Abstract
Spasmodic dysphonia (SD) is a primary focal dystonia characterized by involuntary spasms in the laryngeal muscles during speech production. Although recent studies have found abnormal brain function and white matter organization in SD, the extent of gray matter alterations, their structure–function relationships, and correlations with symptoms remain unknown. We compared gray matter volume (GMV) and cortical thickness (CT) in 40 SD patients and 40 controls using voxel-based morphometry and cortical distance estimates. These measures were examined for relationships with blood oxygen level–dependent signal change during symptomatic syllable production in 15 of the same patients. SD patients had increased GMV, CT, and brain activation in key structures of the speech control system, including the laryngeal sensorimotor cortex, inferior frontal gyrus (IFG), superior/middle temporal and supramarginal gyri, and in a structure commonly abnormal in other primary dystonias, the cerebellum. Among these regions, GMV, CT and activation of the IFG and cerebellum showed positive relationships with SD severity, while CT of the IFG correlated with SD duration. The left anterior insula was the only region with decreased CT, which also correlated with SD symptom severity. These findings provide evidence for coupling between structural and functional abnormalities at different levels within the speech production system in SD.
Keywords: cortical thickness, fMRI, laryngeal dystonia, VBM, voice production
Introduction
Spasmodic dysphonia (SD) is the third most prevalent form of primary focal dystonia (Ludlow et al. 2008). SD is a task-specific disorder, characterized by involuntary spasms in the laryngeal muscles during speech production but not during emotional vocal expressions, such as laughter and cry. The 2 most common forms of SD are adductor SD (ADSD), characterized by involuntary vocal fold closure, producing breaks during vowel production, and abductor SD (ABSD), characterized by prolonged vocal fold opening, leading to breathy breaks during production of voiceless consonants.
Although the exact pathophysiology of SD and other forms of primary focal dystonia has not been fully elucidated, recent studies have identified several functional abnormalities in the sensorimotor cortical and subcortical regions that may contribute to the pathophysiology of this disorder. Using functional magnetic resonance imaging (fMRI), abnormal brain activity in SD patients was found in the primary laryngeal/orofacial sensorimotor cortex, basal ganglia, ventral thalamus, and cerebellum during symptomatic voice production (Haslinger et al. 2005; Ali et al. 2006; Simonyan and Ludlow 2010) with activation of the primary somatosensory cortex remaining abnormally increased also during production of asymptomatic laryngeal tasks (Simonyan and Ludlow 2010). The presence of functional abnormalities during both symptomatic and asymptomatic tasks suggests that the underlying brain structure may be altered in SD. In line with this assumption, a recent diffusion tensor imaging study in SD has shown white matter (WM) abnormalities within the laryngeal motor cortical circuits, such as decreased WM integrity and coherence in the genu of the internal capsule and increased water diffusivity along the corticobulbar/corticospinal tract, basal ganglia, ventral thalamus, and cerebellum (Simonyan et al. 2008). Furthermore, postmortem tissue examination in one SD patient has revealed focal axonal degeneration and demyelination in the genu of the internal capsule and mineral accumulations in the basal ganglia, thalamus, and cerebellum (Simonyan et al. 2008) as well as clusters of microglial activation in the brainstem nuclei associated with the laryngeal control in 2 SD patients (Simonyan et al. 2010). However, despite this recent progress in identifying brain abnormalities in SD, several questions about its pathophysiological characteristics remain open. Specifically, it is unknown what gray matter (GM) abnormalities are present in SD, how they relate to the functional brain activation changes, and whether these abnormalities relate to the severity of SD symptom expression. This missing information is critical for a complete characterization of the extent of brain abnormalities in SD.
In the present study, we combined high-resolution MRI with fMRI to determine the relationship between brain structure and function in the same cohort of SD patients. We hypothesized that SD patients compared with healthy subjects will exhibit GM structural abnormalities within the laryngeal sensorimotor circuits, which may relate to the altered functional activation in these regions, and that the brain regions exhibiting abnormal structure–function relationships may correlate with SD symptom production. To examine GM organization, we used 2 complimentary methods: voxel-based morphometry (VBM) and cortical thickness (CT) analyses. VBM measures the quantity of tissue within a voxel and, hence, is dependent on local CT and/or cortical surface area (Hutton et al. 2009). In contrast, CT measurements are based on determination of the inner and outer cortical boundaries or surfaces (Fischl and Dale 2000) and have been reported to have a higher sensitivity in detecting cortical changes compared with VBM measures (Hutton et al. 2009). While the VBM provides information about the volumetric organization of the entire brain, including subcortical structures and cerebellum, the CT measurements are more limited to the cortical ribbon. Thus, the choice to conduct both VBM and CT analyses was made to yield the most informative result about the GM organization in SD. To investigate the relationship between the structural and functional brain organization in SD, we further correlated VBM and CT measurements with our previously reported findings of functional brain activation abnormalities during symptomatic syllable production (Simonyan and Ludlow 2010) in the same SD patients, who also participated in the present VBM-CT study. Finally, the obtained measurements were used to assess the relationships between GM abnormalities and symptom severity and duration in these patients.
