Abstract
The relative contribution of intrinsic and extrinsic cues in the regulation of cortical neurogenesis remains a crucial challenge in developmental neurobiology. We previously reported that a transient population of glutamatergic neurons, the cortical plate (CP) transient neurons, migrates from the ventral pallium (VP) over long distances and participate in neocortical development. Here, we show that the genetic ablation of this population leads to a reduction in the number of cortical neurons especially fated to superficial layers. These defects result from precocious neurogenesis followed by a depletion of the progenitor pools. Notably, these changes progress from caudolateral to rostrodorsal pallial territories between E12.5 and E14.5 along the expected trajectory of the ablated cells. Conversely, we describe enhanced proliferation resulting in an increase in the number of cortical neurons in the Gsx2 mutants which present an expansion of the VP and a higher number of CP transient neurons migrating into the pallium. Our findings indicate that these neurons act to maintain the proliferative state of neocortical progenitors and delay differentiation during their migration from extraneocortical regions and, thus, participate in the extrinsic control of cortical neuronal numbers.
Keywords: neocortical development, neurogenesis, neuronal numbers, progenitors, tangential migration, transient glutamatergic neurons
Introduction
The generation of an appropriate number of neurons in the central nervous system relies on a strict balance between onset of neurogenesis and maintenance of the progenitor pool (Caviness et al. 2003; Guillemot et al. 2006). At early stages of development, neuroepithelial and radial glia (RG) progenitors are restricted to the ventricular zone (VZ) and divide symmetrically at the apical side to expand the progenitor pool (Gotz and Barde 2005). At the onset of neurogenesis, VZ progenitors begin dividing asymmetrically to generate postmitotic neurons as well as intermediate progenitors (IPs or basal progenitors), which undergo a limited number of divisions in the basal VZ/subventricular zone (SVZ) and, thus, contribute to amplify the number of cortical neurons (Gotz and Huttner 2005; Pontious et al. 2008). Thereafter, newly born neurons migrate radially from the VZ/SVZ to organize in a laminated structure according to an “inside out” sequence whereby early-born neurons reside in deep layers and late-born neurons in more superficial layers (Berry et al. 1964; Bystron et al. 2008). Although the progenitors in the VZ and SVZ undergo both proliferative and neurogenic divisions, their relative contribution to the final number of cortical neurons in deep and superficial layers is still controversial (Pontious et al. 2008; Kowalczyk et al. 2009; Puzzolo and Mallamaci 2010). Nevertheless, the control of their mode of divisions is crucial for the generation of an appropriate number of neuronal subtypes.
An increasing number of molecules regulating cell cycle properties, either the length of specific phases or the timing of cell cycle exit, have been shown to directly influence the mode of divisions of cortical progenitors (Dehay and Kennedy 2007; Glickstein et al. 2009; Pilaz et al. 2009; Salomoni and Calegari 2010). Notably, several of the transcription factors involved in area patterning have also been implicated in the control of cell cycle regulators providing an intrinsic control of progenitors' proliferation (Muzio et al. 2005; Holm et al. 2007; Faedo et al. 2008; O'Leary and Sahara 2008). In addition, recent evidence suggests that the secretion of growth factors and morphogens by postmitotic neurons, some of which reach the developing cortex by tangential migration, or by ingrowing thalamic axons provide an extrinsic control for cortical development (Dehay and Kennedy 2007; Komada et al. 2008; Seuntjens et al. 2009; Griveau et al. 2010). However, the mechanisms coordinating intrinsic versus extrinsic processes and their relative contribution to the final cortical cytoarchitecture remain elusive.
We have previously identified a novel population of transient glutamatergic neurons generated by Dbx1-expressing progenitors in the ventral pallium (VP) at the pallial/subpallial boundary (PSB) at E12.5 which progressively invade the pallium by tangential migration to distribute homogeneously in the neonatal cortex (Teissier et al. 2010). We showed that specific genetic ablation of these VP-derived cortical plate (CP) transient neurons, using E1-Ngn2/CRE;Dbx1DTA animals, leads to a decrease in cortical thickness. In this manuscript, we report that ablation results in a reduced number of cortical neurons which affects predominantly superficial layers and is due to a precocious differentiation and a progressive depletion of the progenitor pools. Conversely, we also describe that Gsx2 mutants, which exhibit an expanded VP (Yun et al. 2001; Stenman, Wang, et al. 2003), display an enhancement in proprogenitors proliferation as well as in the number of cortical neurons generated correlating with an increase in the number of the CP transient neurons. Our results show that these neurons contribute in a non-cell autonomous manner to maintain the neocortical progenitor pools and, thus, participate in the fine-tuning of cortical neuronal numbers.
Materials and Methods
Animals
All animals were kept in C57BL/6J background and use of mice in this study was approved by the Veterinary Services of Paris. The conditional ablation of Dbx1-derived cells was performed by crossing the Dbx1loxP-stop-loxP-DTA mouse line (Bielle et al. 2005) with the E1-Ngn2/CRE(iresGFP) strain (Berger et al. 2004) expressing the CRE recombinase and the GFP under the control of the E1 enhancer element of the Ngn2 gene. The Dbx1iresCRE animals, the Gsx2RA/+ animals, and the CagCat(CC)-EGFP animals were generated as previously described (Bielle et al. 2005; Nakamura et al. 2006; Waclaw et al. 2010). The Gsx2RA/+ animals were crossed with the CagCat(CC)-EGFP or Dbx1CRE/+ animals to generate Gsx2RA/+;CagCat(CC)-EGFP and Gsx2RA/+;Dbx1CRE/+ mice (Waclaw et al. 2010). Embryos and postnatal animals were genotyped by PCR using primers specific for the different alleles. For staging of animals, midday of vaginal plug was considered as embryonic day 0.5 (E0.5).
Tissue Preparation, Immunohistochemistry, and In Situ Hybridization
Embryos and postnatal animals collection and fixation were performed as previously described (Bielle et al. 2005). Embedded tissues were sectioned with a cryostat with a 12–14 μm step for embryonic and 18 μm for postnatal stages. Fluorescent immunohistochemistry, Nissl staining, and in situ hybridization were performed as previously described (Bielle et al. 2005). For whole-mount in situ hybridization, the following modifications were applied: brains were pretreated with 10 μg/mL proteinase K for 30 min, the prehybridization and hybridizations were done at 70 °C in the presence of 3 μg/mL digoxygenin-labeled probes. Primary antibodies produced in mice were anti-Pax6 (DSHB, 1:50), anti-Reelin (Calbiochem, G10, 1:500), and anti-TuJ1 (BAbCo, 1:1000); primary antibodies produced in rabbit were anti-BLBP (AbCys, 1:2000), anti-Cux1/2 (Santa Cruz, CDP M222, 1:600), anti-FoxP2 (Abcam, 1:5000), anti-Ki67 (AbCam, 1:1000), anti-Mef2c (ProteinTech Group, 1:2000), anti-PH3 (Millipore, Ser10, 1:1000), anti-Tbr1 (Chemicon, 1:4000), and anti-Tbr2 (Chemicon, 1:8000). We also used rat anti-Ctip2 (AbCam, 1:600) and chick anti-GFP (AvesLab, 1:2000). For BrdU pulse experiments, embryos were obtained from females injected intraperitoneally with a single dose of BrdU (Sigma, 50 mg/kg) 1 h prior to collection. BrdU staining was performed using rat anti-BrdU (AbD Serotec, 1:400) after 5 min of 4 M HCl treatment and 10 min fixation in 4% paraformaldehyde (PFA), 0.1 M phosphate buffer (PB) pH 7.3. For IdU pulse experiments, embryos were obtained from females injected intraperitoneally with a single dose of IdU (Sigma, 60 mg/kg). For BrdU- and IdU double-labeling experiments, the slides were microwaved 6 min at 270 W in 0.1 M citric acid, phosphate-buffered saline (PBS) preheated 4 min at 740 W. Slides were subsequently cooled down for 30 min at room temperature (RT) and rinsed 3 times in PBS before being incubated with 2 M HCL in PBS for 30 min at RT. After several PBS washes, the slides were fixed for 10 min in 4% PFA, 0.1 M PB and rinsed again in PBS before incubation in PBS, 0.1% Triton, 1% horse serum. The BrdU signal was specifically detected using rat anti-BrdU specific (AbD Serotec, 1:400), whereas the IdU signal was detected using a mouse antibody recognizing both IdU and BrdU (BD Biosciences, 1:100). The sections were incubated overnight with the 2 primaries antibodies diluted in PBS, 0.1% Triton, 1% horse serum and revealed using the appropriate conjugated secondary antibodies (Alexa488-conjugated anti-mouse and Cy3-conjugated anti-rat, Jackson ImmunoResearch). This method identifies IdU-only cells (labeled only by the mouse primary antibody) and IdU+BrdU+ double-labeled cells (labeled by both the mouse and the rat antibodies). For triple staining with Tbr1, the primary and secondary antibodies' reactions to reveal Tbr1 were performed starting the preceding night and were fixed 10 min in 4% PFA, 0.1 M PB before starting the treatments for detection of the BrdU/IdU labeling. Nuclei staining was performed using Vectashield Mounting with 4',6'-diamidino-2-phénylindole (DAPI) from Biovalley except for the BrdU and BrdU/IdU experiments which were incubated for 20 min at RT in bis benzemide (Hoeschst 33342, Coger). All fluorescent secondary antibodies were purchased from Jackson ImmunoResearch.
