PCA when expressed in Xenopus laevis oocytes. PEZ1 localizes to the plasma membrane in rice root cells, as well as in rice root hairs and onion epidermal cells. The pez1-2 mutant accumulated more Fe in the roots, but not in the leaves, compared to wild-type (WT) plants; the differences were greater in the presence of Cd, while no difference was observed in the accumulation of other metals. No significant difference was observed in zinc, manganese (Mn), and copper concentration between WT and pez1-2, in both the roots and shoots, with or without Cd. Fe concentration in the xylem sap was lower than in the WT, while no difference was observed for xylem Cd and Mn. Significant differences in the localization of insoluble Fe were observed when leaf samples were stained with Perl's solution to examine the localization of Fe. These results suggested a clear role of PEZ1 in solubilizing precipitated apoplasmic Fe.21
Secretion of excess PCA strongly solubilizes Fe precipitated in the stele, leading to symptoms of Fe excess. The analysis of PEZ1 overexpression lines confirmed this hypothesis. PEZ1 overexpression lines accumulated higher amounts of Fe in roots and leaves owing to the high solubilization of precipitated apoplasmic stele Fe, and as a result, the growth of these lines was severely restricted. In contrast, PEZ1 overexpression lines grew better than the WT in calcareous soil, showing that in these lines, PCA-solubilized Fe is available under Fe-limiting conditions.
The expression of PEZ1 is regulated by Cd, and both of the PEZ1 knockdown mutants accumulated higher Cd amounts in leaves and seeds when grown in soil, without compromising morphological or physiological characteristics, like the SPAD value, leaf dry weight, yield, and the concentration of other metals in seeds. Why pez1 accumulates Cd is not clear. PCA has a lower affinity for Cd compared to glutathione, and PEZ1 does not transport Cd.21 Cd is partly transported through the Fe uptake system in plants.22–26 Thus, in pez1, Cd accumulation seems to be triggered by the upregulation of OsIRT1. OsIRT1 localization in the phloem, its substrate specificity, and increased expression in pez1 mutants suggests that Fe and Cd uptake and translocation in pez1 mutants could be enhanced through OsIRT1,11 and that an increased Cd accumulation in pez1 mutants may be due to the increase in OsIRT activity in a decreased Fe environment in which Cd will have reduced competition. PEZ1 localizes to the stele in root cells. The localization of different genes involved in Fe transport is summarized in Figure 1.
Figure 1.
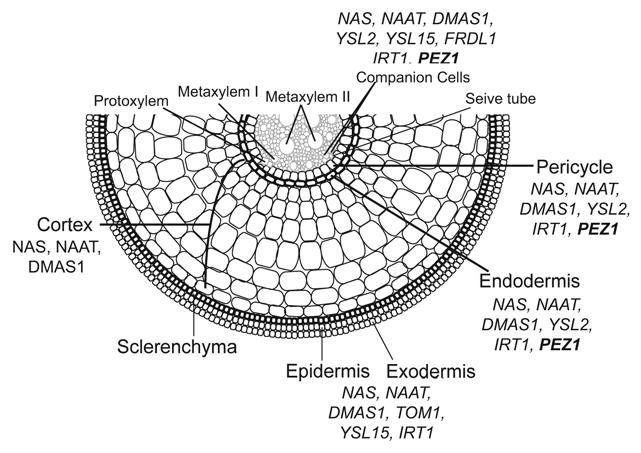
Tissue-specific expression of Fe homeostasis-related genes in rice root.
In short, phenolics secretion affects Fe acquisition in rice. Reduced secretion of PCA in the pez1-2 mutant impairs the solubilization of precipitated apoplasmic Fe in the stele, and thus, the low availability of Fe leads to the induction of OsIRT1. As PEZ1 and OsIRT1 co-localize in the stele, the PCA secretion may complement Fe2+ uptake by OsIRT1 and seems to be an integral part of the Fe2+ uptake system in rice (Fig. 2). In contrast, the increase in phenolics secretion in PEZ1-overexpressing plants increases the solubilization of apoplasmic Fe, and plants showed an increased tolerance to Fe deficiency in alkaline soils. The identification of PEZ1 is an important step that helps in better understanding the solubilization of apoplasmic Fe and will generate research on phenolics efflux mechanisms in other plants.