Subjects and Methods
Subjects
Forty patients with SD (mean age 56.9 ± 10.6 years; 15 males/25 females; 25 ADSD/15 ABSD) and 40 healthy subjects (52.5 ± 10.5 years; 23 males/17 females) underwent high-resolution MRI for VBM and CT analyses. To determine the GM structure–function relationship in SD, the functional activation differences during symptomatic syllable production were examined in the fMRI datasets of 15 SD patients (mean age 54.1 ± 10.1 years; 7 males/8 females; 9 ADSD/6 ABSD) and 15 healthy controls (mean age 49.5 ± 13.3 years; 7 males/8 females) from the same cohort. Univariate analysis of the fMRI data in 14 patients and 11 controls was reported earlier (Simonyan and Ludlow 2010). Neither VBM and CT analyses nor correlations between the structural and functional brain organization in SD patients were previously performed or reported.
All patients and controls underwent history and physical examination and fiber-optic nasolaryngoscopy to confirm normal laryngeal anatomy and function in healthy subjects and the diagnosis of SD in patients. All subjects were right-handed (except one ADSD patient) and monolingual native English speakers. No participant had a history of neurological (other than SD in the patient group), psychiatric, voice, swallowing, or respiratory problems. All patients had isolated SD, that is, they did not show dystonic features in other body segments (e.g., face, neck, and limbs). Mean duration of SD was 14.4 years, ranging from 1.5 to 45 years. The SD patients were neither actively enrolled in voice and speech therapy at the time of participation and/or in the recent past nor reported receiving benefits from voice and speech therapy as is usually the case in SD (Ludlow et al. 2008). All patients (except one ABSD and 2 ADSD) received regular botulinum toxin injections into their laryngeal muscles to manage SD symptoms. All patients were fully symptomatic at the time of scanning; those, who received botulinum toxin treatment, had at least a 3-month interval between their last injection and study participation. All patients provided written informed consent before participation in both the MRI and fMRI studies, which were approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Data Acquisition and Analysis
Two high-resolution anatomical MRI volumes were collected per subject. VBM analysis was conducted using the first of 2 volumes, while CT analysis required the average of 2 volumes for enhancement of signal-to-noise ratio.
Voxel-Based Morphometry
Whole-brain high-resolution T1-weighted images (3D-magnetization prepared rapid gradient echo: time to inversion = 450 ms, time echo [TE] = 3 ms, time repetition [TR] = 7.4 s, flip angle [FA] = 10 degrees, slice thickness = 1.3 mm without gap, matrix 256 × 256 mm, field of view [FOV] = 240 mm) were acquired in 40 SD patients and 40 controls (3.0-T GE Excite scanner, 8-channel head coil). Data were analyzed using VBM8 toolbox in the SPM8 software (Wellcome Department of Imaging Neuroscience Group, London, UK) running on MATLAB version 7.4 (Mathworks, MA). Raw images were bias corrected for MRI inhomogeneities and noise; tissue classified into GM, WM, and cerebrospinal fluid (CSF), and registered using linear and nonlinear transformations within a unified model (Ashburner and Friston 2005). Spatial normalization was performed using a diffeomorphic nonlinear registration tool (diffeomorphic anatomical registration using exponentiated lie algebra [DARTEL]) to improve intersubject registration (Ashburner 2007). This was followed by modulation of GM volume (GMV) through multiplication with nonlinear components derived from the normalization matrix to preserve tissue volume after warping. The resulting images were smoothed with a 10-mm full-width at half-maximum isotropic Gaussian kernel (final smoothness values: x = 9.7; y = 10.3; z = 9.6). Voxelwise statistical differences in GMV between patients and controls were examined using an independent t-test with age, gender, and total intracranial volume (TIV) as nuisance effects. The TIV was not significantly different between the patient and control groups (mean ± standard deviation [SD] in patients vs. controls: 1377 ± 76.6 vs. 1386 ± 116.2, P = 0.67). SD clinical characteristics (e.g., number of breaks) were not included as covariates because these measures were not available in all SD patients. To avoid possible edge effects between different tissue types, the absolute threshold masking was used to exclude voxels with GM values of less than 0.1. The cluster-level significance was assessed using a nonstationary cluster extent correction (Hayasaka et al. 2004) at P ≤ 0.01 to correct for multiple comparisons.
CT
The same high-resolution raw T1-weighted images were submitted to CT analysis. Automated surface reconstruction and CT determination in each subject were performed following the standard procedures using FreeSurfer software (Fischl and Dale 2000). After motion correction and averaging of 2 structural volumes in each subject, a single high signal-to-noise averaged volume was processed for the removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (Segonne et al. 2004); automated Talairach–Tournoux transformation; segmentation into GM, WM, and CSF; tessellation of the GM boundary; automated topology correction followed by visual inspection of each individual subject’s dataset, and surface deformation. The deformable procedures included surface inflation, registration to a spherical atlas of optimally aligned gyral patterns to match cortical geometry across subjects, and parcellation of the cortex into units based on gyral and sulcal structure. The resultant images were smoothed using a 10-mm full-width at half-maximum Gaussian kernel (final smoothness values: x = 9.7; y = 10.7; z = 9.8). CT measurements were obtained based on the shortest distance from the GM/WM boundary to the GM/CSF boundary at each vertex on the tessellated surface (Fischl and Dale 2000). To account for variations in CT dependent on age and gender (Sowell et al. 2007), statistical differences between the SD and control groups were assessed using a general linear model at each vertex with age and gender as nuisance effects. Statistical significance was set at P ≤ 0.01 after correction for multiple comparisons using the Monte Carlo cluster-wise simulation implemented in FreeSurfer.