Data Collection
Counts of cells labeled by immunofluorescence and measures of the thickness of the deep and superficial layers were performed manually on calibrated pictures using ImageJ software.
rD and cL levels determination: At embryonic stages, the rostrodorsal (rD) level was identified as the most dorsal domain on coronal sections selected by the presence of the lateral ganglionic eminence (LGE) and septum but not the medial ganglionic eminence. The caudolateral (cL) level was identified as the domain located just above the morphological hinge at the PSB on coronal sections selected by the presence of the caudal ganglionic eminence and the cortical hem. At E18.5 and P2 stages, the rD level was identified at the level of the prospective primary motor area on coronal sections, whereas the cL level was identified at the level of the prospective secondary sensory area, according to the Paxinos Mouse Atlases. For each experiment, the sections from the E1-Ngn2/CRE;Dbx1DTA or Gsx2−/− mutants were compared with sections from control littermates and processed simultaneously.
Measures of deep and superficial layers: The thickness of deep and superficial layers, in the P2 E1-Ngn2/CRE;Dbx1DTA mutant and control animals, was measured on sections stained by Nissl (Fig. 1A). The layers of the CP were delineated based on the differential cell density. Since the Gsx2−/− animals do not survive at birth and Nissl staining is not precise enough to delineate cortical layers at E18.5, the thickness of the deep and superficial layers in the E18.5 Gsx2−/− mutant and control littermates were measured based on coronal sections processed for in situ hybridization for Tbr1 mRNA (Fig. 8A). In all cases, the CP thickness of deep and superficial layers was measured in dorsal and lateral domains of 3 coronal sections for each specimen (only quantifications for rostrodorsal and caudolateral domains are shown in Figs 1A and 8A).
PH3+ cells quantifications: For quantifications of VZ and SVZ mitosis at E12.5, E14.5, and E16.5 (Figs 3 and 7), the number of PH3+ cells was counted through the thickness of the pallium and normalized for 100-μm-wide strips at both rD and cL levels on coronal sections. The number of PH3+ cells in the VZ corresponds to labeled mitosis located at the border of the ventricle and that for the SVZ corresponds to the abventricular ones.
Tbr2+ cells quantifications: Tbr2+ cells were counted through the thickness of the pallium and normalized for 100-μm-wide strips at both rD and cL levels on coronal sections (Fig. 5).
VZ cell density: The cell density of the VZ was estimated on coronal sections of 14 μm thickness in E16.5 E1-Ngn2/CRE;Dbx1DTA mutant and control animals by counting the number of DAPI+ cells in 100 × 100 μm boxes at dorsal and lateral levels. At least 5 boxes were counted for each level in 3 animals.
Quantification of the increase in CP transient neurons: The Dbx1CRE/+;CagCat(CC)-EGFP mice in the Gsx2RA/RA mutant and Gsx2+/+ control backgrounds were generated by crossing Gsx2RA/+;CagCat(CC)-EGFP with Gsx2RA/+;Dbx1CRE/+ mice. The total number of GFP+Tbr1+ and GFP+Mef2c+ cells in the CP and MZ (corresponding to CR cells) of the neocortex were counted on coronal sections in 3 specimens for each background.
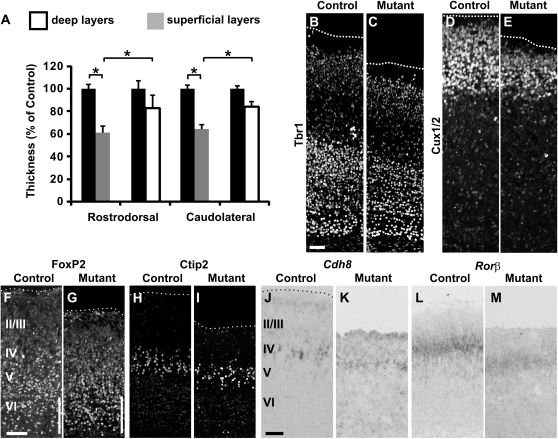
Figure 1.
Decrease in the number of cortical neurons is more pronounced in superficial layers upon Dbx1-derived CP transient neurons ablation. (A) Graphs show CP thickness in E1-Ngn2/CRE;Dbx1DTA mutants relative to wild-type littermates at both rostrodorsal (rD) and caudolateral (cD) levels of P2 brains and for both deep (white columns) and superficial layers (gray columns) based on Nissl staining. The superficial layers are more affected than the deep layers at both cL and rD levels (rD deep layers: 17.38 ± 13.32%, P = 0.085; rD superficial layers: 38.98 ± 11.24%, P = 0.043; cL deep layers: 16.04 ± 9.01%, P = 0.064; cL superficial layers: 35.77 ± 14.58%, P = 0.010). Graphs represent means ± standard error of the mean (SEM). *P < 0.05. (B–E) Immunostaining for Tbr1 (B,C) and Cux1/2 (D,E) shows that both deep and superficial layers at rD levels are affected in E1-Ngn2/CRE;Dbx1DTA mutants (C,E) compared with wild-type littermates (B,D), although the effect is more pronounced for the superficial layers. (F–G) Immunostaining for FoxP2 at E18.5 reveals a small increment in the number of neurons located in layer VI (white bars in F,G). (H–M) Immunostaining for Ctip2 (H,I) and in situ hybridization for Cdh8 (J,K) and Rorβ (L,M) confirm a reduced neuronal numbers specifically affecting the superficial layers. Scale bars: B,F,J, 100 μm.
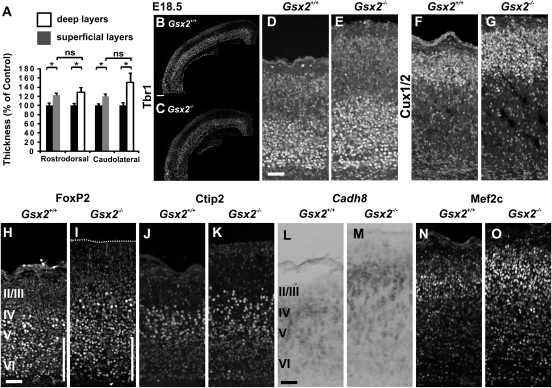
Figure 8.
E18.5 Gsx2 mutants present an increase in the thickness of both deep and superficial cortical layers. (A) Graphs represent the thickness of deep (white) and superficial (gray) layers in Gsx2 mutants relative to the thickness of Gsx2+/+ control animals in rostrodorsal and caudolateral matched territories based on Tbr1 mRNA differential expression in deep versus superficial layers (rD deep layers: 128.68%, P = 0.0478; superficial layers: 122.45%, P = 0.0122; n = 5 and cL deep layers: 150.13%, P = 0.0146; superficial layers: 120.19%, P = 0.0121). Notably, both deep and superficial layers appear to be thickened in the mutant cortices in a similar manner (rD: P = 0.380; cL: P = 0.20). We controlled that the thickening of cortical layers was not the consequence of a reduction in neuronal density by counting the number of DAPI+ nuclei cells in the CP and we rather observed the opposite effects (120 ± 4.82% mutant/control, n = 3), therefore suggesting that the defects in thickness were likely underestimated. Graphs represents mean ± SEM. *P < 0.05. (B–G) Immunostaining for Tbr1 (B–E) and Cux1/2 (F,G) labeling deep and superficial layers, respectively, confirms a thicker CP comprising both the deep and the superficial layers in E18.5 Gsx2 mutant (C,E,G) compared with Gsx2+/+ control (B,D,F) littermates. (H–O) Immunostaining at rD levels for FoxP2 (H,I), Ctip2 (J,K), and Mef2c (N,O) and in situ hybridization for Cdh8 (L,M), labeling layers V/VI, layer V, and layers II–IV. Except for neurons located in layer VI whose number appears reduced (white bars in H,I), all other markers show a pronounced increase in Gsx2 mutants (I,K,M,O) compared with controls (H,J,L,N). Scale bars: B, 200 μm; D,H,L, 100 μm.
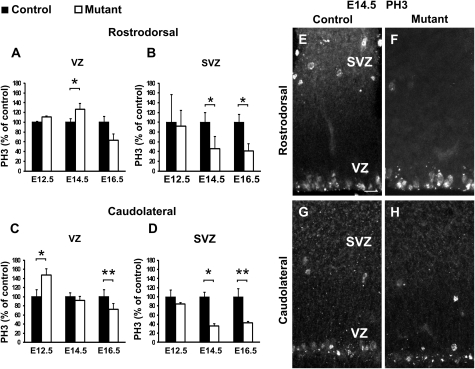
Figure 3.
Temporal and spatial dynamics of proliferation defects follow the expected trajectory of the CP transient neurons in ablated embryos. (A–D) Graphs show percentages of mitotic PH3+ cells in mutant (white bars) relative to control (black bars) embryos in the VZ (A,C) and SVZ (B,D) at rD (A,B) and cL (C,D) levels at E12.5, E14.5, and E16.5. Quantifications of the number of PH3+ cells show a transient increase in VZ proliferation of cL regions at E12.5 (P = 0.02402; n = 3) and of rD regions at E14.5 (P = 0.02487; n = 4) followed by a decrease at all levels by E16.5 (rD: P = 0.15084; cL: P = 0.0017; n = 4). A reduction of SVZ proliferation is detected at E14.5 and E16.5 at both levels (rD: E14.5, P = 0.01482 and E16.5, P = 0.03273; cL: E14.5, P = 0.03167 and E16.5, P = 0.00100). Graphs represent means ± SEM. *P < 0.05 and **P < 0.005. (E–H) Immunostaining for PH3 at both rD (E,F) and cL (G,H) levels at E14.5 shows more numerous mitoses in the VZ with fewer mitoses in the SVZ of mutants (F) at rD levels. At cL levels, both VZ and SVZ display reduced number of mitosis in mutant animals (H) compared with controls (G). Scale bars: E, 20 μm.