Figure 2.
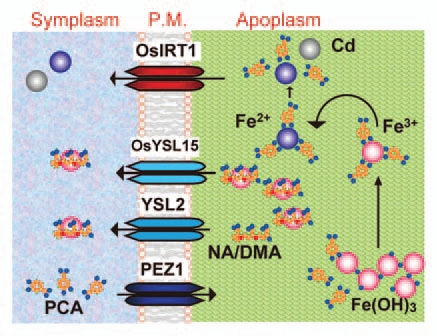
Model of Fe and Cd uptake mechanisms in rice xylem. P.M., plasma membrane.
Acknowledgments
This work was supported by the Programme for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) and a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, GMB0001).
Abbreviations
- IRT1
iron-regulated transporter
- PCA
protocatechuic acid
- PEZ1
phenolics efflux zero 1
References
- 1.Guerinot ML, Ying Y. Iron: Nutritious, noxious and not readily available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marschner H. Mineral nutrition of higher plants. London: Academic Press; 1995. [Google Scholar]
- 3.Römheld V, Marschner H. Mechanism of iron uptake by peanut plants I. FeIII reduction, chelate splitting and release of phenolics. Plant Physiol. 1983;71:949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong J, Guerinot ML. Homing in on iron homeostasis in plants. Trends Plant Sci. 2009;14:280–285. doi: 10.1016/j.tplants.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil. 2010;329:1–25. [Google Scholar]
- 6.Jeong J, Connolly EL. Iron uptake mechanisms in plants: Functions of the FRO family of ferric reductases. Plant Sci. 2009;176:709–714. [Google Scholar]
- 7.Bashir K, Ishimaru Y, Nishizawa N. Iron uptake and loading into rice grains. Rice. 2010;3:122–130. [Google Scholar]
- 8.Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, et al. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem. 2006;281:32395–32402. doi: 10.1074/jbc.M604133200. [DOI] [PubMed] [Google Scholar]
- 9.Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, et al. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem. 2011;286:5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, et al. Rice OsYSL15 Is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem. 2009;284:3470–3479. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- 11.Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ Plant J. 2006;45:335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoshino M, Murakami K. Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Anal Biochem. 1998;257:40–44. doi: 10.1006/abio.1997.2522. [DOI] [PubMed] [Google Scholar]
- 13.Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007;144:278–285. doi: 10.1104/pp.107.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios JL, Recio MC, Escandell JM, Andujar I. inhibition of transcription factors by plant-derived compounds and their implications in inflammation and cancer. Curr Pharm Design. 2009;15:1212–1237. doi: 10.2174/138161209787846874. [DOI] [PubMed] [Google Scholar]
- 15.Perron N, Brumaghim J. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 16.Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, et al. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–1681. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 17.Popa VI, Dumitru M, Volf I, Anghel N. Lignin and polyphenols as allelochemicals. Ind Crops Prod. 2008;27:144–149. [Google Scholar]
- 18.Sijmons PC, Kolattukudy PE, Bienfait HF. Iron deficiency decreases suberization in bean roots through a decrease in suberin-specific peroxidase activity. Plant Physiol. 1985;78:115–120. doi: 10.1104/pp.78.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, et al. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in arabidopsis. PLoS Genet. 2009;5:1000492. doi: 10.1371/journal.pgen.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci. 2006;27:587–593. doi: 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ishimaru Y, Kakei Y, Shimo H, Bashir K, Sato Y, Sato Y, et al. A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J Biol Chem. 2011;286:24649–24655. doi: 10.1074/jbc.M111.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14:1347–1357. doi: 10.1105/tpc.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa I, Nakanishi H, Mori S, Nishizawa N. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil. 2009;325:97–108. [Google Scholar]
- 24.Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr. 2006;52:464–469. [Google Scholar]
- 25.Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, et al. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot. 2011;62:4843–4850. doi: 10.1093/jxb/err136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, et al. IRT1, an arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]