Functional Magnetic Resonance Imaging
For identification of brain structure–function relationships in SD, we reanalyzed the fMRI data in 15 SD patients during symptomatic syllables (i.e.,/i·i/in adductor SD and/ihi/in abductor SD) compared with 15 healthy controls. The details on data acquisition and processing can be found in our previous paper (Simonyan and Ludlow 2010). Briefly, an event-related sparse sampling design was used with a gradient-weighted echo planar imaging pulse sequence (TE = 30 ms; TR = TA + delay = 2 s + 8.6 s; FA = 90°; FOV = 240 × 240 mm; matrix 64 × 64 mm; in-plane resolution 3.75 mm; 35 sagittal slices; slice thickness 4 mm, no gap) and blood oxygen level–dependent (BOLD) contrast on the 3.0-T GE scanner. An additional high-resolution T1-weighted image was collected within each subject’s fMRI session for anatomical reference.
Functional image analysis was performed using the AFNI software package (Cox 1996). After initial preprocessing and smoothing of images at 10-mm full-width at half-maximum Gaussian kernel (final smoothness values: x = 10.2; y = 9.8; z = 9.7), the task-related responses were analyzed using multiple linear regression with a task regressor convolved with a canonical hemodynamic response function. The resultant images were converted to the standard Talairach–Tournoux space. To reduce the variance of the beta values due to potentially increased tissue volume in some brain regions in SD patients and to clarify whether the increased activation in certain brain regions is a functional increase or simply proportional to increased tissue volume, we used the GMV of these subjects as a covariate in voxelwise independent t-test between control and patient groups. The GMV measures in 15 SD patients and 15 controls were obtained through the separate high-dimensional DARTEL normalization and segmentation of each individual’s T1-weighted images collected during the fMRI scanning session using VBM8 toolbox in the SPM8 software. The GMV was not significantly different between the patient and control groups (mean ± SD in patients vs. controls: 620.4 ± 60.1 vs. 588.0 ± 85.1, P = 0.24). Statistical significance of between-group analysis was set at P ≤ 0.01, corrected for multiple comparisons using Monte Carlo simulations implemented in AFNI’s AlphaSim program (minimal cluster volume of 283 mm3 at a voxelwise threshold of P ≤ 0.001).
Correlation Analyses between Brain Structural and Functional Measures in SD
Similar to the procedure described earlier (Casanova et al. 2007), voxelwise whole-brain correlation analyses between VBM and fMRI datasets and between CT and fMRI datasets were conducted using AFNI software to examine the relationships between structural and functional abnormalities in the same 15 SD patients, who underwent both fMRI and VBM/CT studies. Before correlation analyses, each patient’s VBM dataset (i.e., a smoothed modulated for the nonlinear components DARTEL warped segmented GM image) was transformed from the Montreal Neurological Institute standard space into the Talairach–Tournoux standard space using AFNI software to match the space transformation of the fMRI and CT datasets. Although the CT datasets (i.e., surface values of cortical ribbon resampled to a volume) were already normalized into the Talairach–Tournoux space, 3dAllineate program and visual inspection of the results were used to confirm the proper alignment of all volumes (VBM, CT, and fMRI) in each patient. To create a single 3D + subjects volume for each of the VBM, CT, and fMRI datasets, the respective images were concatenated across all subjects. The resultant single volumes of VBM and CT datasets were resampled to match the same grid spacing (2 × 2 × 2 mm) and orientation (RAI: right anterior inferior) of the fMRI dataset. Pearson’s correlation coefficients were computed between the corresponding voxels of each VBM-fMRI and CT-fMRI dataset pairs using AFNI’s 3dTcorrelate program. The resultant maps were thresholded at P ≤ 0.025, corrected for multiple comparisons using Monte-Carlo simulations (AlphaSim program, minimal cluster volume of 50 mm3).
Correlation Analyses between Brain Abnormalities and SD Characteristics
Separate multiple regression and Pearson’s correlation analyses were conducted to assess the relationships of structural and functional abnormalities (i.e., VBM, CT, fMRI) with SD duration and symptom severity at a corrected P ≤ 0.025 using minimal cluster volumes of 252 mm3 for functional (AFNI software) and 80 mm3 for structural correlations (SPM8 and FreeSurfer software), respectively. Information about SD duration was collected in all 40 patients. SD symptom severity was measured based on the voice and speech recordings in 37 patients; the recordings were not made in the remaining 3 patients due to technical problems. The recordings contained production of 2 sets of sentences: 20 sentences with a high number of glottal stops preceding vowels to elicit symptoms of ADSD, and 20 sentences with a high number of voiceless consonants, such as/f/s/h/p/t/k/, to elicit symptoms of ABSD. Recordings were made before the scanning session using microphone positioned at 2-inch distance from the corner of the mouth. The identifying information on the recorded samples was removed, and the samples were randomized before voice and speech analyses made by the an experienced speech–language pathologist. The presence of SD-characteristic voice breaks was measured by counting the number of ADSD and ABSD breaks in the recorded sentences (Bielamowicz and Ludlow 2000; Barkmeier et al. 2001).
