Abstract
Stopping an initiated response is an essential function, investigated in many studies with go/no-go and stop-signal paradigms. These standard tests require rapid action cancellation. This appears to be achieved by a suppression mechanism that has “global” effects on corticomotor excitability (i.e., affecting task-irrelevant muscles). By contrast, stopping action in everyday life may require selectivity (i.e., targeting a specific response tendency without affecting concurrent action). We hypothesized that while standard stopping engages global suppression, behaviorally selective stopping engages a selective suppression mechanism. Accordingly, we measured corticomotor excitability of the task-irrelevant leg using transcranial magnetic stimulation while subjects stopped the hand. Experiment 1 showed that for standard (i.e., nonselective) stopping, the task-irrelevant leg was suppressed. Experiment 2 showed that for behaviorally selective stopping, there was no mean leg suppression. Experiment 3 directly compared behaviorally nonselective and selective stopping. Leg suppression occurred only in the behaviorally nonselective condition. These results argue that global and selective suppression mechanisms are dissociable. Participants may use a global suppression mechanism when speed is stressed; however, they may recruit a more selective suppression mechanism when selective stopping is behaviorally necessary and preparatory information is available. We predict that different fronto–basal–ganglia pathways underpin these different suppression mechanisms.
Keywords: motor-evoked potential, primary motor cortex, response inhibition, stop signal task, subthalamic nucleus
Introduction
The ability to stop is essential in every day life. For example, one must immediately stop an impending movement to step into the street when a car suddenly appears. Experimentally, this behavior has been operationalized using stop-signal and go/no-go tasks. These require participants to try to quickly stop a response whenever a signal occurs (reviewed by Verbruggen and Logan 2009). As these paradigms typically require rapid action control, it is likely that human subjects use the easiest and fastest suppression mechanism available. By contrast, everyday life often requires behavioral stopping that is selective to a particular tendency. This could engage a selective rather than global suppression mechanism. Here, we aimed to dissociate these putative stopping mechanisms physiologically using single-pulse transcranial magnetic stimulation (TMS).
Evidence for a fast suppression mechanism that has widespread effects on the motor system comes from both behavioral and TMS studies. In one behavioral study, participants initiated 2 responses together on each trial and then, whenever an infrequent signal occurred, tried to stop one response while continuing with the other (Coxon et al. 2007). It was found that the movement of the continuing hand was severely delayed, possibly due to a widespread suppression of motor tendencies that also affected the continuing hand. This interpretation is supported by TMS, which can probe the corticomotor excitability of both task-relevant and -irrelevant muscles. Several studies have shown that stopping a particular manual response suppresses not only the task-relevant muscle but also the task-irrelevant muscles of the same hand (Leocani et al. 2000; Sohn et al. 2002; Coxon et al. 2006; van den Wildenberg et al. 2010a), the homologous muscles of the opposite hand (Coxon et al. 2006; Badry et al. 2009), and, most impressively, the task-irrelevant leg (Badry et al. 2009). Here, we refer to this widespread effect over task-irrelevant muscles as “global” suppression. At the neural systems level, it is possible that this global suppressive effect arises from recruitment of the subthalamic nucleus (STN) of the basal ganglia (via the “hyperdirect signaling pathway” from the cortex). Imaging, lesion, and neurophysiology implicate the STN in standard forms of stopping (reviewed by Eagle and Baunez 2010), and the STN sends a broad output to the pallidum that is thought to have widespread effects on the motor system by reducing thalamocortical drive (Mink 1996; Gillies and Willshaw 1998; Nambu et al. 2002).
Yet, many everyday situations call for the selective stopping of a particular response. It seems unlikely that this is achieved using the abovementioned global suppression mechanism. For example, it is possible to continue speaking even while one stops an initiated manual movement, and it is possible to keep walking even while one cancels the tendency to say something. Moreover, skilled tasks such as playing music or sports often require selective, often independent, control of one’s hands and feet. For example, an American football player can continue running even as he cancels a throw of the ball. Thus, the suppression used in all these cases could engage a mechanism that is selectively targeted at a particular response tendency rather than one that is generally targeted at many muscle representations.
Using a novel behavioral paradigm, we attained preliminary evidence that global and selective mechanisms for stopping are dissociable (Aron and Verbruggen 2008; Claffey et al. 2010). We adapted the method mentioned above, where 2 responses are initiated on each trial and where one must be stopped whenever a signal occurs while the other is quickly continued. The reaction time (RT) delay to continue this other response on stop trials was taken as a measure of the selectivity of stopping. A key innovation in these recent studies was that in one condition, participants were cued in advance about which response to stop (e.g., Maybe Stop Left), while, in the other condition, no cue was given. By cuing people in advance, we encouraged them to engage behaviorally selective stopping because they could prepare which response to stop. Consequently, stopping was indeed more selective but also slower relative to the case when advanced information was not given. We interpreted these findings as indicating different neural pathways for stopping—a selective system that is slower (perhaps because it uses more synapses) versus a global system that is faster (perhaps on account of the hyperdirect signaling pathway). Here, we aimed to dissociate these putative stopping mechanisms physiologically using TMS.