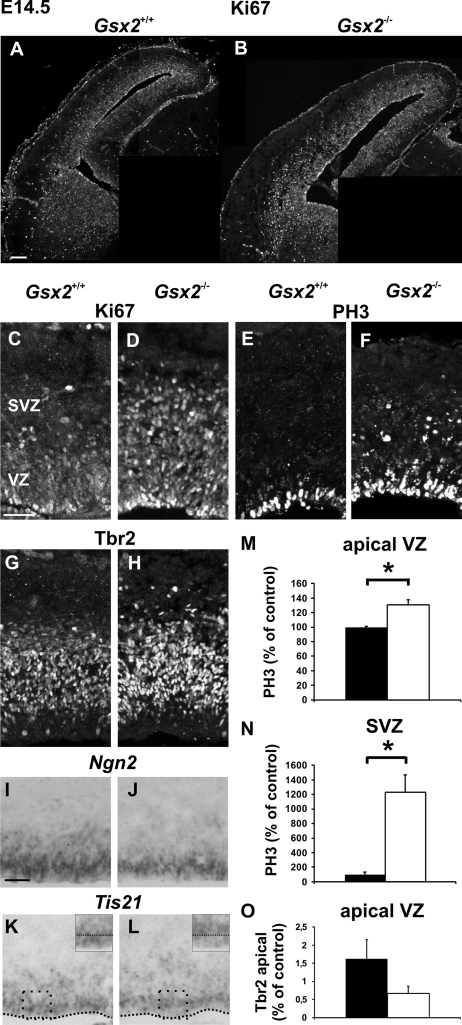
Figure 7.
Increased proliferation of pallial progenitors at midneurogenesis in Gsx2 mutants. (A–D) Immunostaining for Ki67 on E14.5 telencephalons reveals an increase in the number of proliferative cells in the pallium of Gsx2 mutants (B,D) compared with Gsx2+/+ controls (A,C) affecting both the VZ and the SVZ. (E–H) Immunostaining for PH3 (E,F) and Tbr2 (G,H) on the same animals showing an increment in the number of cycling cells in mitosis in both VZ and SVZ, as well as an increase in the number of IPs and/or of young neurons in Gsx2−/− mutants brains (F,H). (I–L) In situ hybridization for Ngn2 (I,J) and Tis21 (K,L) confirms a decreased fraction of neurogenic divisions in Gsx2−/− mutants in both VZ and SVZ. High magnifications of boxes in (K,L) show that the decrease in Tis21 staining is more pronounced in the apical VZ. (M,N) Graphs show percentages of mitotic PH3+ cells in mutant (white columns) relative to control (black columns) embryos in the apical VZ (M) and SVZ (N) at rD levels at E14.5. Values for the VZ are 27.39 ± 0.38 (control) and 35.88 ± 1.92 (mutant) (P = 0.0497) and for the SVZ 1.15 ± 0.38 (control) and 14.27 ± 2.70 (mutant) (P = 0.0406). (O) Graphs represent the number of Tbr2+ cells at the apical VZ normalized for 100-μm-wide strips at rD levels at E14.5 in controls (black columns) and mutants (white columns). The number of Tbr2+ cells is reduced, although not statistically significant (1.625 ± 0.53 (control) and 0.666 ± 0.20 (mutant)) (P = 0.094). Graphs represent means ± SEM. *P < 0.05. Scale bars: A, 100 μm; C,I, 50 μm.
Figure 5.
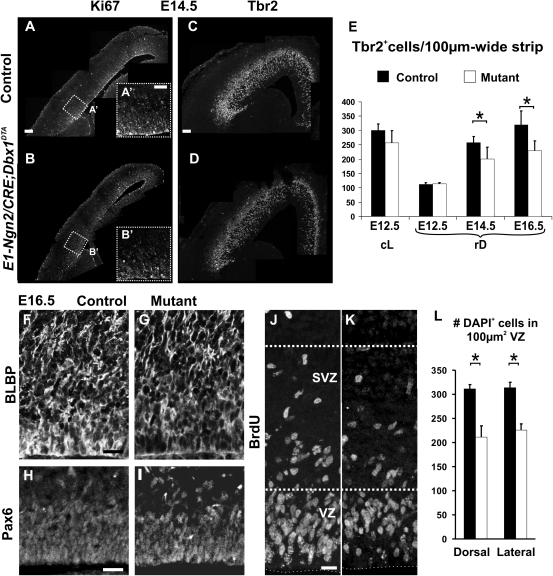
Depletion of RG and IP progenitor pools in E1-Ngn2/CRE;Dbx1DTA animals. (A–B′) Immunohistochemistry for Ki67 shows a global decrease in cycling progenitors in mutant E1-Ngn2/CRE;Dbx1DTA (B,B′) compared with control (A,A′) cortices at E14.5. (C,D) Immunohistochemistry for Tbr2 reveals fewer Tbr2+ cells in the pallium of E14.5 mutant (D) compared with control (C) littermates. (E) Graphs represent the number of Tbr2+ cells through the thickness of the pallium and normalized for 100-μm-wide strips at E12.5 at both cL and rD levels and E14.5 and E16.5 at rD levels in controls (black columns) and mutants (white columns). The number of Tbr2+ cells is reduced, although not statistically significant, at cL levels of E12.5 mutant embryos. At rD levels, a 22% decrease is observed at E14.5 and E16.5 (E14.5: P = 0.023539; E16.5: P = 0.023532). (F–K) Immunohistochemistry for BLBP (F,G) and Pax6 (H,I) on E16.5 control (F,H) and mutant (G,I) animals shows a reduction in the size of the RG pool in the VZ of mutant animals. (J,K) A pulse of BrdU 2 h before sacrifice labeling cells in S-phase reveals fewer cycling progenitors in both VZ and SVZ of E16.5 mutant (K) compared with control (J) littermates. (L) Graph shows the number of DAPI+ nuclei per 100 μm2 boxes of 14-μm-thick coronal sections in the VZ at dorsal and lateral levels in E16.5 mutants (white columns, dorsal: 211.66 ± 22.98, lateral: 225.87 ± 12.87) and controls (black columns, dorsal: 311.62 ± 8.3, lateral: 313.56 ± 11.43) (P = 0.026 in dorsal and P = 0.036 in lateral). Graphs represent means ± SEM. *P < 0.05. Scale bars: A,C, 100 μm; A′,F,H, 50 μm; and J, 20 μm.
IdU, BrdU Injections, and Cell Cycle Analysis
Both IdU and BrdU are thymidine analogs which are incorporated during the S-phase of the cell cycle with a clearance in the brain of ∼3 h. If we assume that progenitor cells in the telencephalic neuroepithelium 1) progress through the cell cycle asynchronously and 2) have similar cell cycle durations, we can conclude that in such a population of proliferating cells, the fraction of cells in a given phase of the cell cycle is directly proportional to the length of that phase relative to the total length of the cell cycle (Nowakowski et al. 1989). Therefore, upon one single IdU or BrdU injection, all the cells in S-phase at that time will be labeled, and therefore, their proportion among progenitors represents Ts/Tc. If we wait for a ΔT < 3 h for the mice to be sacrificed, the number of labeled cells will therefore represent the proportion of cells that have been in S-phase not only at the time of injections but also during the ΔT and therefore allow to estimate (Ts + ΔT)/Tc (Nowakowski et al. 1989).
Cell Cycle Length
Pregnant mice were injected intraperitoneally with IdU (60 mg/kg), followed by a BrdU injection (50 mg/kg) 1.5 h later and were sacrificed 3 h after the IdU injection to collect the embryos at E12.5 or E14.5. Considering that some DAPI+ cells located in the VZ may be differentiated, we controlled that the proportion of TuJ1+ cells was small and that the differences between controls and mutants were negligible as compared with the total number of DAPI+ cells at all levels and stages studied (E12.5: rD = 5.65% in control vs. 5.8% in mutant animals; cL = 7.13% vs. 11.26%; E14.5: rD = 9.42% vs. 9.39%; cL = 9.73 vs. 12.25%; n = 2). In addition, previous studies have shown that a prolonged pulse of BrdU will label virtually all VZ cells at E12.5 (Estivill-Torrus et al. 2002), allowing us to assume that all the DAPI+ cells in the VZ were proliferating (Pcells). Cells that have been labeled upon the IdU injection and that have exited the cell cycle at the time of the BrdU injection will be labeled by IdU and not by BrdU, and their number in the VZ is designated as the leaving fraction (Lcells). The number of cells labeled with BrdU is designated as Scells. Therefore, the length of the S-phase is calculated using the formula: Ts/ΔT = Scells/Lcells and the length of the cell cycle is estimated from the formula: Tc/Ts = Pcells/Scells (Martynoga et al. 2005; Fukumitsu et al. 2006; Quinn et al. 2007).
Q Fraction
Pregnant mice were injected intraperitoneally once with IdU (60 mg/kg), then 7 times with BrdU (50 mg/kg) every 2 h and sacrificed 15 h after the initial IdU injection. Embryos were stained as described above. Since the normal duration of a cell cycle at E14.5 is ∼17 h but Tc − Ts is ∼14.5 h for cortical progenitors (Miyama et al. 1997; Calegari et al. 2005), all the proliferative cells have incorporated some IdU or BrdU. Cells located in the VZ/SVZ that have been labeled upon the IdU injection and that have exited the cell cycle at the time of the first BrdU injection will be labeled by IdU and not by BrdU. Therefore, they are considered as the cells that have exited the cell cycle during one cycle and their proportion among the total number of proliferative cells (IdU+ or BrdU+) is designated as the Q fraction (Takahashi et al. 1996; Tarui et al. 2005).
Statistical Analysis
For all experiments, results have been obtained from at least 3 pairs of control and mutant littermates, and the number of counted cells is indicated in the Figure Captions. For all quantifications, normal distribution was confirmed and unpaired, 2-tailed t-test on group means was performed for statistical analysis, using Microsoft Excel software (*P < 0.05, **P < 0.005).
Image Acquisition
Brightfield images of brain sections were acquired using a color camera (Zeiss Axiocam HRc) coupled to a Zeiss Axiovert 200 microscope and immunofluorescence images using an inverted confocal microscope (Leica TCS SP5 AOBS Tandem resonant Scanner).