Results
Structural and Functional Abnormalities in SD
Routine neuroradiological evaluation of high-resolution MRI did not identify any gross abnormalities in either controls or SD patients.
Voxel-Based Morphometry
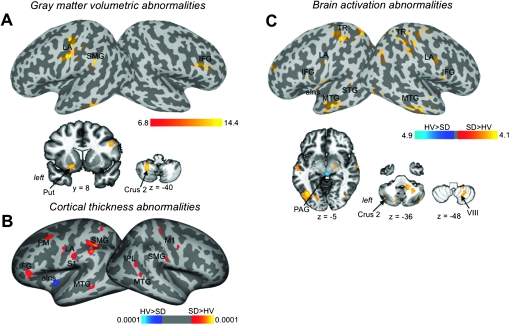
Compared with healthy subjects, SD patients showed increased cortical GMV in the left primary sensorimotor cortex, including the larynx area, supramarginal gyrus (SMG) and right inferior frontal gyrus (IFG). Subcortical GMV increases in SD were found in the left dorsal putamen as well as in the bilateral cerebellum (Crus 2) (Fig. 1A, Table 1). No GMV decreases were found in SD patients compared with controls (corrected P ≤ 0.01).
Figure 1.
Regions of abnormal GMV (A), CT (B), and brain activation during symptomatic syllable production (C) in SD patients compared with healthy subjects are presented on the inflated cortical surfaces; differences between the groups in the subcortical regions and cerebellum are shown on the series of axial and coronal brain images. The color bars represent F values (A), P value (B), and t-values (C) and reflect the significance of abnormalities of GMV, CT, and activation extent, respectively. LA, larynx area; TR, trunk area; PM, premotor cortex; STG, superior temporal gyrus; Put, putamen; PAG, periaqueductal gray; M1, primary motor cortex; S1, primary somatosensory cortex; aIns, anterior insula; IPL, inferior parietal lobule.
Table 1.
VBM-derived GMVs in SD patients compared with healthy controls
| Anatomical region | Cluster peak coordinates (x, y, z) | Cluster peak levels |
|
| F | Z | ||
| L primary sensorimotor cortex | −39, −18, 46 | 8.9 | 4.4 |
| R IFG | 51, 17, 28 | 8.7 | 4.3 |
| L SMG | −60, −39, 24 | 8.2 | 4.2 |
| L putamen | −18, 3, −12 | 10.3 | 4.7 |
| L cerebellum (Crus 2) | −34, −42, −60 | 14.4 | 5.6 |
Note: Coordinates are given in the Montreal Neurological Institute standard space. Statistical significance is set at a corrected P ≤ 0.01. R, right; L, left.
CT
SD patients exhibited increased CT in the bilateral primary sensorimotor cortex, including the larynx area, premotor cortex, middle temporal gyrus (MTG), SMG, left IFG, and the right inferior parietal cortex. The only CT decrease was observed in the left anterior insula (corrected P ≤ 0.01) (Fig. 1B, Table 2).
Table 2.
CT-derived GM thickness in SD patients compared with healthy controls
| Anatomical regions | Cluster peak coordinates (x, y, z) | Cluster peak level, t |
| L primary motor/premotor cortex | −41, −10, 34 | 2.3 |
| R primary motor/premotor cortex | 51, −10, 36 | 2.9 |
| L primary somatosensory cortex | −57, −17, 34 | 2.8 |
| R primary somatosensory cortex | 31, −30, 55 | 3.3 |
| L premotor cortex | −35, 9, 55 | 2.1 |
| L IFG | −49, 33, 1 | 3.2 |
| L MTG | −58, −43, −4 | 3.1 |
| R MTG | 43, −43, 6 | 3.9 |
| L anterior insula | −34, −10, −8 | 2.9 |
| L SMG | −40, −43, 38 | 4.5 |
| −55, −37, 44 | 4.0 | |
| R SMG | 50, −38, 23 | 3.0 |
| R inferior parietal cortex | 48, −43, 24 | 2.5 |
Note: Coordinates are given in the Talairach–Tournoux standard space. Statistical significance is set at a corrected P ≤ 0.01. L, left; R, right.
Functional Magnetic Resonance Imaging
Increased brain activation during symptomatic syllable production in SD patients compared with controls was similar to previously reported (Simonyan and Ludlow 2010) and was found in the bilateral primary sensorimotor cortex, including the larynx and trunk areas, IFG, MTG, cerebellum, left STG, anterior insula, and parietal operculum. Activation in the midbrain, including periaqueductal gray, was decreased in SD patients compared with controls (corrected P ≤ 0.01) (Fig. 1C, Table 3).