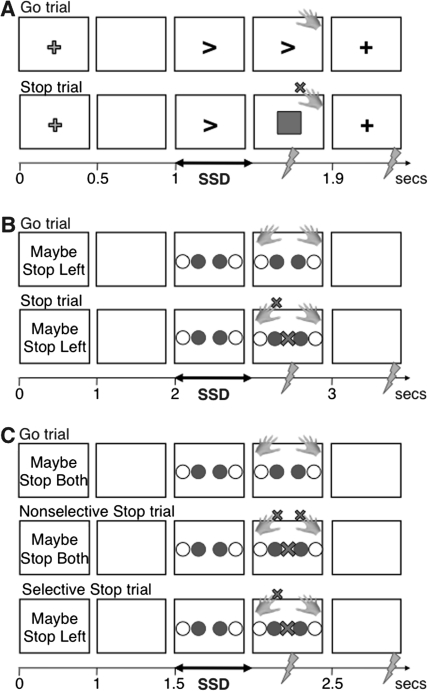
Our key assumption was that if a global suppression mechanism is used to stop the hand, there would be observable suppression of the task-irrelevant leg (cf. Badry et al. 2009). By contrast, if a selective suppression mechanism is used to stop the hand, there would be no leg suppression. We tested these predictions in a series of experiments using TMS to probe the cortico-motor excitability of the task-irrelevant leg while subjects stopped the hand in conditions that did or did not emphasize behavioral selectivity. In Experiment 1 (Fig. 1A), we aimed to replicate the earlier observation (Badry et al. 2009) that stopping the hand in a standard stop signal task leads to suppression of the leg. In Experiment 2 (Fig. 1B), we used a task that emphasized behaviorally selective stopping (cf. Aron and Verbruggen 2008; Claffey et al. 2010; Cai et al. 2011). We predicted less leg suppression than in Experiment 1. In Experiment 3 (Fig. 1C), we intermixed trials requiring selective behavioral stopping (stopping one hand, continuing with the other) and nonselective stopping (stopping both hands). Again, we predicted less leg suppression for the selective compared with nonselective case.
Figure 1.
Task designs. (A) Experiment 1. A left or right arrow indicated the Go response, followed by a red box as a stop signal on 1/3 of trials. TMS was delivered during the Go response time at “Practice” GoRT-100 ms or, on a minority of trials, during the ITI 300 ms before the onset of the next trial. (B) Experiment 2. A cue informed the participant which hand may need to stop. Blue circles indicated the Go response (inner = index; outer = little fingers), followed by a central red X as a stop signal on 1/3 of trials. On these stop trials, participants continued one response while stopping the other. TMS was delivered as in Experiment 1. (C) Experiment 3. Trials began with a cue indicating possible stopping of both hands (Nonselective: 50% of trials) or either the left or right hand (Selective: each 25%). TMS was delivered either 200, 220, or 240 ms after the stop signal on Stop trials or at comparative times on Go trials. On a minority of trials, TMS was delivered 200 ms before the onset of the next trial.
Materials and Methods
Participants
There were 25 participants in total (Experiment 1: 7 total, 4 females, mean age, 20.6 ± 1.6 years, all right handed; Experiment 2: 7 total, 3 females, mean age, 20.7 ± 2.7 years, 6 right handed; Experiment 3: 11 total, 6 females, mean age, 21.91 ± 1.8 years, all right handed). All participants provided written consent in accordance with the Institutional Review Board guidelines of the University of California, San Diego. They also completed a TMS safety-screening questionnaire based on recommendations from the International Workshop on the Safety of Repetitive TMS (Wassermann 1998). This questionnaire excluded any neurological or psychiatric disorder.
EMG Recording Preparation
Surface electromyography (EMG) recordings were obtained from the tibialis anterior (TA) muscle of both legs using pairs of 10-mm silver electrodes. This muscle is responsible for dorsoflexion of the foot and was completely irrelevant for the behavioral task. A ground electrode was placed over the lateral malleoli (outer ankle protuberance) of both legs.
A Grass QP511 Quad AC Amplifier System (Glass Technologies, West Warwick, RI) amplified the EMG signal using a 30 Hz to 1 kHz band-pass filter and a 60Hz notch filter. A CED Micro 1401 mk II acquisition system sampled the data at a frequency of 2 kHz. The data from both legs were displayed in 2 channels and recorded using CED Signal v4 (Cambridge Electronic Design, Cambridge, UK).
TMS Preparation
TMS was delivered with a MagStim 200-2 system (Magstim, Whitland, UK) and a batwing coil; type no. 15411. The study began with a thresholding procedure. Participants were seated comfortably with heels placed on the floor and toes raised 5 cm upon a platform. This leg arrangement increased the likelihood of obtaining a motor-evoked potential (MEP) in the TA muscles. The coil was initially placed over the vertex of the head (midline and halfway between the nasion and the inion), approximately close to the midline M1 representations of the TA muscles. With single-pulse stimulation, the TMS coil was incrementally repositioned to elicit a maximal response in whichever of the 2 TA muscles that was most easily stimulated. Participants were requested to slightly activate their legs in order to increase the likelihood of finding the TA motor hotspot. This location averaged about 1 cm laterally over the right motor cortex in 22 participants (stimulating the left leg) and the left motor cortex in 3 participants (stimulating the right leg—note this was only in Experiment 1). After this location was identified and marked, the participant was asked to rest. The lowest stimulation intensity required to elicit MEP amplitudes of at least 0.05 mV in at least 5 of 10 trials was determined as the resting motor threshold (RMT). The stimulation intensity for each subject in each experiment was approximately 110% of the RMT (Tables 1 and 2). This level elicited consistent responses between 0.05 and 0.2 mV.
Table 1.
Comparison of Experiment 1 (standard stopping) and 2 (selective stopping)
| Experiment 1 (N = 7) | Experiment 2 (N = 7) | |
| RMT (%)* | 62.5 ± 4.1 | 55.6 ± 6.1 |
| Experimental intensity (%)* | 71.8 ± 4.1 | 61.7 ± 6.6 |
| Mean stimulation time (ms)* | 307 ± 61 | 404 ± 51 |
| Behavior | ||
| Accuracy on Go trials (%) | 93.5 ± 8.5 | 88.4 ± 2.3 |
| Probability of stopping (%) | 41.4 ± 11.9 | 42.6 ± 7.2 |
| Direction error rate (%) | N/A | 8.2 ± 4.8 |
| RT on Go trials (ms) | 507 ± 131 | 472 ± 49 |
| Mean SSD (ms) | 247 ± 192 | 168 ± 52 |
| SSRT (ms) | 288 ± 127 | 294 ± 32 |
| RT of continuing response (ms) | N/A | 545 ± 70 |
| Stopping Interference Effect (ms) | N/A | 74 ± 47 |
| Raw motor-evoked potential amplitude | ||
| Correct Go trials (μV) | 517 ± 188 | 459 ± 165 |
| Successful Stop trials (μV) | 436 ± 139 | 447 ± 128 |
| Failed Stop trials (μV) | 529 ± 211 | 458 ± 156 |
| Intertrial interval (μV) | 533 ± 239 | 349 ± 201 |
| Percent Leg Modulation (%)* | −13.6 ± 10.2 | –0.3 ± 14.8 |
Note: All values given as mean ± standard deviation.