Results
Reduction of Cortical Neurons' Numbers Especially in Superficial Layers upon CP Transient Neurons Ablation
We have previously shown that the specific ablation of the CP transient neurons derived from Dbx1-expressing progenitors at the VP/PSB in E1-Ngn2/CRE(iresGFP);Dbx1DTA mutant animals leads to a ∼20% reduction in CP thickness throughout the neocortex at E18.5, without affecting either cell density or death (Teissier et al. 2010). This population invades the preplate (PP) and the SVZ/intermediate zone (IZ) by tangential migration with a caudolateralhigh to rostromediallow gradient starting at E12.5 and is redistributed homogeneously in the CP along the rostrocaudal (RC) and mediolateral (ML) axes at birth.
In order to understand how the loss of Dbx1-derived CP transient neurons leads to a reduction of the CP thickness in E1-Ngn2/Cre(iresGFP);Dbx1DTA animals, we analyzed the defects at caudolateral (cL) and rostrodorsal (rD) levels which represent cortical territories at short and long distances from their generation site, respectively (Teissier et al. 2010). Nissl staining analysis at P2 confirmed that the entire cortex showed a decrease in CP thickness, as previously described at E18.5, with respect to wild-type cortices (rD: 24.32% and cL: 23.87%) (Teissier et al. 2010 and data not shown) and also revealed that this reduction was clearly more pronounced for superficial layers (Fig. 1A, rD: 38.98%; cL: 35.77%) than for deep layers (rD: 17.38%; cL: 16.04%) at both rD and cL levels. No significant differences were observed in the extent of the defects between cL and rD levels. These results were confirmed by specifically labeling deep and superficial layers using Tbr1 and Cux1/2 immunostaining, respectively. We observed in both cL and rD regions a small decrease in the thickness of Tbr1+ deep layers in addition to a severe reduction in that of Cux1/2+ superficial layers in mutant brains (Fig. 1B–E). Laminar fate and positioning of cortical neurons appeared to be preserved as observed by immunohistochemistry for FoxP2 (deep layers) and Ctip2 (prospective layer V subpopulations) (Fig. 1F–I) as well as in situ hybridization for Cdh8 (prospective layers II–IV and subpopulation of layer V) and Rorβ (prospective layer IV) (Fig. 1J–M). In addition, an increase in the number of FoxP2 neurons was detected in deeper portions of layer VI (Fig. 1F,G, white bars), whereas that of neurons fated to layer V, as identified by Ctip2 labeling (Fig. 1H,I), was slightly decreased in mutant compared with control littermates. A more pronounced decrement of Cdh8 and Rorβ staining confirmed that superficial layers are more highly affected as revealed by Cux1/2 labeling (Fig. 1J–M). Finally, since another population of tangentially migrating cells, namely Cajal–Retzius (CR) cells, are also generated from Dbx1-expressing progenitors at the VP prior to the CP transient neurons and have been shown to influence cortical patterning (Griveau et al. 2010), we performed whole-mount in situ hybridization for Lmo4 and Cdh8 mRNAs on P0 animals (Supplementary Fig. S1). We observed no differences in area patterning between ablated and control littermates cortices as expected from the absence of defects in CR cells distribution previously reported in the E1-Ngn2/CRE;Dbx1DTA mutants (Teissier et al. 2010) and the late migration and homogeneous distribution of the CP transient neurons.
We conclude that the specific genetic ablation of the CP transient glutamatergic neurons generated from Dbx1-expressing progenitors in the VP leads to a homogeneous deficit in the total number of neurons, although this is more pronounced for the superficial layers, throughout the early postnatal neocortex.
Defects in Differentiation and Proliferation Progressively Affect the Entire Pallium along the Expected Trajectory of the Ablated CP Transient Neurons
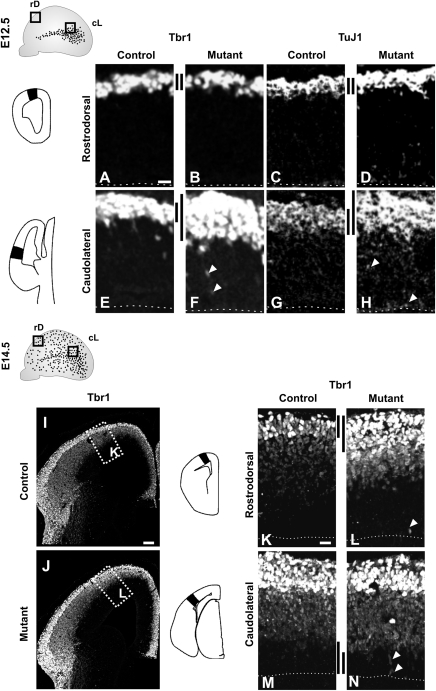
Since the CP transient neurons progressively invade the pallium from caudolateral regions starting at E12.0 and reach the rostrodorsal territories by E14.5 (Teissier et al. 2010), we began by analyzing neuronal differentiation at E12.5 and E14.5 using immunostaining for TuJ1 and Tbr1, markers of young postmitotic neurons (Memberg and Hall 1995) and early differentiated glutamatergic cortical neurons (Englund et al. 2005), respectively. We observed an increase in the number of both Tbr1+ and TuJ1+ differentiating neurons in the CP (Fig. 2E–H, black bars) together with ectopically positioned labeled cells in the VZ (arrowheads) in cL regions of E12.5 ablated telencephalons with respect to control littermates. No defects in differentiation were observed in rD regions at this stage (Fig. 2A–D) correlating with the presence of numerous Dbx1-derived CP transient neurons in cL but not in rD territories at E12.5 (Teissier et al. 2010). Interestingly, by E14.5, a similar increase in differentiation was observed in the rD region of mutant telencephalons (Fig. 2I–L and data not shown), whereas no differences were detected in the CP of the cL region, with the exception of ectopic Tbr1+ neurons in the VZ (Fig. 2M,N, arrowheads). Moreover, at E14.5 a slight thinning of the VZ was apparent especially in mutant cL territories (Fig. 2M,N, black bars).
Figure 2.
Transient increase in differentiation progressively affects the entire pallium of E1-Ngn2/CRE;Dbx1DTA mutants. (A–H) Immunohistochemistry performed on E12.5 embryos shows no differences for Tbr1 (A,B) and TuJ1 (C,D) staining between control (A,C) and E1-Ngn2/CRE;Dbx1DTA (B,D) embryos at rD levels, whereas there is a strong increase for both in cL regions in mutants (F,H, black bars) compared with control littermates (E,G). Moreover, ectopic Tbr1+ and TuJ1+ cells are observed in the mutant VZ (arrowheads in F,H). (I–N) By E14.5, Tbr1 staining is increased at rD levels in mutants (J,L), whereas no differences are detected at cL levels (N) with respect to control littermates (I,K,M). Ectopic Tbr1+ neurons (arrowheads in L,N) are observed in the VZ of mutants at both levels as well as a thinning of the VZ at cL levels (black bars in M,N). K and L represent high magnifications of boxed areas in I and J, respectively. Scale bars: A,K, 20 μm; I, 100 μm.
In order to assess if the increase in differentiation was due to defects in proliferation occurring upon ablation, we quantified the number of mitotic cells, as labeled by PH3 staining, in the rD and cL regions of the developing pallium at E12.5, E14.5, and E16.5 (Fig. 3A–D). We detected an initial increase in the number of PH3+ mitotic progenitors in the cL VZ at E12.5 followed by a reduction clearly observed at E16.5 (Fig. 3C). At E14.5, corresponding to the time of arrival of the CP transient neurons in rD territories in control animals, enhanced VZ proliferation was observed in rD regions of mutant cortices (Fig. 3A,E,F), followed by a small decrease by E16.5 (Fig. 3A). Notably, the number of PH3+ mitotic progenitors in the SVZ was strongly reduced at both cL and rD levels starting at E14.5 (Fig. 3B,D,E–H).
Taken together, these results show that the loss of the CP transient neurons leads initially to an increment in the number of mitosis in the VZ and of differentiating neurons (at E12.5 in cL and E14.5 in rD regions), followed by a decrement in the number of both VZ and SVZ mitosis. Notably, changes in proliferation and differentiation appear to progress from the caudolateral to the rostrodorsal cortex and to correlate with the timing and trajectory of invasion of pallial regions by the CP transient neurons.
CP Transient Neurons Ablation Results in Precocious Neurogenesis
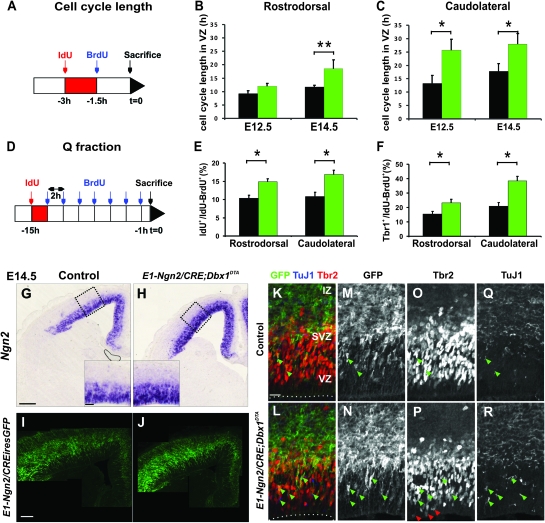
The defects observed in ablated animals could result from either a change in cell cycle length, an increase in the fraction of neurogenic divisions and/or a variation in the size of the progenitor pools. In order to discriminate between these possibilities, we started by measuring the length of the cell cycle using IdU and BrdU injections 3 and 1.5 h before collection of the embryos (see Materials and Methods) (Fig. 4A–C). At E12.5, we observed lengthening of the cell cycle in progenitors located in the VZ in cL (Tc = 13.31 h ± 2.91 in controls and Tc = 25.71 h ± 4.02 in mutants) but not in rD territories (Tc = 9.19 h ± 1.07 in controls and Tc = 11.96 h ± 1.09 in mutants). By E14.5, VZ progenitors at both cL and rD levels displayed a clear increase in Tc (rD: Tc = 11.74 h ± 0.54 in controls and Tc = 18.51 h ± 3.27 in mutants; cL: Tc = 17.81 h ± 2.80 in controls and Tc = 27.96 h ± 3.91 in mutants). Therefore, defects in proliferation and differentiation correlate with a lengthening of the cell cycle in E1-Ngn2/Cre;Dbx1DTA mutant embryos.