Table 3.
fMRI-derived brain activation levels in SD patients compared with healthy controls
| Anatomical regions | Cluster peak coordinates (x, y, z) | Cluster peak level, t |
| L primary somatosensory cortex | −43, −33, 54 | 4.01 |
| R primary somatosensory cortex | 27, −41, 34 | 3.49 |
| L primary motor cortex | −47, −13, 32 | 3.57 |
| R primary motor cortex | 43, −7, 30 | 4.09 |
| L IFG | −48, 3, 23 | 3.89 |
| R IFG | 60, 2, 15 | 3.54 |
| L parietal operculum | −59, −3, 14 | 3.97 |
| L anterior insula | −32, −10, −10 | 3.60 |
| L superior temporal gyrus | −55, −37, 12 | 3.76 |
| L MTG | −53, −15, −6 | 4.06 |
| R MTG | 59, −19, −18 | 4.95 |
| Midbrain/periaqueductal gray | 1, −25, −4 | 3.74 |
| L cerebellum (Crus 2) | −45, −62, −34 | 2.98 |
| R cerebellum (VIII) | 27, −53, −38 | 3.90 |
Note: Coordinates are given in the Talairach–Tournoux standard space. Statistical significance is set at a corrected P ≤ 0.01. L, left; R, right.
Relationship between Structural and Functional Abnormalities in SD
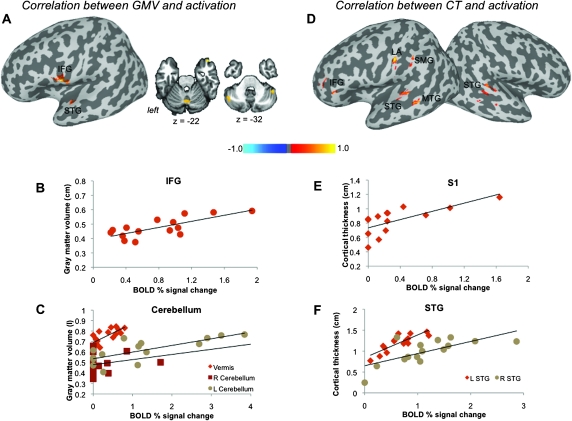
Positive significant correlations (all P ≤ 0.025) between GMV and brain activation abnormalities in SD patients were found in the left IFG (r = 0.75), STG (r = 0.67), and bilateral cerebellum (Crus 2 and vermis, r > 0.64) (Fig. 2A–C, Table 4). Positive correlations between CT and brain activation abnormalities (all P ≤ 0.025) were observed in the bilateral STG (r > 0.69), left laryngeal primary somatosensory cortex, including the larynx area (r = 0.70), IFG (r = 0.71), SMG (r = 0.75), and MTG (r = 0.72) (Fig. 2D–F, Table 4).
Figure 2.
Relationships of brain activation during symptomatic syllable production with the GMV (A) and CT (D) in SD patients are shown on inflated cortical surfaces; correlations in the cerebellum are shown on the coronal images. The color bar represents r values. The plots depict correlations between the GMV (in liter) and BOLD percent signal change (B, C) and between CT (in cm) and BOLD percent signal change (E, F) in the representative regions. STG, superior temporal gyrus; LA, larynx area of the sensorimotor cortex.
Table 4.
Correlation between functional and structural measures
| Anatomical regions | Cluster peak coordinates (x, y, z) | Cluster peak level, r |
| GMV and brain activation | ||
| L IFG | −55, 3, 10 | 0.84 |
| L superior temporal gyrus | −52, −15, 2 | 0.67 |
| R cerebellum (Crus 2) | 47, −43, −34 | 0.79 |
| R cerebellum (VIII) | 35, −51, −54 | 0.78 |
| CT and brain activation | ||
| L IFG | −45, 39, 12 | 0.74 |
| L primary somatosensory cortex | −55, −19, 38 | 0.85 |
| L SMG | −57, −25, 29 | 0.75 |
| L superior temporal gyrus | −57, −26, 5 | 0.73 |
| R superior temporal gyrus | 65, −23, 12 | 0.75 |
| L MTG | −55, −33, 2 | 0.82 |
Note: Coordinates are given in the Talairach–Tournoux standard space. Statistical significance is set at a corrected r ≥ 0.6, P ≤ 0.025. L, left; R, right.
Relationship between Brain Abnormalities and SD Characteristics
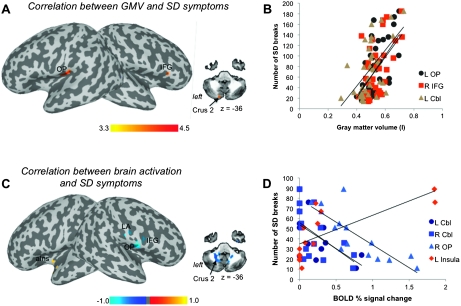
Symptom severity and duration of SD were unrelated (r = 0.18, P = 0.29). The SD severity, measured by the number of voice breaks, showed positive significant relationships (all P ≤ 0.025) with the GMV in the left parietal operculum (r = 0.58), the right IFG (r = 0.54), and left cerebellum (Crus 2) (r = 0.57) (Fig. 3A,B, Table 5).
Figure 3.
Relationships of SD severity with the GMV (in liter) (A) and BOLD percent signal change during symptomatic syllable production (C) are shown on the inflated cortical surfaces; correlation in the cerebellum is shown on an axial image. The color bar represents t-values (A) and r values (C). (B, D) The plots depict representative correlations of (A) and (B). OP, parietal operculum; LA, larynx area; aIns, anterior insula; Cbl, cerebellum.
Table 5.