Significantly different between experiments (P < 0.05).
Table 2.
Results of Experiment 3 (N = 11)
| Nonselective trials | Selective trials | |
| RMT (%) | 56.0 ± 4.6 | 56.0 ± 4.6 |
| Experimental intensity (%) | 62.7 ± 3.6 | 62.7 ± 3.6 |
| Mean stimulation time (ms) | 478 ± 62 | 451 ± 93 |
| Behavior with TMS | ||
| Accuracy on Go trials (%)* | 95.6 ± 3.2 | 89.9 ± 4.2 |
| Probability of stopping (%) | 54.6 ± 6.6 | 45.2 ± 10.8 |
| Direction error rate (%) | N/A | 4.6 ± 2.8 |
| RT on Go trials (ms) | 518 ± 47 | 529 ± 39 |
| Mean SSD (ms) | 257 ± 61 | 239 ± 107 |
| SSRT (ms) | 246 ± 38 | 271 ± 100 |
| RT of continuing response (ms) | N/A | 663 ± 57 |
| Stopping Interference Effect (ms) | N/A | 134 ± 60 |
| Raw motor-evoked potential amplitude | ||
| Correct Go trials (μV) | 648 ± 380 | 660 ± 389 |
| Successful Stop trials (μV) | 596 ± 367 | 694 ± 382 |
| Failed Stop trials (μV) | 675 ± 364 | 700 ± 389 |
| Intertrial interval (μV) | 599 ± 397 | 599 ± 397 |
| Percent Leg Modulation (%)* | −8.0 ± 10.1 | 6.8 ± 17.3 |
| At 200 ms (%)* | −11.9 ± 18.3 | 3.6 ± 12.1 |
| At 220 ms (%)* | −16.1 ± 11.8 | 9.8 ± 23.2 |
| At 240 ms (%) | 2.5 ± 21.1 | 0.9 ± 28.0 |
Note: All values given as mean ± standard deviation.
Significantly different between conditions (P < 0.05).
Experiment 1
Behavioral Task
The behavioral task (see Fig. 1A) was a standard nonselective stopping paradigm based on Badry et al. (2009). Participants placed left and right index fingers on the “Z” and “/” keys of a standard keyboard. Stimuli were presented on an iMac (19 in monitor). Each trial began with a yellow fixation “+” for 500 ms. This then disappeared, leaving a blank (black) screen for 500 ms. An imperative Go signal, an arrow, was then presented. This pointed to the left or right with equal frequency (“<” or “>”), cueing the participant to respond with either the left or right index finger. On Go trials (67% of all trials), this arrow cue remained either until the participant made a response or for a maximum of 900 ms. On Stop trials (33% of all trials), a red square stop signal appeared over the arrow after a short delay and remained for the duration of the trial. In the inter-trial interval (ITI), a white fixation + was presented for 4 to 6 s (mean 5.1 s). There were 5 blocks of 105 trials each (70 Go trials and 35 Stop trials). The first block was practice without TMS.
The time interval between the Go signal (the arrow) and the Stop signal (the red square) is known as the stop signal delay (SSD). This was varied dynamically throughout the experiment depending on the subject’s performance. The SSD increased by 50 ms with every successful stop and decreased by 50 ms with every failed stop, leading to an overall stopping probability of approximately 50%. This converging “tracking” method is optimal for calculating the stop signal reaction time (SSRT, see below) (Band et al. 2003). Two separate SSD staircases were used for left and right-hand response trials. SSD values began at 150 ms for both staircases.
TMS Delivery
TMS was delivered either during the response period (on 90 of the 105 trials, 60 Go trials, and 30 Stop trials) or during the ITI (before 15 of the 105 trials, 10 Go trials, and 5 Stop trials). For the response period, stimulation occurred 100 ms before the mean Go RT of the practice session (GoRT-100 ms) (based on Badry et al. 2009). For the ITI, stimulation occurred 300 ms before the onset of the next trial (i.e., before the yellow fixation).
Experiment 2
Behavioral Task
The behavioral task (see Fig. 1B) was a selective stopping paradigm based on Aron and Verbruggen (2008). Participants placed the index and little fingers of each hand on a standard keyboard (left hand: little finger on Z and index on “V”; right hand: index on “M” and little finger on /). Each experimental trial began with a cue (“Maybe Stop Right” or “Maybe Stop Left”) written in white text on a black background for 1 s. The cue then disappeared leaving a blank (black) screen for 1 s.
An imperative Go stimulus, 4 horizontally arranged circles, was then presented. Two of the circles on each trial, either the 2 inner circles or the 2 outer circles, were colored blue, whereas the other 2 were colored gray. The circles were 2.3° visual angles in diameter. The 2 inner circles were separated from each other by a 4.6° visual angle and from the 2 outer circles by a 1.2° visual angle. If the 2 inner circles were blue, participants responded with both index fingers simultaneously, whereas if the outer circles were blue, participants responded with both little fingers. Inner (index) and outer (little) finger responses were equiprobable. Failure to respond with both hands simultaneously (defined as a difference > 70 ms) resulted in a textual “Decoupled” warning presented for 2 s.
On Go trials (69% of all trials), the circles remained until either the participant made a response or for a maximum of 1 s. On stop trials (31% of all trials), a red “X” appeared in the center of the screen (between the inner circles) after a short delay (the SSD) and remained until the end of the trial. Participants were required to stop the response of the hand previously cued in the beginning of the trial (e.g., Maybe Stop Left) while quickly continuing with the other hand.