Figure 4.
Elongated cell cycle length and enhanced neurogenic divisions in progenitors upon ablation of the CP transient neurons. (A) Schematic illustration of the double S-phase–labeling paradigm for the estimation of cell cycle length. (B) Graphs show cell cycle length of progenitors in the VZ at both rD (B) and cL (C) levels calculated using IdU and BrdU injections (for details see Materials and Methods) in E12.5 and E14.5 wild-type (black bars) and mutant (green bars) embryos. At E12.5, an increase in Tc is detected specifically at cL levels in mutant compared with wild-type embryos (Tc = 13.31 ± 2.9 h in controls and Tc = 25.71 ± 4.02 h in mutants; P = 0.0347), whereas the Tc is similar at rD levels (Tc = 9.19 ± 1.07 h in controls and Tc = 11.96 ± 1.09 h in mutants; P = 0.088). At E14.5, the Tc is increased throughout the pallium in mutant embryos (cL: Tc = 17.81 ± 2.8 h in controls and Tc = 31.12 ± 0.82 h in mutants; P = 0.0127; rD: Tc = 11.74 ± 0.54 h in controls and Tc = 18.51 ± 3.27 h in mutants; P = 0.0010). Graphs represent means ± SEM. *P < 0.05 and **P < 0.005. (D) Schematic illustration of the double S-phase–labeling paradigm for the estimation of the Q fraction. (E) Graphs show the Q fraction corresponding to the number of cells that have exited the cell cycle within the 2-h interval between the IdU and the first BrdU injection relative to the total number of cycling cells at both rD and cL levels at E14.5. The pallial neuroepithelium of E1-Ngn2/CRE;Dbx1DTA embryos displays an increased Q fraction (rD: 10.33 ± 0.85% in controls with respect to 14.86 ± 1.62% in mutants, P = 0.049; cL: 10.82 ± 1.15% with respect to 16.83 ± 0.71%, P = 0.007). (F) Graphs represent the percentage of Tbr1+ among the double-labeled cells that have, thus, been generated during the 15 h of the experiment (rD: 15.37 ± 1.87% in controls with respect to 23.08 ± 2.64% in mutants, P = 0.030; cL: 20.83 ± 2.63% in controls with respect to 38.45 ± 3.08% in mutants, P = 0.007). (G,H) In situ hybridization for Ngn2 mRNA suggests an increment in neurogenic division at both VZ and SVZ levels in E14.5 E1-Ngn2/CRE;Dbx1DTA mutants (H) compared with control littermates (G). (I,J) Immunohistochemistry for GFP on coronal sections of E14.5 E1-Ngn2/CRE(iresGFP);Dbx1DTA mutants (J) compared with E1-Ngn2/CRE(iresGFP) control littermates (I) reveals a greater activation of the E1 enhancer of the Ngn2 gene in mutants. (K–R) Immunohistochemistry for GFP (K–N), Tbr2 (K,L,O,P), and TuJ1 (K,L,Q,R) at rD levels on E14.5 control (K,M,O,Q) and E1-Ngn2/CRE(iresGFP);Dbx1DTA (L,N,P,R) telencephalons. The Tbr2+Tuj1+ cells located in the VZ often correspond to GFP+ cells (green arrowheads in K–R) and their number is more numerous in mutants. In addition, ectopic Tbr2+ cells are observed at the apical VZ in mutants (red arrowheads in P). Scale bars: G,I, 200 μm; blow up in G, 50 μm; K, 20 μm.
A lengthening of the cell cycle has been shown to characterize differentiative divisions (Miyama et al. 1997; Calegari et al. 2005). To address whether an increased fraction of cells exited the cell cycle (Q fraction) in E14.5 E1-Ngn2/Cre;Dbx1DTA animals, we performed a single injection of IdU followed by multiple injections of BrdU (every 2 h) for a total of 15 h (Fig. 4D) and quantified the number of cells which failed to reentered the S-phase during the time of the experiment (IdU+BrdU−) among actively cycling cells (IdU+BrdU+ or IdU−BrdU+) (see Materials and Methods). We observed that an increased proportion of cells exited the cell cycle in ablated cortices compared with controls at both cL and rD levels (Fig. 4E). Evaluation of the total number of postmitotic neurons generated during one cell cycle by counting the number of Tbr1+ cells relative to the size of the progenitor pool (IdU+BrdU+ or IdU−BrdU+) also confirmed an enhanced fraction of neurogenic divisions in mutant compared with control littermates (Fig. 4F). Furthermore, we detected an increase in the expression of Ngn2, a proneural gene detected at high levels in neurogenic divisions (Shimojo et al. 2008; Ochiai et al. 2009), in the VZ and SVZ of mutant compared with control littermates (Fig. 4G,H). Together, these experiments suggested that there was an augmentation in the number of progenitors undergoing neurogenic instead of proliferative divisions in both the VZ and SVZ of ablated embryos.
To further analyze whether the cortical neuroepithelium was undergoing precocious neurogenesis, we performed coimmunostaining for Tbr2, a gene expressed in both IP cells and early postmitotic neurons in the developing pallium (Englund et al. 2005) and TuJ1. We observed an increase in the number of TuJ1+ cells in the VZ of E14.5 E1-Ngn2/Cre(iresGFP);Dbx1DTA mutant embryos and especially in that of TuJ1+Tbr2+ cells in the VZ (green arrowheads in Fig. 4K–R). We also noticed that ectopic Tbr2+ cells were detected in the apical VZ almost at the ventricle in mutant animals (red arrowheads in Fig. 4P). Notably, a general increase in expression of the GFP, corresponding to the activity of the E1 enhancer element of the Ngn2 gene, was also detected in mutants particularly at the apical VZ (Fig. 4I–N) and correlated with the enhanced Ngn2 staining previously described. All GFP+ cells were Tbr2+ in the VZ, but not reciprocally, in both control and mutant E1-Ngn2/Cre(iresGFP);Dbx1DTA cortices (Fig. 4K–P), suggesting that the GFP labels a specific subpopulation of the Tbr2+ cells. Moreover, the TuJ1+Tbr2+ cells in the apical VZ observed in control as well as the ectopic ones observed in mutant animals were almost always associated with GFP expression (green arrowheads in Fig. 4K–R), suggesting that the Tbr2+GFP+ cells in the apical VZ are postmitotic neurons. Accordingly, we observed a higher proportion of Tbr2+GFP+ cells among Tbr2+ cells at E12.5 in cL territories (cL: 20.62 ± 2.65% in controls compared with 51.42 ± 2.96% in mutants, the GFP is not expressed at rD level at this stage). A similar increase was detected in both cL and rD regions at E14.5 (cL: 31.85 ± 1.79% in controls compared with 52.76 ± 0.18% in mutants; rD: 29.39 ± 11.79% in controls compared with 63.19 ± 3.03% in mutants) and maintained at E16.5 (cL: 15.06 ± 1.40% in controls compared with 45.82 ± 9.68% in mutants; rD: 15.75 ± 0.78% in controls compared with 24.67 ± 0.46% in mutants). We conclude that an enhanced fraction of Tbr2+ cells corresponds to postmitotic neurons upon ablation of the CP transient neurons.
Thus, together these experiments demonstrate that progenitors throughout the neocortical neuroepithelium undergo precocious neurogenic divisions in E1-Ngn2/CRE;Dbx1DTA animals by midneurogenesis.
Depletion of Neocortical Progenitor Pools in Ablated Mutants
An increase in the neurogenic fraction of progenitor divisions and a decrease in that of proliferative divisions should lead to a progressive depletion of the progenitor pool. Indeed, the observed decrease in the number of mitosis in E14.5 and E16.5 in E1-Ngn2/Cre;Dbx1DTA embryos together with a reduction in Ki67 staining, a marker of proliferative cells, at caudal levels of E14.5 mutant pallium compared with control littermates (Fig. 5A–B′) strongly suggested a global depletion of the progenitor pools.
Since the decrease in the proliferation was most pronounced in the SVZ at E14.5, we first tested whether the pool of IPs was affected. We quantified the number of Tbr2+ cells within one column spanning the thickness of the developing pallium and observed a reduction starting in the cL region of mutant telencephalons at E12.5 (Fig. 5C–E). Strong defects were observed in rD territories at E14.5 and E16.5 but not at E12.5. Therefore, together with the enhanced number of postmitotic Tbr1+ and Tbr2+ neurons at E12.5 and E14.5 in cL and rD regions, respectively (Figs 2E,F,I–L and 4K–P) and the reduction in SVZ proliferation (Fig. 3B,D), these results show that the decrease in the total number of Tbr2+ cells corresponds to a depletion in the pool of IPs rather than of early postmitotic neurons (also Tbr2+).
In order to determine if the pool of RGs was also affected, we analyzed the expression of BLBP and Pax6 proteins, 2 specific markers of RG cells, at E16.5. We observed a decrease in their expression in mutant cL cortices (Fig. 5F–I) correlating with a ∼25% reduction in cell density, as measured by DAPI staining, in the VZ (Fig. 5L). In addition, we observed at E16.5 a decrease in BrdU labeling (upon a 2 h pulse) in the VZ and SVZ of both rD and cL regions (Fig. 5J,K). We conclude that a depletion of both the IP and the RG progenitors' pools occurs in the pallium of the E1-Ngn2/Cre;Dbx1DTA animals.