Correlation between brain structure, SD severity, and duration
| Anatomical region | Cluster peak coordinates (x, y, z) |
| GMV and SD severity | |
| L parietal operculum | −44, −14, 15 |
| L cerebellum (Crus 2) | −11, −76, −36 |
| R IFG | 56, 21, 4 |
| CT and SD severity | |
| L anterior insula | −36, −11, −2 |
| L inferior parietal lobule | −41, −67, 39 |
| R IFG | 15, −48, 8 |
| Brain activation and SD severity | |
| L anterior insula | −36, 3, −7 |
| R primary sensorimotor cortex | 54, −9, 25 |
| R operculum | 57, −3, 12 |
| R IFG | 50, −3, 15 |
| L cerebellum (VIII) | −21, −48, −36 |
| R cerebellum (VIII) | 19, −47, −48 |
| SD duration | |
| L IFG | −43, 21, 20 |
| L superior temporal gyrus | −53, −31, −11 |
Note: Coordinates are given in the Talairach–Tournoux standard space. Statistical significance is set at a corrected P ≤ 0.01 for all correlations. R, right; L, left.
A positive relationship between the functional activation during symptomatic syllable production and SD symptoms was observed in the left anterior insula (r = 0.73), while negative correlations were found in the right primary laryngeal sensorimotor cortex, extending to the parietal operculum and IFG (r > −0.81), as well as in the bilateral cerebellum (Crus 2) (r = −0.60) (all P ≤ 0.025) (Fig. 3C,D, Table 5).
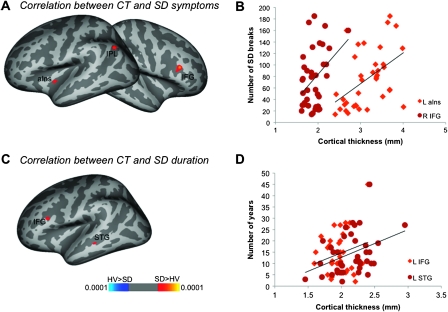
SD symptoms were positively correlated with CT measures in the left anterior insula (r = 0.58), inferior parietal lobule, and the right IFG (r = 0.49) (P ≤ 0.025) (Fig. 4A,B, Table 5). Furthermore, SD duration showed significant positive relationship with CT in the left IFG and STG (P ≤ 0.025) (Fig. 4C,D, Table 5). No significant relationship of disorder duration was observed with either GMV or functional activation.
Figure 4.
Relationships of CT (in mm) with SD symptoms (A) and duration (D). (B, D) The plots show representative correlations between SD clinical characteristics and CT. aIns, anterior insula; STG, superior temporal gyrus. The color bar represents P values.
Discussion
Brain regions commonly showing both functional and structural GM increases in SD patients compared with healthy subjects included the laryngeal primary sensorimotor cortex, IFG, STG/MTG, and cerebellum (Fig. 1). All these regions are critical components of the speech control system (Hickok and Poeppel 2007; Simonyan et al. 2009) and may play a role in the pathophysiology of SD. While brain abnormalities in the laryngeal sensorimotor cortex, IFG, and STG/MTG have not been reported in other forms of primary focal dystonia (Draganski et al. 2003; Garraux et al. 2004; Delmaire et al. 2007; Obermann et al. 2007), the cerebellum has been found to be one of the most commonly affected brain regions in patients with other dystonias (Galardi et al. 1996; Ceballos-Baumann et al. 1997; Odergren et al. 1998; Ibanez et al. 1999; Hutchinson et al. 2000; Preibisch et al. 2001; Draganski et al. 2003; Delmaire et al. 2007). These observations imply that brain abnormalities in SD may be categorized into SD specific (with the involvement of the laryngeal sensorimotor cortex, IFG, STG/MTG) and common across different forms of dystonia (with the involvement of the cerebellum) (Table 6).
Table 6.
Common structural and functional abnormalities in SD
| SD specific | Dystonia common | |
| Structural and functional abnormalities | LMSC, IFG, STG/MTG | Cerebellum |
| Structure–function correlations | LSC, IFG, STG, SMG | Cerebellum |
| Structure/function-symptom correlations | IFG | Cerebellum |
| Structure/function-duration correlations | IFG |
Note: Regions of common brain abnormalities and correlations across the VBM, CT, and fMRI datasets. LMSC, laryngeal motor and sensory cortex; STG, superior temporal gyrus; LSC, laryngeal somatosensory cortex.
The mechanisms involved in the GM abnormalities in SD could include both the primary pathophysiology and be a consequence of the disorder. Precise cellular mechanisms and causes of GM abnormalities revealed by brain imaging remain unknown (Bandettini 2009). A series of studies have shown that motor training and exercise may give rise to structural and functional GM increases in certain brain regions (Draganski et al. 2004; Ceccarelli et al. 2009; Quallo et al. 2009; Granert et al. 2011). In SD, voice and speech therapy might be considered as motor training, although SD patients do not typically respond to voice and speech therapy (Ludlow et al. 2008). In the present study, none of SD patients were either actively enrolled in voice and speech therapy at the time of study participation and near past or had indicated receiving any benefits from voice and speech therapy in the past. Thus, our findings are likely to reflect disorder-related dynamic reshaping of cortical organization rather than a result of motor training and exercise, such as voice and speech therapy.