An ITI blank screen lasted for 2 to 4 s (mean 2.6 s). Each block consisted of 78 trials (54 Go trials and 24 Stop trials). Participants took part in either 4 or 5 total blocks of the experiment proper as well as a single practice block without TMS. SSDs varied dynamically as in Experiment 1.
TMS Delivery
TMS was delivered during the response period (on 72 of the 78 trials, 48 Go trials, and 24 Stop trials) or during the ITI (before the 6 remaining Go trials). As in Experiment 1, stimulation onset was set at practice GoRT-100 ms. For the ITI, stimulation was delivered 300 ms before the onset of the next trial. Since ITI stimulation was informative as to the subsequent trial type, these trials were excluded from the behavioral analysis.
Experiment 3
Behavioral Task
This was a modification of the task used in Experiment 2 with a key difference (see Fig. 1C). In order to test the effects of Nonselective stopping in the same design as Selective stopping, a third stopping cue, “Maybe Stop Both,” was used in addition to the original 2 (Maybe Stop Right and Maybe Stop Left). On Maybe Stop Both trials, subjects were required to stop both fingers if a stop signal occurred. The nonselective “Both” cue was presented on 50% of all trials, whereas the selective “Left” and “Right” cues were presented with equal frequency on the other 50% of trials. The experiment was otherwise similar to Experiment 2 except that the stop signal probability was 33%, the blank screen after the cue was 500-ms long, the ITI blank screen was 1.5-s long, and there were trials with and without TMS.
The entire experiment was a maximum of 816 trials long (408 Nonselective and 408 Selective trials), with trial types pseudorandomized throughout. Participants completed from 8 to a maximum of 16 blocks (mean = 13) within the allotted time. Each block had 51 trials.
SSD varied dynamically as before. Trials had independent SSD staircases based on which of the 3 stopping cues preceded them and whether TMS was delivered during the trial or not (for details, see below). This led to a total of 6 independent SSD staircases.
TMS Delivery
Of the 816 total trials possible, TMS was delivered during the response period on 66% of trials (540 total, 270 Nonselective, and 270 Selective). Stimulation was also delivered in the ITI 200 ms before the presentation of the cue in 4% of trials (36 total, 18 Nonselective, and 18 Selective). No TMS was delivered in the remaining 30% of trials (240 total, 120 Nonselective, and 120 Selective). This preserved a subset of trials without TMS delivery that were used to accurately estimate SSRT. As the estimates for SSRT were discovered to be very similar to the TMS trials, these non-TMS trials are not further analyzed or discussed.
On Stop trials, stimulation was delivered at either 200, 220, or 240 ms after the stop signal (60 total at each interval, 30 Nonselective, and 30 Selective). On Go trials, stimuli were delivered in a yoked fashion, that is, relative to the timing on Stop trials in the respective Nonselective or Selective condition (120 at each interval, 60 Nonselective, and 60 Selective).
Behavioral Indices
For Experiment 1, the following indices were calculated: accuracy of Go trials; probability of stopping; RT on Go trials, that is, the mean correct Go RT; Mean SSD; and SSRT. For this and the other experiments, SSRT was calculated using the Integration method (see Verbruggen and Logan 2009). The 2 SSD values with the greatest number of trials and a stopping probability between 40% and 60% were determined. For each of these, the failed stop rate was calculated and used to determine the corresponding point in the rank-ordered distribution of Go trials. SSRT was then estimated by subtracting SSD from this Go RT value. The 2 SSRTs were then averaged.
Some additional indices were also calculated for Experiments 2 and 3. These were: the direction error rate, that is, how often the subject stopped the wrong hand on stop trials; RT of the alternative response, that is, the mean RT of the continuing hand on correct stop trials; and the Stopping Interference Effect, that is, the difference between the alternative response RT and the mean correct Go RT (see Aron and Verbruggen 2008).
MEP Analysis
MEP analysis involved amplitude calculation, trial rejection, trimming, and averaging within condition. An auxiliary analysis examined pre-TMS EMG activity.
Peak-to-peak MEP amplitude was determined using custom software developed in Matlab. Trials were rejected if 1) there was no stable baseline tracing in the period leading up to TMS delivery or 2) the MEP was less than 0.05 mV (average trials removed per subject: Experiment 1 = 7; Experiment 2 = 12; Experiment 3 = 7). MEP amplitudes in Correct Go, Successful Stop, and Failed Stop trials, as well as in the ITI period, were trimmed (by removing upper and lower 10% of values) and then averaged. In order to verify that the muscle of interest was equally at rest before stimulation, the root mean square of the EMG trace from 200 to 100 ms before TMS onset was calculated.
Relative MEP amplitudes were calculated for Correct Go, Successful Stop, and Failed Stop conditions by dividing by the baseline ITI MEP. In addition, a primary measure of importance was the percentage of leg modulation when stopping. This was calculated as (Successful Stop MEP – Correct Go MEP) × 100/Correct Go MEP. Negative leg modulation indicates suppression. In Experiment 3, these values were calculated separately for Nonselective and Selective trials.
Stimulation was delivered to one side of the body while participants made bilateral hand responses. Differential hand movement (moving one hand and not the other) occurred on Go trials in Experiment 1 (based on the direction of the Go cue) or on successful Selective stopping trials in Experiment 2 and 3 (where stopping one hand was coupled to a response in the other hand). To explore the laterality of leg modulation during stopping, we separated trials depending on the recording leg’s relationship with the hand that stopped (i.e., whether the hand on the same side as the leg stopped or continued). Percent Leg Modulation was calculated separately for each case.