Increased Number of Dbx1-Derived CP Transient Neurons in the Pallium of Gsx2 Mutants
Since the E1-Ngn2/CRE;Dbx1DTA mutants represent a specific ablation of Dbx1-expressing progenitors at the VP, we sought to analyze Gsx2 null mice which have been reported to display opposite effects, namely an expansion of the Dbx1 expression domain in the VP (Yun et al. 2001; Carney et al. 2009). Notably, these mutants have recently been shown to present an increase in the number of Dbx1-derived glutamatergic neurons migrating into the lateral amygdala (Waclaw et al. 2010). The role of Gsx2 in dorsoventral patterning and neurogenesis of the subpallium has been analyzed in several studies (Corbin et al. 2000; Yun et al. 2001; Stenman, Toresson, et al. 2003; Yun et al. 2003; Carney et al. 2009). However, no major defects in pallial development had been reported so far.
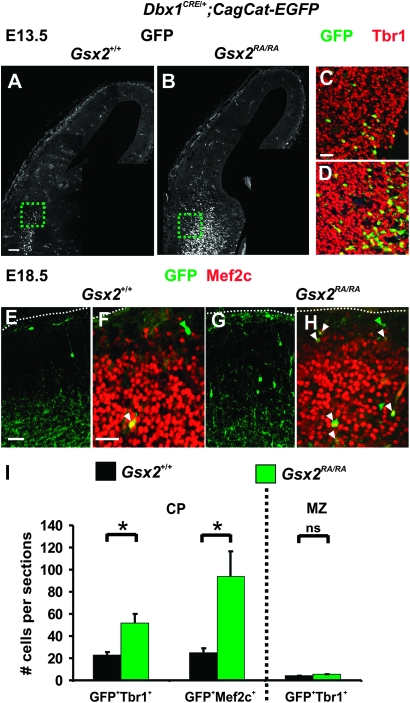
In order to permanently trace Dbx1-derived CP transient neurons in the pallium of the Gsx2 mutants, we crossed Dbx1CRE/+;Gsx2RA/+ with CagCat-EGFP;Gsx2RA/+ animals (Waclaw et al. 2010). By immunostaining for Tbr1 on Dbx1CRE/+;CagCat-EGFP;Gsx2+/+ and Dbx1CRE/+;CagCat-EGFP;Gsx2RA/RA cortices, we observed an increased stream of Dbx1-derived glutamatergic neurons (GFP+Tbr1+) migrating dorsally into the pallium of E13.5 homozygous mutants (Fig. 6B,D) compared with control littermates (Fig. 6A,C). In addition, quantification of the number of GFP+ cells coexpressing Tbr1 and/or Mef2c, a gene which labels VP-derived neurons migrating into the amygdala (Waclaw et al. 2010), revealed a 124 ± 3.18% and 274.66 ± 5.72% increase in the CP of E18.5 Gsx2 mutants (Fig. 6E–I). Most supernumerary Dbx1-derived neurons were positioned in deep layers as expected from their early birthdate. No significant differences in the number or location of Dbx1-derived Reelin+ CR cells was observed in E13.5 and E18.5 Dbx1CRE/+;CagCat-EGFP;Gsx2+/+ and Dbx1CRE/+;CagCat-EGFP;Gsx2RA/RA cortices (data not shown and Fig. 6I). We conclude that Gsx2 mutant animals display a specific increase in the number of CP transient neurons migrating into the pallium from early stages of development.
Figure 6.
Increased number of Dbx1-derived CP transient neurons migrating in the developing pallium of Gsx2 mutants. (A–D) Immunohistochemistry for GFP and Tbr1 shows an increase in the number of GFP+ cells migrating tangentially into the pallium of E13.5 Dbx1CRE/+;CagCat-EGFP;Gsx2RA/RA mutant animals (B,D) compared with Dbx1CRE/+;CagCat-EGFP;Gsx2+/+ control littermates (A,C). A higher number of Dbx1-derived GFP+Tbr1+ neurons is detected in the pallium of mutants compared with controls (C,D magnification of dashed boxes in A,B, respectively). (E–H) Immunohistochemistry for GFP and Mef2c on E18.5 Dbx1CRE/+;CagCat-EGFP;Gsx2RA/RA mutant animals (G,H) compared with Dbx1CRE/+;CagCat-EGFP;Gsx2+/+ control littermates (E,F) confirms an increment in the number of GFP+Mef2c+ neurons in the CP at dorsolateral levels. (I) Graph represents the total number of GFP+Tbr1+ and GFP+Mef2c+ neurons in the CP of coronal sections of mutant and control cortices at E18.5, corresponding to the number of Dbx1-derived CP transient neurons, as well as the total number of GFP+Tbr1+ neurons in the MZ, corresponding to Dbx1-derived CR cells. These results confirm a strong increment in the number of Dbx1-derived glutamatergic neurons in mutants (GFP+Tbr1+: 23 ± 2.51 neurons per sections in controls vs. 51.66 ± 8.01 in mutants, P = 0.026; GFP+Mef2c+: 25 ± 4.04 neurons per sections in controls vs. 93.66 ± 23.14 in mutants, P = 0.043) as compared with a slight increase in the number of Dbx1-derived CR cells (GFP+Tbr1+: 3.83 ± 0.16 neurons per sections in controls vs. 5.18 ± 0.49 in mutants, P = 0.060). Graphs represent means ± SEM. *P < 0.05. Scale bars: A, 100 μm; E,F, 50 μm.
Enhanced Proliferation and Neurogenesis in the Pallium of Gsx2 Mutants
We started by analyzing the progression of differentiation using Tbr1 and TuJ1 immunostaining in E12.5 Gsx2 mutants and observed a global decline affecting both cL and rD regions, but this was more pronounced at cL levels (Supplementary Fig. S2). We also detected a concomitant enhanced proliferation in both the VZ and the SVZ, as revealed by Ki67 and BrdU staining (Supplementary Fig. S2). At E14.5, a strong increment in Ki67 and PH3 immunostaining was detected in both the VZ and the SVZ of Gsx2 mutants at cL and rD levels (Fig. 7A–F and data not shown). Quantification of PH3+ cells at the apical VZ and the SVZ confirmed a higher number of ventricular and abventricular mitosis in these mutants (Fig. 7M,N). In addition, we observed more numerous Tbr2+ cells in the SVZ (Fig. 7G,H) but not at the apical VZ (Fig. 7G,H,O), showing that both RG and IP progenitors display enhanced proliferation. Finally, we observed a reduction in Ngn2 staining in both the VZ and the SVZ of mutant animals (Fig. 7I,J) as well as a reproducible slight decrease in Tis21 expression, especially observed in the apical portion of the VZ (Fig. 7K,L), strongly arguing for a decrease in the fraction of neurogenic divisions. Notably, the number of Tbr1+ neurons in the CP of E14.5 Gsx2 mutants was similar to that of control embryos (data not shown). Therefore, together these results suggest that an amplification of both RGs and IPs progenitor pools and a delayed neurogenesis occur in the pallium of Gsx2 mutants. These defects are opposite with respect to the precocious differentiation and depletion of the progenitor pools described in the E1-Ngn2/CRE;Dbx1DTA mutants at corresponding stages.
We then analyzed the phenotype at E18.5, the latest stage possible for Gsx2 mutants which die at birth and observed an increase in the thickness of the CP at both rD (25.49%) and cL (30.96%) levels. Tbr1 and Cux1/2 immunostaining showed that both deep and superficial layers were thicker (Fig. 8B–G) and appeared to be affected in a similar manner (Fig. 8A), although the supernumerary CP transient neurons were positioned in deep layers. Finally, we observed a strong increase of neuronal numbers in layers V and II–IV upon immunostaining for FoxP2, Ctip2 and Mef2C, as well as by in situ hybridization for Cdh8 (Fig. 8H–O). In conclusion, we have shown that Gsx2 mutants present a surplus number of CP transient neurons and display opposite non-cell autonomous pallial defects to those observed upon their ablation in E1-Ngn2/CRE;Dbx1DTA animals, namely excessive proliferation of both VZ and SVZ progenitors and a consequent increased number of cortical neurons fated to both deep and superficial layers.
Discussion
We have shown that the specific ablation of CP transient neurons derived from Dbx1-expressing progenitors results in a decrement in the final number of cortical neurons as a consequence of the precocious differentiation of cortical progenitors. The kinetics of the defects observed in ablated mutants follow the expected trajectory of these cells, which migrate tangentially from the VP at the border of the developing pallium (Teissier et al. 2010). On the contrary, a gain of CP transient neurons, as observed in Gsx2 mutants, correlates with a delayed onset of neurogenesis in the pallium resulting in an increase in the size of the progenitor pools and in the total number of cortical neurons. Therefore, all together these results show that these transient glutamatergic neurons from the postmitotic compartment appear to control the mode of divisions of cortical progenitors in a non-cell autonomous manner over long distances from their generation site. Their dynamics of invasion of the CP together with their role in delaying differentiation might represent a novel mechanism to refine neurogenetic gradients during cortical growth via cell migration.