The other possible causes for the GM increases may be the formation of new connections by dendritic spine growth and axonal remodeling and strengthening of existing connections (Chklovskii 2004; Chklovskii et al. 2004; Sur and Rubenstein 2005; Holtmaat et al. 2006). In line with this view, recent studies in SD have suggested that voice-controlling networks may be altered in this disorder (Simonyan et al. 2008; Simonyan and Ludlow 2010). SD is characterized by laryngeal motor cortical control deficits without any articulatory, hearing, or cognitive impairments. Our findings, nonetheless, indicate that both structural and functional GM abnormalities affect more than a single brain region of motor execution (e.g., laryngeal motor cortex), extend further to the brain regions involved in motor preparation (e.g., IFG) (Petersen et al. 1988; Hirano et al. 1996; Horwitz et al. 2003; Ozdemir et al. 2006) and may even interact with regions processing auditory information during speech production (e.g., STG/MTG) (Houde and Jordan 1998; Burnett and Larson 2002; Tourville et al. 2008). Furthermore, the laryngeal somatosensory cortex, IFG, STG/MTG, and cerebellum showed significant correlation between functional and structural abnormalities in SD patients (Fig. 2, Table 6). Abnormal structure–function relationship in these brain regions points again to the multilevel alterations of the speech-controlling networks, involving sensorimotor output (laryngeal sensory cortex, IFG, cerebellum) and auditory monitoring (MTG/STG). It is important, however, to note that not all brain regions showing statistically significant relationships between functional and structural measures in SD patients were found to have both structural and functional abnormalities when compared with controls. For example, the left IFG showed correlation both between GMV and activation and between CT and activation; however, compared with controls, SD patients had increased functional activation and CT but no volumetric abnormalities (Fig. 1B,C). Similar discrepancies were observed in the right cerebellum (increased activation but not volume), left STG (increased activation but not volume), and left SMG (increased CT but not functional activation) (Figs 1 and 2). Methodological limitations, such as higher sensitivity of CT analysis in detection of cortical changes compared with VBM analysis (Hutton et al. 2009), may possibly explain the presence of abnormalities in functional activation and CT but not in volume in some brain regions (e.g., left IFG). On the other hand, some of these relationships might not indicate a primary pathophysiology specific to SD. Functional activation increases without VBM or CT differences in the brain regions, such as in the left STG, may be secondary to abnormalities in other brain regions or have compensatory character without underlying structural changes.
In addition to abnormal structure–function relationships, the IFG and cerebellum showed significant correlations between SD clinical characteristics and GM abnormalities. Structural and functional increases in the IFG and cerebellum demonstrated statistically significant correlation with SD symptoms, while increased CT in the IFG was additionally related to SD duration. These findings suggest that structural abnormalities may relate to functional changes in these regions, which, in turn, may be related to SD symptom expression. However, the relationships between the SD symptoms and brain abnormalities in these structures had different directions. While structural increases in the both IFG and cerebellum exhibited positive correlations with symptom production, functional brain activation during symptomatic voice production showed an inverse relationship with SD symptoms. The relationships of the IFG and cerebellum with clinical characteristics of SD may suggest that evaluation of these structures may be considered for assessment of SD severity and treatment responses in SD patients.
The left anterior insula was the only region that showed decreased CT, increased functional activation, positive relationships between CT measurements/brain activation, and SD symptom severity but no volumetric changes. The insula is involved in a wide variety of behaviors, including voice and speech control, due, in part, to its direct connections with the laryngeal motor cortex, IFG, auditory, and cingulate cortices (Simonyan and Jurgens 2003, 2005; Cauda et al. 2010). The role of the anterior insula in speech motor control remains controversial. While several studies have initially suggested its involvement in motor planning of speech production (Dronkers 1996; Nestor et al. 2003; Bohland and Guenther 2006; Ogar et al. 2006), this view has been recently expanded by associating the anterior insula with the temporal processing of auditory stimuli and the control of automatic aspects of speech production, such as the regulation of respiratory activity (for review, see Ackermann and Riecker 2010). Our findings of anterior insular changes and their correlations with symptom severity in SD patients may point to its possible role in SD pathophysiology.
Limitations of the Study
A limitation affecting the interpretation of neuroimaging findings in SD and other primary focal dystonias is the lack of postmortem specimens from these patients. Recent neuropathological examination of the cerebellum in one SD patient has demonstrated structural pathology, such as parenchymal clusters of calcium, potassium, and iron depositions (Simonyan et al. 2008). In cervical dystonia, Bergman gliosis, patchy loss of Purkinje cells, torpedo fibers, and ubiquitinated inclusions between the Purkinje and molecular layers have been found in 4 patients (Zerrate et al. 2007). Future studies are needed to determine whether GM abnormalities in the laryngeal somatosensory cortex, IFG, STG/MTG, and SMG in SD patients are associated with cortical pathology in this disorder.