Statistical Analysis
In Experiments 1 and 2, one-sample t-tests and nonparametric Wilcoxon tests were performed to assess the change in leg excitability from baseline (test value: 0). To compare leg excitability and behavior between Experiments 1 and 2, independent-samples t-tests and nonparametric Mann–Witney tests were performed. In Experiment 3, paired-samples t-tests were used to compare between selective and nonselective conditions for leg excitability and behavior. In Experiment 3, an analysis of variance was also performed with the factors pulse time (200, 220, 240) and condition (selective, nonselective). Significant results were followed up with paired-samples t-tests using Bonferroni correction. All tests had an alpha level of 0.05.
Results
Experiment 1
Participants performed the same standard stop signal task known to engage a global suppressive mechanism (Badry et al. 2009). As predicted, leg excitability was suppressed on successful stops, a significant modulation of −13.6% (one-sample t6 = 3.539, P = 0.012) (Fig. 2A). Strikingly, this suppression was evident in each of the 7 participants (the result was also significant when using a nonparametric Wilcoxon test, P = 0.018). This finding successfully replicates the Badry et al. (2009) finding that stopping the hand leads to suppression of the task-irrelevant leg. Behavioral performance was typical for a standard stop signal task (Table 1). Importantly, the suppression was not due to differences in pre-TMS leg excitability for Go and Stop trials (Go = 1.2 μV; Stop = 1.1 μV; n.s.). Furthermore, the leg was suppressed regardless of whether the stopped hand was on the same or opposite side of the recorded leg (same side = −15.4%, opposite side = −13.4%, t6 < 1), providing further evidence of global suppression.
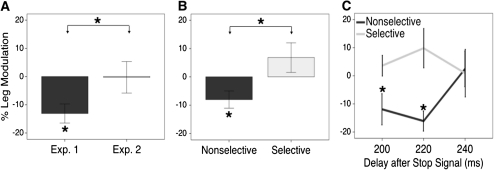
Figure 2.
Leg modulation when stopping. (A) Leg Modulation was negative in Experiments 1 and absent in Experiment 2. (B) Leg Modulation was negative in Nonselective trials and nonsignificant in Selective trials in Experiment 3. (C) Leg Modulation in Experiment 3 was temporally specific. Nonselective modulation was significantly negative at 200 and 220 ms after the stop signal but not at 240 ms. Percent Leg Modulation calculated as (Successful Stop MEP – Correct Go MEP) × 100/Correct Go MEP. Error bars indicate one standard error from the mean.
Experiment 2
Participants performed a behaviorally selective stopping task that putatively engages a selective suppressive mechanism (Aron and Verbruggen 2008; Claffey et al. 2010; Cai et al. 2011). Consistent with our prediction, behaviorally selective stopping did not modulate mean leg excitability from baseline (Leg Modulation = −0.3%, one-sample t6 < 1) (Fig. 2A). Again, pre-TMS leg excitability did not differ for Go and Stop trials (Go = 8.9 μV; Stop = 8.4 μV; n.s.). Behavioral performance on the selective stopping task was similar to our prior reports (Aron and Verbruggen 2008; Claffey et al. 2010) (Table 1). Importantly, participants were highly selective in performing the task, as the delay in continuing the other hand response on Stop trials (the Stopping Interference Effect) was short—only 74 ms on average.
The apparent absence of leg suppression contrasts with Experiment 1 where simple stopping did globally suppress the leg. An independent samples t-test showed significantly greater leg suppression in Experiment 1 than in Experiment 2, as hypothesized (Experiment 1: −13.6%; Experiment 2: −0.3%; t12 = 1.97, P = 0.04, one-tailed, Cohen’s d = 1.07) (Fig. 2A). This difference was marginally significant when using a nonparametric Mann–Whitney Test, P = 0.055. Comparing the 2 experiments behaviorally, there was no difference in Go accuracy (t12 = 1.513, n.s.), Stopping probability (t12 < 1), Go RT (t12 < 1), SSD (t12 = 1.041, n.s.), or SSRT (t12 < 1). However, stimulation was delivered earlier in Experiment 1 (t12 = 3.231, P < 0.01). Experiment 3 controls for this.
Finally, we note that in Experiment 2, participants were required to stop one response while continuing the other. This raises the possibility of an alternative explanation of our findings. Behaviorally, selective stopping may also engage the global suppression mechanism (which affects the leg as in Experiment 1), but this suppression may subsequently be masked by reinitiation of the continuing action (which elevates leg excitability). This global-suppression-plus-reinitiation account predicts that the masking effect is greater when the leg is on the same side as the continuing hand (i.e., if leg excitability is increased when the hand responds, one would expect this to occur more strongly when hand and leg are in the same hemisphere). Our task paradigm allowed us to test this prediction by comparing leg modulation when the recorded leg was on the same side as the continuing hand or the same side as the stopped hand (Fig. 3). The leg was significantly suppressed when it was on the side of the continuing hand (–11.5%, one-sample t6 = 2.999, P = 0.024), whereas there was no reliable leg modulation when the leg was on the side of the stopped hand (6.8%, one-sample t6 < 1) (Fig. 3B). This contradicts the global-suppression-plus-reinitiation account and will be further discussed below.
Figure 3.
Lateralized leg modulation when stopping and going. (A) Successful Stop trials in Experiments 2 and 3 were separated as to whether the stimulated leg was on the same side as the hand that “stopped” or the hand that “continued.” (B) The leg is negatively modulated in Experiment 2 when on the same side as the alternative hand movement (hand continues). (C) Leg modulation in Experiment 3 Selective Stop trials was similar in direction to that in Experiment 2. Error bars indicate one standard error from the mean.
Experiment 3
Experiment 3 compared behaviorally nonselective and selective stopping with a randomized within-block design. The task-irrelevant leg was significantly suppressed in the Nonselective condition (Leg Modulation = −8.0%, one-sample t10 = 2.648, P = 0.024) but not in the Selective condition (Leg Modulation = 6.8%, one-sample t10 = 1.303, P = 0.222). A paired-sample t-test showed more leg suppression in the Nonselective compared with Selective condition (t10 = 4.205, P = 0.002, Cohen’s d = 1.56) (Fig. 2B).