The Proliferation/Differentiation Transition of Cortical Progenitors Depends on the Number of CP Transient Neurons in Developing Pallial Territories
The specific ablation of Dbx1-derived CP transient neurons results in an early increase in the leaving fraction and a lengthening of the cell cycle together with an enhanced expression of markers of neurogenic divisions. Correlating with the observed precocious neurogenesis, the generation of FoxP2+ layer VI neurons is incremented in E1-Ngn2/CRE;Dbx1DTA animals. In addition, proliferation in the SVZ is reduced suggesting a defect in IPs generation from VZ progenitors. Thus, the early onset of differentiation leads to a progressive depletion of both VZ and SVZ progenitor pools and results in a general reduction of neuronal production although this is more pronounced for neurons fated to superficial layers. On the contrary, in Gsx2 mutants, which display an excessive generation of VP-derived CP transient neurons, the numbers of cycling VZ and SVZ progenitors and of cortical neurons are amplified. Thus, a delayed differentiation resulting in an enlarged progenitor pool occurs in Gsx2 mutants. However, unlike E1-Ngn2/CRE;Dbx1DTA mutants, Gsx2 mutants display a fairly homogeneous increase of both superficial and deep layer neurons. This is likely due to the fact that E1-Ngn2/CRE;Dbx1DTA mutants present only a partial (around 50%) ablation of the CP transient neurons (Teissier et al. 2010) because of a late onset of recombination starting after E11.5 whereas VP expansion is already well established at this stage in Gsx2 mutants (Yun et al. 2001). Nevertheless, our results show that E1-Ngn2/CRE;Dbx1DTA and Gsx2 mutants represent mouse models for studying loss and gain of function of Dbx1-derived CP transient neurons, respectively. In addition, this is the first time that a pallial phenotype is described for Gsx2 mutants. Indeed, previous studies have mostly focused on the role of Gsx2 on dorsoventral patterning and have described a considerably smaller striatum resulting from patterning defects that lead to a specific decrease in proliferation in the LGE, notably affecting the SVZ at E12.5 (Corbin et al. 2000; Toresson et al. 2000; Toresson and Campbell 2001). This cell autonomous phenotype appears opposite to the one we describe in the pallium and supports an indirect function for Gsx2 in the development of the cortical primordium through the inhibition of expression of the Dbx1 gene (Yun et al. 2001; Winterbottom et al. 2010).
All together, our results strongly suggest that CP transient neurons have an active role in enhancing progenitor proliferative divisions and, thus, decreasing the probability of cell cycle exit. In addition, both E1-Ngn2/CRE;Dbx1DTA and Gsx2 mutants are strongly affected in SVZ proliferation, and present defects in both deep and superficial layers, supporting the hypothesis that IPs generate neurons fated to all layers and have a role in the amplification of RGs progeny rather than on neuronal fate (Pontious et al. 2008; Kowalczyk et al. 2009; Puzzolo and Mallamaci 2010). Accordingly, both CyclinD2 and Tbr2 mutants specifically affecting SVZ proliferation display a reduced number of neurons in both deep and superficial layers (Arnold et al. 2008; Sessa et al. 2008; Glickstein et al. 2009) although this is more pronounced in superficial layers. However, this appears to be in the absence of precocious differentiation of VZ progenitors. On the contrary, mutants that do result in premature differentiation, such as Pax6 and Tlx mutants, also display a depletion of the progenitor pool correlated with an increased leaving fraction and a consequent reduction in the thickness of superficial layers as described for E1-Ngn2/CRE;Dbx1DTA animals (Land and Monaghan 2003; Roy et al. 2004; Tuoc et al. 2009; Georgala et al. 2011). Interestingly, mutants for both Pax6 (Sey/Sey) and Tlx present a loss of Dbx1 expression at the VP/PSB (Yun et al. 2001; Stenman, Yu, et al. 2003; Roy et al. 2004), and cell death has been reported to specifically occur at the PSB of Tlx mutants (Roy et al. 2004). Although both the Pax6 and the Tlx genes have been shown to control cell autonomously the mode of divisions of cortical progenitors (Quinn et al. 2007; Osumi et al. 2008; Zhang et al. 2008) it cannot be excluded that part of the phenotype in their respective mutants is dependent on alterations in the generation or specification of the CP transient neurons due to VP defects.
CP Transient Neurons Migration and “Neurogenetic Gradients”
Gradients of proliferation (rostrolateralhigh to caudomediallow) and differentiation (lateralhigh to mediallow) have been suggested to regulate timing of early neurogenesis and, thus, neuronal numbers in distinct cortical territories (Bayer et al. 1991; Polleux, Dehay, and Kennedy 1997; Carney et al. 2007). Neurogenesis has been shown to be initiated rostrolaterally at E10.5 and to expand caudomedially but still maintaining a strong lateralhigh to dorsallow gradient between E12.5 and E14.5 (Miyama et al. 1997; Calegari et al. 2005). Accordingly, at midneurogenesis, the lateral regions of the pallium already start generating neurons fated to superficial layers while the rostrodorsal ones still generate exclusively neurons fated to deep layers (Polleux, Dehay, and Kennedy 1997; Polleux, Dehay, Moraillon, et al. 1997; Takahashi et al. 1999). We have shown that the CP transient neurons are generated with a peak at E12.5 and migrate tangentially to reach the rostrodorsal pallium by E14.5 along caudal to rostral and lateral to dorsal migratory pathways (Teissier et al. 2010). Therefore, CP transient neurons' migration parallels the neurogenetic gradient occurring between E12.5 and E14.5. Strikingly, while the defects in proliferation follow the expected trajectory of ablated cells in E1-Ngn2/CRE;Dbx1DTA animals (i.e., starting at the caudal PSB at E12.0–E12.5 and reaching the dorsal cortex by E14.5), the decrease in neuronal generation is homogeneous along the RC axis despite delayed onset of ablation effects in rostrodorsal regions, suggesting that the neurogenetic gradients are unperturbed in ablated animals. Since the pattern of the E1/Ngn2 enhancer activity also correlates with gradients of differentiation (Berger et al. 2004) and the expression of Ngn2 increases in mutant animals, our data strongly suggest that ablation results in a switch to neurogenic divisions at the expense of proliferative divisions (Iacopetti et al. 1999; Kawaguchi et al. 2004). Dbx1-derived CP transient neurons might act by delaying differentiation possibly by maintaining low Ngn2 expression in progenitors. This occurs in the absence of defects in laminar fate and arealization, strongly suggesting that CP transient neurons do not affect the intrinsic sequence which leads progenitors to produce the whole set of layer-specific neurons once they have initiated neurogenesis (Shen et al. 2006; Gaspard et al. 2008). Most likely, they appear to time the exit from the cell cycle and, thus, to increase the total number of self-renewing cell cycle per progenitor.
CP Transient Neurons Migration Provides an Extrinsic Mechanism to Control the Mode of Divisions of Cortical Progenitors
We have shown that both RGs and IPs progenitors are affected upon modifications in the number of CP transient neurons. Our results indicate that the CP transient neurons might influence progenitors proliferation/differentiation transition while invading the pallium. Since these neurons migrate through both the IZ/SVZ and the developing PP at early stages of development (E12.5–E14.5) (Teissier et al. 2010), they could interact at this location by cell-cell–mediated contacts with the basal processes of RG cells or with IPs as well as by the secretion of signaling factors. Notably, the neurogenic gradient observed in the developing cortex has already been suggested to be initiated by signaling molecules secreted by the patterning centers and to progress by cell–cell contact through hemichannels propagating calcium waves. This process has been shown to be both necessary and sufficient to promote RGs proliferation (Bittman et al. 1997; Caviness et al. 2009; Liu et al. 2010). In addition, newly generated neurons and IPs are involved by presenting Delta ligands in maintenance of RGs proliferation through the activation of Notch signaling (Yoon et al. 2008). Lastly, an increasing number of evidence suggests that the secretion of diffusible molecules provides a feedback control from the postmitotic compartment to regulate the proliferation properties of cortical progenitors (Polleux et al. 1998; Dehay et al. 2001; Viti et al. 2003; Agasse et al. 2006; Dehay and Kennedy 2007; Seuntjens et al. 2009; Griveau et al. 2010). However, it is unlikely that Dbx1-derived CP transient neurons would use the same molecules expressed by all differentiating glutamatergic neurons to communicate with cortical progenitors, for example, Nt3/Fgf9 as in the case of Sip1 mutants (Seuntjens et al. 2009) or Notch ligands (Yoon et al. 2008). Indeed, they represent a small percentage of the neurons in the postmitotic compartment at any given stage and especially in rD at E14.5 when the effect upon ablation is already observed (Teissier et al. 2010) and their ablation would further be compensated by the precocious differentiation, initially generating more neurons.
Patterning, growth, and cell fate, by “mobile signaling cells/structures,” such as TCA axons and migrating cells (CR neurons, CP transient neurons, neural crests, meninges, and blood vessels) (Etchevers et al. 1999; Dehay et al. 2001; Schneider et al. 2001; Creuzet et al. 2004; Vasudevan et al. 2008; Javaherian and Kriegstein 2009; Siegenthaler et al. 2009; Stubbs et al. 2009; Griveau et al. 2010) depend critically on the timing of their generation, migration, and arrival in different cortical regions. Their early arrival (E10.5–E12.5) will affect regionalization and later arrival (E12.5–E13.5) the onset of neurogenesis in a tight interplay with intrinsic developmental programs of cortical progenitors. Therefore, it appears that multiple temporal, spatial, and qualitative levels of control underlie the role of these mobile “signaling” cells/structures in cortical development. The identification of this novel population and its crucial non-cell autonomous role in progenitor pool maintenance will now allow further studies aiming at the molecular characterization of how migrating CP transient neurons contributes to the extrinsic control of corticogenesis.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Agence Nationale de la Recherche (ANR-07-NEURO-046-01); Fondation pour le Recherche Médicale (INE20060306503); Association pour la Recherche sur le Cancer (grant # 4940); Ville de Paris (2006 ASES 102 to A.P.); National Institutes of Health (grant NS044080 to K.C.).