Another limitation of the current study is that, although we included both ADSD and ABSD patients, we did not examine possible differences between their GMV and CT abnormalities because the low and unequal number of patients in each separate group (25 ADSD and 15 ABSD) would not allow for achieving statistically meaningful results. As the primary aim of the current study was to identify the structure–function relationships in SD patients compared with healthy controls, future research should focus on determining whether there are characteristic differences in GM abnormalities between ADSD and ABSD. Based on our recent findings of minimal differences in the extent of brain activation in the primary sensorimotor cortex and cerebellum between ADSD and ABSD patients (Simonyan and Ludlow 2010), we expect that GM structural differences would be largely similar in both types of SD.
Summary
The present study determined that the laryngeal primary sensorimotor cortex, IFG, STG, SMG, and cerebellum exhibit abnormal structure–function relationships in SD patients. Among these structures, IFG and cerebellum showed both structural and functional relationships with SD symptom production, while the IFG showed an additional correlation with disorder duration. It appears that speech-controlling networks rather than single region (e.g., laryngeal sensorimotor cortex) are altered in SD, which may play an important role in the task specificity and pathophysiology of this disorder.
Funding
National Institute on Deafness and Other Communication Disorders; National Institutes of Health (R00DC009620 to K.S.); Intramural Program of National Institute of Neurological Disorders and Stroke (Z01NS00298 to C.L.L.).
Acknowledgments
We thank Sandra B. Martin, M.S., for help with patient recruitment, Pamela R. Kearney, M.D., for assistance with medical examination of the subjects, Kimberly Finnegan, M.S., for evaluation of patient’s speech samples, Richard Reynolds, M.S., for assistance with data analysis, and Mark Hallett, M.D., for collaboration on this project. Conflict of Interest: None declared.
References
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2010;214:419–433. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Ali SO, Thomassen M, Schulz GM, Hosey LA, Varga M, Ludlow CL, Braun AR. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49:1127–1146. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bandettini PA. What's new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkmeier JM, Case JL, Ludlow CL. Identification of symptoms for spasmodic dysphonia and vocal tremor: a comparison of expert and nonexpert judges. J Commun Disord. 2001;34:21–37. doi: 10.1016/s0021-9924(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Bielamowicz S, Ludlow CL. Effects of botulinum toxin on pathophysiology in spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2000;109:194–203. doi: 10.1177/000348940010900215. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Burnett TA, Larson CR. Early pitch-shift response is active in both steady and dynamic voice pitch control. J Acoust Soc Am. 2002;112:1058–1063. doi: 10.1121/1.1487844. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.11.049. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp. A PET study. Brain. 1997;120(Pt 4):571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, Filippi M. Cognitive learning is associated with gray matter changes in healthy human individuals: a tensor-based morphometry study. Neuroimage. 2009;48:585–589. doi: 10.1016/j.neuroimage.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB. Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron. 2004;43:609–617. doi: 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, Meunier S, Terrier A, Lehericy S. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. 2007;69:376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardi G, Perani D, Grassi F, Bressi S, Amadio S, Antoni M, Comi GC, Canal N, Fazio F. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand. 1996;94:172–176. doi: 10.1111/j.1600-0404.1996.tb07049.x. [DOI] [PubMed] [Google Scholar]
- Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736–739. doi: 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, Deuschl G, Zeuner KE, Siebner HR. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage. 2011;54:32–41. doi: 10.1016/j.neuroimage.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hirano S, Kojima H, Naito Y, Honjo I, Kamoto Y, Okazawa H, Ishizu K, Yonekura Y, Nagahama Y, Fukuyama H, et al. Cortical speech processing mechanisms while vocalizing visually presented languages. Neuroreport. 1996;8:363–367. doi: 10.1097/00001756-199612200-00071. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, Braun AR. Activation of Broca’s area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia. 2003;41:1868–1876. doi: 10.1016/s0028-3932(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279:1213–1216. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, Eidelberg D. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology. 2000;55:673–677. doi: 10.1212/wnl.55.5.673. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer's cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Adler CH, Berke GS, Bielamowicz SA, Blitzer A, Bressman SB, Hallett M, Jinnah HA, Juergens U, Martin SB, et al. Research priorities in spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, Gizewski ER, Diener HC, Maschke M. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–1123. doi: 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer's cramp. Mov Disord. 1998;13:497–508. doi: 10.1002/mds.870130321. [DOI] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97:343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M. Cerebral activation patterns in patients with writer's cramp: a functional magnetic resonance imaging study. J Neurol. 2001;248:10–17. doi: 10.1007/s004150170263. [DOI] [PubMed] [Google Scholar]
- Quallo MM, Price CJ, Ueno K, Asamizuya T, Cheng K, Lemon RN, Iriki A. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc Natl Acad Sci U S A. 2009;106:18379–18384. doi: 10.1073/pnas.0909751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Afferent subcortical connections into the motor cortical larynx area in the rhesus monkey. Neuroscience. 2005;130:119–131. doi: 10.1016/j.neuroscience.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an FMRI study. Cereb Cortex. 2010;20:2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL, Vortmeyer AO. Brainstem pathology in spasmodic dysphonia. Laryngoscope. 2010;120:121–124. doi: 10.1002/lary.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ostuni J, Ludlow CL, Horwitz B. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci. 2009;29:14912–14923. doi: 10.1523/JNEUROSCI.4897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39:1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerrate MC, Pardo CA, Jinnah HA. Neuropathology in idiopathic cervical dystonia. Movement Disord. 2007;22:S114–S114. [Google Scholar]