The Nonselective and Selective conditions did not differ behaviorally in Go RT (P = 0.35), SSD (P = 0.47), or SSRT (P = 0.27), although Go accuracy was greater on Nonselective trials (Nonselective = 95.6%, Selective = 89.9%, t10 = 4.715, P = 0.001) (Table 2). Additionally, there were no differences in stimulation onset after the Go signal (P = 0.19) or in pre-TMS leg excitability before Go trials (Nonselective = 3.3 μV; Selective = 3.5 μV; P = 0.44) and Stop trials (Nonselective = 3.2 μV; Selective = 3.6 μV; P = 0.25).
We further examined the temporal specificity of the nonselective and selective stopping processes because we had stimulated at 3 different timepoints after stop signal onset, that is, after 200, 220, and 240 ms. There was a significant interaction between stimulation time and stopping condition (F10,2 = 5.920, P = 0.023). Subsequent analysis revealed that Nonselective suppression was greater than Selective suppression at 200 and 220 ms (t10 = 3.275, P = 0.008, Cohen’s d = 1.05 and t10 = 3.569, P = 0.005, Cohen’s d = 1.15, respectively) but not at 240 ms (t10 < 1) (Table 2, Fig. 2C). Moreover, Nonselective stopping led to suppression from baseline at the first 2 timepoints (200 ms: t10 = 2.155, P = 0.057; 220 ms: t10 = 4.533, P = 0.001) but not at 240 ms (t10 < 1). Selective stopping did not effect leg modulation at any timepoint (200 ms: t10 < 1, P = 0.35; 220 ms: t10 = 1.406, P = 0.19; 240 ms: t10 < 1).
As in Experiment 2, we examined leg modulation on Selective Stop trials as a function of whether the recorded leg was on the same side as either the continuing or stopped hand. Again, as in Experiment 2, the leg was slightly suppressed when it was on the side of the continuing hand (although not significantly this time, −1.4%, t10 < 1, n.s.) but not when it was on the side of the stopped hand (8.3%, t10 = 1.171, n.s.) (Fig. 3C). Again this contradicts the global-suppression-plus-reinitiation account and will be discussed further below.
Discussion
In 3 experiments, we measured the corticomotor excitability of the task-irrelevant leg while participants performed manual stop signal tasks. In Experiment 1, we showed that stopping the hand in the standard stop signal paradigm is accompanied by suppression of the task-irrelevant leg. In Experiment 2, we used a behavioral paradigm that emphasized selective stopping of the hand. In this case, there was no mean leg suppression. In Experiment 3, we directly compared trials requiring behaviorally selective and nonselective stopping. In the behaviorally selective condition (stop one hand, continue with the other), there was no leg suppression, while in the behaviorally nonselective condition (stop both hands), there was leg suppression. Moreover, we observed that the leg suppression in the nonselective condition was temporally specific, occurring at 200 and 220 ms after the stop signal but not at 240 ms.
Experiment 1: Stopping in the Standard Paradigm Is Associated with Leg Suppression
The suppression of the task-irrelevant leg in Experiment 1 replicates Badry et al. (2009). It is also consistent with several earlier studies showing that behaviorally nonselective stopping is associated with widespread corticomotor excitability reductions and increased γ-aminobutyric acidergic inhibition across M1 (Leocani et al. 2000; Sohn et al. 2002; Coxon et al. 2006). This global effect may involve the STN of the basal ganglia, which has been implicated in stopping during stop-signal and go/no-go studies (Aron and Poldrack 2006; van den Wildenberg et al. 2006; Isoda and Hikosaka 2008; Li et al. 2008; Eagle and Baunez 2010; Hershey et al. 2010). Furthermore, the STN is a node of the hyperdirect pathway with direct connections from prefrontal regions involved in stopping, such as the right inferior frontal gyrus and the presupplementary motor area (Nambu et al. 1997; Aron et al. 2007; Forstmann et al. 2010). Since the STN is known to have a very broad inhibitory effect on basal ganglia output (Mink 1996; Gillies and Willshaw 1998; Nambu et al. 2002), the global suppression we observe in the leg could be a “side effect” of using this fast STN-mediated hyperdirect stopping mechanism.
We speculate that in Experiment 1 and in most stopping studies, subjects resort to using this global stopping mechanism because there is no cost to the global side effect (i.e., this mechanism may be the quickest and easiest to use). However, this global mechanism is unlikely to be used in all situations in which stopping is required. In everyday life, selectively stopping one action while continuing others is important, and global suppression of motor excitability in such situations would interfere with these continuing actions. Thus, we set out to show that an alternative mechanism of selective suppression exists. We provide evidence for this selective suppressive mechanism in Experiment 2.
Experiment 2: Behaviorally Selective Stopping Is Not Associated with Leg Suppression
In this experiment, participants responded with both hands and stopped the response of one hand upon stop signal presentation while continuing with the other hand as quickly as possible. Since the stop signal contained no information about which hand to stop, participants were forced to use the advance information provided by the initial cue. As we have shown before, this advance information is key for behaviorally selective stopping. In one study, we showed that those participants with greater knowledge of the cue (tested after the trial was complete) were those who stopped more selectively (Claffey et al. 2010). In another study, we showed that the advance information (e.g., Maybe Stop Right) is manifest in reduced motor excitability of the right hand even before the go signal occurs, and moreover, the extent of this suppression predicts the subsequent selectivity of stopping (Cai et al. 2011). Yet in the current experiment, in contrast to Experiment 1, there was no mean suppression of the leg when the hand was stopped. This is consistent with our hypothesis that behaviorally selective stopping recruits a more selective suppression mechanism. Experiment 3 provided further support for this.