Supplementary Material
Acknowledgments
We thank M. Barber and F. Causeret for critical reading of the manuscript, S. Karaz and L. Vigier for their technical support, Y. Arai for advices on the analysis of the cell cycle length, and U. Borello for the Tis21 in situ probe. We are also grateful to the animal house and especially A. Djemat and the ImagoSeine facility at the IJM. A.T. was the recipient of a fellowship from the French Ministry of Education. A.G. was the recipient of fellowships from the French Ministry of Education and the Association pour la Recherche sur le Cancer. A.P. is a CNRS (Centre National de la Recherche Scientifique) Investigator. Author contributions: A.T., A.G., and A.P conceived the study and wrote the manuscript. A.T. carried out most of the experiments. A.G. initiated and performed part of the analysis of the E1-Ngn2/CRE;Dbx1DTA mutant animals. R.W. and K.C. generated and participated in the analysis of the Gsx2 mutants. A.P. supervised the project. Conflict of Interest : None declared.
References
- Agasse F, Benzakour O, Berjeaud JM, Roger M, Coronas V. Endogenous factors derived from embryonic cortex regulate proliferation and neuronal differentiation of postnatal subventricular zone cell cultures. Eur J Neurosci. 2006;23:1970–1976. doi: 10.1111/j.1460-9568.2006.04739.x. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Dai XF, Simmons JA. Cell migration in the rat embryonic neocortex. J Comp Neurol. 1991;307:499–516. doi: 10.1002/cne.903070312. [DOI] [PubMed] [Google Scholar]
- Berger J, Eckert S, Scardigli R, Guillemot F, Gruss P, Stoykova A. E1-Ngn2/Cre is a new line for regional activation of Cre recombinase in the developing CNS. Genesis. 2004;40:195–199. doi: 10.1002/gene.20081. [DOI] [PubMed] [Google Scholar]
- Berry M, Rogers AW, Eayrs JT. Pattern of cell migration during cortical histogenesis. Nature. 1964;203:591–593. doi: 10.1038/203591b0. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bittman K, Owens DF, Kriegstein AR, LoTurco JJ. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RS, Bystron I, Lopez-Bendito G, Molnar Z. Comparative analysis of extra-ventricular mitoses at early stages of cortical development in rat and human. Brain Struct Funct. 2007;212:37–54. doi: 10.1007/s00429-007-0142-4. [DOI] [PubMed] [Google Scholar]
- Carney RS, Cocas LA, Hirata T, Mansfield K, Corbin JG. Differential regulation of telencephalic pallial-subpallial boundary patterning by Pax6 and Gsh2. Cereb Cortex. 2009;19:745–759. doi: 10.1093/cercor/bhn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Nowakowski RS, Bhide PG. Neocortical neurogenesis: morphogenetic gradients and beyond. Trends Neurosci. 2009;32:443–450. doi: 10.1016/j.tins.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci U S A. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- Dehay C, Savatier P, Cortay V, Kennedy H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129:455–466. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, Le Douarin NM. Anterior cephalic neural crest is required for forebrain viability. Development. 1999;126:3533–3543. doi: 10.1242/dev.126.16.3533. [DOI] [PubMed] [Google Scholar]
- Faedo A, Tomassy GS, Ruan Y, Teichmann H, Krauss S, Pleasure SJ, Tsai SY, Tsai MJ, Studer M, Rubenstein JL. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18:2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26:13218–13230. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Georgala PA, Manuel M, Price DJ. The generation of superficial cortical layers is regulated by levels of the transcription factor Pax6. Cereb Cortex. 2011;21:81–94. doi: 10.1093/cercor/bhq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, Monaghan JA, Koeller HB, Jones TK, Ross ME. Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortex. J Neurosci. 2009;29:9614–9624. doi: 10.1523/JNEUROSCI.2284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Barde YA. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46:369–372. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Griveau A, Borello U, Causeret F, Tissir F, Boggeto N, Karaz S, Pierani A. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortex neuroepithelium. PLoS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Molnar Z, Tarabykin V, Stoykova A. Molecular mechanisms of cortical differentiation. Eur J Neurosci. 2006;23:857–868. doi: 10.1111/j.1460-9568.2006.04626.x. [DOI] [PubMed] [Google Scholar]
- Holm PC, Mader MT, Haubst N, Wizenmann A, Sigvardsson M, Gotz M. Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Mol Cell Neurosci. 2007;34:99–119. doi: 10.1016/j.mcn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Iacopetti P, Michelini M, Stuckmann I, Oback B, Aaku-Saraste E, Huttner WB. Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division. Proc Natl Acad Sci U S A. 1999;96:4639–4644. doi: 10.1073/pnas.96.8.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A, Kriegstein A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex. 2009;19(Suppl 1):i70–i77. doi: 10.1093/cercor/bhp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A, Ogawa M, Saito K, Matsuzaki F, Okano H, Miyata T. Differential expression of Pax6 and Ngn2 between pair-generated cortical neurons. J Neurosci Res. 2004;78:784–795. doi: 10.1002/jnr.20347. [DOI] [PubMed] [Google Scholar]
- Komada M, Saitsu H, Kinboshi M, Miura T, Shiota K, Ishibashi M. Hedgehog signaling is involved in development of the neocortex. Development. 2008;135:2717–2727. doi: 10.1242/dev.015891. [DOI] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land PW, Monaghan AP. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex. 2003;13:921–931. doi: 10.1093/cercor/13.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HK, Wang Y, Belz T, Bock D, Takacs A, Radlwimmer B, Barbus S, Reifenberger G, Lichter P, Schutz G. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Dividing neuron precursors express neuron-specific tubulin. J Neurobiol. 1995;27:26–43. doi: 10.1002/neu.480270104. [DOI] [PubMed] [Google Scholar]
- Miyama S, Takahashi T, Nowakowski RS, Caviness VS., Jr. A gradient in the duration of the G1 phase in the murine neocortical proliferative epithelium. Cereb Cortex. 1997;7:678–689. doi: 10.1093/cercor/7.7.678. [DOI] [PubMed] [Google Scholar]
- Muzio L, Soria JM, Pannese M, Piccolo S, Mallamaci A. A mutually stimulating loop involving emx2 and canonical wnt signalling specifically promotes expansion of occipital cortex and hippocampus. Cereb Cortex. 2005;15:2021–2028. doi: 10.1093/cercor/bhi077. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Ochiai W, Nakatani S, Takahara T, Kainuma M, Masaoka M, Minobe S, Namihira M, Nakashima K, Sakakibara A, Ogawa M, et al. Periventricular notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol Cell Neurosci. 2009;40:225–233. doi: 10.1016/j.mcn.2008.10.007. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26:1663–1672. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- Pilaz LJ, Patti D, Marcy G, Ollier E, Pfister S, Douglas RJ, Betizeau M, Gautier E, Cortay V, Doerflinger N, et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci U S A. 2009;106:21924–21929. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Kennedy H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J Comp Neurol. 1997;385:95–116. [PubMed] [Google Scholar]
- Polleux F, Dehay C, Kennedy H. Neurogenesis and commitment of corticospinal neurons in reeler. J Neurosci. 1998;18:9910–9923. doi: 10.1523/JNEUROSCI.18-23-09910.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Moraillon B, Kennedy H. Regulation of neuroblast cell-cycle kinetics plays a crucial role in the generation of unique features of neocortical areas. J Neurosci. 1997;17:7763–7783. doi: 10.1523/JNEUROSCI.17-20-07763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Puzzolo E, Mallamaci A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural Dev. 2010;5:8. doi: 10.1186/1749-8104-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JC, Molinek M, Martynoga BS, Zaki PA, Faedo A, Bulfone A, Hevner RF, West JD, Price DJ. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2007;302:50–65. doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 2010;20:233–243. doi: 10.1016/j.tcb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuntjens E, Nityanandam A, Miquelajauregui A, Debruyn J, Stryjewska A, Goebbels S, Nave KA, Huylebroeck D, Tarabykin V. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci. 2009;12:1373–1380. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Yu RT, Evans RM, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130:1113–1122. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs D, DeProto J, Nie K, Englund C, Mahmud I, Hevner R, Molnar Z. Neurovascular congruence during cerebral cortical development. Cereb Cortex. 2009;19(Suppl 1):i32–i41. doi: 10.1093/cercor/bhp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr. Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui T, Takahashi T, Nowakowski RS, Hayes NL, Bhide PG, Caviness VS. Overexpression of p27 Kip 1, probability of cell cycle exit, and laminar destination of neocortical neurons. Cereb Cortex. 2005;15:1343–1355. doi: 10.1093/cercor/bhi017. [DOI] [PubMed] [Google Scholar]
- Teissier A, Griveau A, Vigier L, Piolot T, Borello U, Pierani A. A novel transient glutamatergic population migrating from the pallial-subpallial boundary contributes to neocortical development. J Neurosci. 2010;30:10563–10574. doi: 10.1523/JNEUROSCI.0776-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128:4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Tuoc TC, Radyushkin K, Tonchev AB, Pinon MC, Ashery-Padan R, Molnar Z, Davidoff MS, Stoykova A. Selective cortical layering abnormalities and behavioral deficits in cortex-specific Pax6 knock-out mice. J Neurosci. 2009;29:8335–8349. doi: 10.1523/JNEUROSCI.5669-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci. 2008;11:429–439. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viti J, Gulacsi A, Lillien L. Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2. J Neurosci. 2003;23:5919–5927. doi: 10.1523/JNEUROSCI.23-13-05919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Ehrman LA, Pierani A, Campbell K. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci. 2010;30:6944–6953. doi: 10.1523/JNEUROSCI.5772-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbottom EF, Illes JC, Faas L, Isaacs HV. Conserved and novel roles for the Gsh2 transcription factor in primary neurogenesis. Development. 2010;137:2623–2631. doi: 10.1242/dev.047159. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Koo BK, Im SK, Jeong HW, Ghim J, Kwon MC, Moon JS, Miyata T, Kong YY. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461:151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.