Experiment 3: Directly Contrasting Selective and Nonselective Behavioral Stopping
This experiment compared behaviorally selective stopping (stopping one hand, continuing with the other) with nonselective stopping (stopping both hands). There was significantly greater leg suppression for behaviorally nonselective than selective stopping. This experiment also provided greater information about the timing of the effect relative to the stop signal. Previous TMS studies have shown that motor suppression occurs toward the end of the SSRT (Coxon et al. 2006; van den Wildenberg et al. 2010a). Thus, we stimulated at 200, 220, and 240 ms after the stop signal. This proved judicious since SSRT averaged approximately 260 ms (Nonselective SSRT was 246 ms and Selective SSRT was 271 ms, a nonsignificant difference but one that was in the same direction and magnitude as our earlier report, see Aron and Verbruggen 2008). In the behaviorally nonselective condition, leg suppression was temporally specific—it was present at both 200 and 220 ms after the stop signal, but it had evidently expired before 240 ms just before SSRT ended.
Discounting the Global-Suppression-Plus-Reinitiation Account
We considered an alternative interpretation of the absent leg suppression in Experiments 2 and 3, that is, the global-suppression-plus-reinitiation account. This account assumes that stopping, even in the behaviorally selective condition, leads to suppression of task-irrelevant muscles such as those of the leg but that the requirement to continue with the other response leads to a reinitiation of that response tendency that also elevates the motor excitability of the leg representation. To address this possibility, we compared leg excitability in the case where the leg was on the same side as the continuing hand to when the leg was on the same side as the stopped hand (and opposite to the continuing hand). Figure 3B,C show that the leg is suppressed when the hand on the same side continues movement, though this suppression is significant only in Experiment 2. By contrast, there is no significant leg modulation on the same side as the stopped hand, opposite to the continuing hand.
If stopping one hand did initially cause leg suppression via a global mechanism, it would be unlikely for reinitiation of the continuing hand to abolish leg suppression on the side “opposite” to that continuing hand while sparing the suppression on the “same” side of that hand (see Fig. 4C). The pattern of data is best explained by selective suppression that targets the hand needing to stop without affecting the leg in the same hemisphere (Fig. 4B).
Figure 4.
Model of global and selective inhibitory control. (A) Global suppression. Standard stopping causes suppression (gray arrows) across the motor cortex, affecting the task-irrelevant legs in both Experiments 1 and 3 (downward pointing black arrows in boxes indicate reduced excitability). (B) Selective suppression. Stopping selectively while continuing an alternative movement likely involves an alternative mechanism in which suppression is only directed toward the effector in question. However, there is also leg suppression on the same side as the continuing hand, which is significant only in Experiment 2. We suggest that this may be the signature of a different mechanism associated with making the continuing movement (rather than stopping), that is, intrahemispheric hand–arm surround inhibition. (C) Global-suppression-plus-reinitiation alternative model. Global suppression (gray arrows in left panel) is subsequently masked by activation from reinitiating the continuing hand (black arrows in right panel), which only affects the leg representation of the opposite hemisphere and spares that of the same hemisphere.
In Experiment 2, the suppression on the same side as the continuing hand movement on selective stop trials may be a manifestation of the phenomenon of “motor surround inhibition”—that is, activating the representations in M1 for one effector suppresses the representations of other effectors in that same hemisphere (documented by Stinear and Byblow 2003; Sohn and Hallett 2004; Coxon et al. 2007; Beck et al. 2009; Shin et al. 2009). Although we do not clearly identify this phenomenon outside of Experiment 2 (it is neither significant in Selective Stop trials in Experiment 3 or unimanual Go trials in Experiment 1), the selective suppression observed in Experiment 3 is at least consistent with that of Experiment 2 to suggest further evidence against the global-suppression-plus-reinitiation account.
Another important consideration against the global-suppression-plus-reinitiation account is that here, as previously (Claffey et al. 2010), there was a very small Stopping Interference Effect in some subjects. In Experiment 2, for example, the mean Stopping Interference Effect was just 74 ms and as low as 9 ms in some participants, indicating almost perfect selectivity when stopping. Yet, the Stopping Interference Effect would likely be larger if stopping employed an initial global suppression followed by a subsequent reinitiation of movement.
Taken together, these observations argue for a selective mechanism of inhibitory control that targets the particular hand that needs to stop while sparing irrelevant effectors such as the leg.
What Are the Neural Mechanisms?
Our findings are compatible with different possible accounts of neural stopping mechanisms. On one account, there are 2 different neural mechanisms for stopping that are implemented via different fronto–basal–ganglia circuits; on another account, there is a single neural mechanism for stopping that can operate in different modes—for example, broad versus focused effects on primary motor cortex. When combined with other evidence, we speculate that our findings point more strongly to the former case, that of 2 different neural mechanisms. Specifically, behaviorally nonselective stopping may be implemented via a hyperdirect frontosubthalamic pathway (as suggested by prior results, see Aron and Poldrack 2006), and we speculate that behaviorally selective stopping is implemented via an indirect fronto–striatal–pallidal pathway. While this requires empirical verification, the preliminary evidence is as follows:
First, in 2 earlier reports, we showed that a condition with more selective stopping was associated with slower SSRT than one that was associated with nonselective stopping (Aron and Verbruggen 2008; Claffey et al. 2010). The difference was around 30 ms (which is close to the difference in the current Experiment 3 [25 ms]). Although the indirect pathway only has 2 or 3 extra synapses compared with the hyperdirect pathway, an approximately 30-ms delay for the indirect pathway has been observed when stimulating the cortex and recording from the basal ganglia (Magill et al. 2004).
Second, while the hyperdirect pathway via the STN may send a massive pulse to the pallidum and broadly affect basal ganglia output (Gillies and Willshaw 1998), the indirect pathway has the requisite neural specificity to target a particular response tendency (Mink 1996). Admittedly, the standard model of the indirect pathway does include the STN; however, the STN’s role in that pathway may not cause the same broad effects as its involvement in the hyperdirect pathway. Alternatively, the indirect pathway may be implemented via striatal–external pallidal–internal pallidal connections that bypass the STN (Mink 1996). Notwithstanding, a key feature of the indirect pathway is striatal involvement. Notably, other research has pointed to the importance of the striatum for selective stopping, such as studies of antisaccade performance (Ford and Everling 2009; Watanabe and Munoz 2010). We predict that neuroimaging or neurophysiology will be able to dissociate global and selective stopping mechanisms to different fronto–basal–ganglia pathways (Aron 2010).
Summary and Implications
Standard stopping, assessed by many stop-signal and go/no-go tasks, may employ an expedient mechanism that leads to a diffuse suppression in both task-relevant and task-irrelevant motor representations. The hyperdirect basal ganglia pathway that signals through the STN may underlie this mechanism due to its speed and diffuse effect in reducing thalamo-cortical drive. However, this form of rapid and global stopping has limitations as a model of human control, which often involves both advance preparation for what to stop, as well as selectivity in stopping (Aron 2010; van den Wildenberg et al. 2010b). Accordingly, we have developed a behavioral paradigm that gives participants advance information of what to stop and “forces” behaviorally selective stopping (Aron and Verbruggen 2008). With this paradigm and using TMS, we have shown that this advance preparation is associated with below-baseline suppression of the effectors that might need to be stopped in the future. Furthermore, the greater this neural suppression, the more selective the subsequent behavioral stopping (Cai et al. 2011). The current results add important information by showing that neural suppression at the time of stopping is mechanistically selective. We predict that this kind of control will be implemented via fronto–striatal interactions and will target the motor system via the indirect pathway of the basal ganglia.
This behavioral and neural model may be useful for research into impulse control disorders such as Tourette syndrome, obsessive compulsive disorder, and attention deficit hyperactivity disorder. All of these are disorders characterized by failures to control particular urges, of which motor response tendencies are an important part, and all have been characterized as involving deficiencies in fronto–striatal circuits (Casey et al. 1997; Fineberg et al. 2010; Mazzone et al. 2010). It is likely that the poor control in these disorders relates at least partly to weaknesses in setting up (or maintaining) particular stopping goals and in implementing these goals to target particular response tendencies.
In the absence of disorder, however, participants can use a stopping goal to setup neural suppression of a particular response tendency. Here, we show that this proactive targeted stopping is not only mechanistically selective but also physiologically dissociable from one that leads to global suppression when participants stop quickly and nonselectively.
Funding
Alfred P. Sloan Foundation; National Institutes of Health National Institute on Drug Abuse (grant DA026452) to A.R.A. (PI); National Science Foundation (grant 0921168).
Acknowledgments
We thank Cathy Stinear for helpful comments on the manuscript. Conflict of Interest : None declared.
References
- Aron AR. From reactive to proactive and selective control: developing a Richer model for stopping inappropriate responses. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychol Sci. 2008;19:1146–1153. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol. 2009;120:1717–1723. doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Beck S, Schubert M, Richardson SP, Hallett M. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol. 2009;107:1513–1518. doi: 10.1152/japplphysiol.91580.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Oldenkamp C, Aron AR. A proactive mechanism for selective suppression of response tendencies. J Neurosci. 2011;31:5965–5969. doi: 10.1523/JNEUROSCI.6292-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Claffey MP, Sheldon S, Stinear CM, Verbruggen F, Aron AR. Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia. 2010;48:541–548. doi: 10.1016/j.neuropsychologia.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Selective inhibition of movement. J Neurophysiol. 2007;97:2480–2489. doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Everling S. Neural activity in primate caudate nucleus associated with pro- and antisaccades. J Neurophysiol. 2009;102:2334–2341. doi: 10.1152/jn.00125.2009. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schafer A, Neumann J, Brown S, Wagenmakers EJ, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci U S A. 2010;107:15916–15920. doi: 10.1073/pnas.1004932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AJ, Willshaw DJ. A massively connected subthalamic nucleus leads to the generation of widespread pulses. Proc Biol Sci. 1998;265:2101–2109. doi: 10.1098/rspb.1998.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Campbell MC, Videen TO, Lugar HM, Weaver PM, Hartlein J, Karimi M, Tabbal SD, Perlmutter JS. Mapping Go-No-Go performance within the subthalamic nucleus region. Brain. 2010;133(Pt 12):3625–3634. doi: 10.1093/brain/awq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bevan MD, Brown P, Bolam JP. Synchronous unit activity and local field potentials evoked in the subthalamic nucleus by cortical stimulation. J Neurophysiol. 2004;92:700–714. doi: 10.1152/jn.00134.2004. [DOI] [PubMed] [Google Scholar]
- Mazzone L, Yu S, Blair C, Gunter BC, Wang Z, Marsh R, Peterson BS. An FMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. Am J Psychiatry. 2010;167:341–349. doi: 10.1176/appi.ajp.2009.08121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Nambu A, Kaneda K, Tokuno H, Takada M. Organization of corticostriatal motor inputs in monkey putamen. J Neurophysiol. 2002;88:1830–1842. doi: 10.1152/jn.2002.88.4.1830. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Inase M, Takada M. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett. 1997;239:13–16. doi: 10.1016/s0304-3940(97)00877-x. [DOI] [PubMed] [Google Scholar]
- Shin H-W, Sohn YH, Hallett M. Hemispheric asymmetry of surround inhibition in the human motor system. Clin Neurophysiol. 2009;120:816–819. doi: 10.1016/j.clinph.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88:333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol. 2003;89:2014–2020. doi: 10.1152/jn.00925.2002. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WPM, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cogn Neurosci. 2010a;22:225–239. doi: 10.1162/jocn.2009.21248. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WPM, Wylie SA, Forstmann BU, Burle B, Hasbroucq T, Ridderinkhof KR. To head or to heed? Beyond the surface of selective action inhibition: a review. Front Hum Neurosci. 2010b;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Munoz DP. Saccade suppression by electrical microstimulation in monkey caudate nucleus. J Neurosci. 2010;30:2700–2709. doi: 10.1523/JNEUROSCI.5011-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]