Abstract
Lung cancer is the most common malignant tumor in the world. Calcium is a ubiquitous cellular signal, which is crucial in cancer. This review presents regulation of calcium signaling in lung cancer. Altered expression of specific Ca2+ channels and Ca2+-binding proteins are characterizing features of lung cancer, which regulate cell signaling pathway leading to cell proliferation or apoptosis. Chemoresistance is frequent in lung cancer. Altered endoplasmic reticulum Ca2+ homeostasis of lung cancer cell is correlated with drug resistance. Hypoxia has a vital role in tumor angiogenesis, metastasis, apoptosis. And Ca2+ channels are open induced by hypoxia with the increase of Ca2+ influx causing tumor growth.
Keywords: calcium, lung cancer, endoplasmic reticulum, calcium channels, calcium-binding protein
Calcium, as the second messenger, is essential signal transduction element involved in cell growth including cell cycle, differenation, proliferation and apoptosis. Calcium signaling is activated in the cell with pathological condition, which leading to intracellular environment changing and cell abnormal reaction. In general, prolonged increases in Ca2+ or long-lasting Ca2+ -oscillations (hours) are believed to trigger proliferation, while short lasting, high amplitude elevations of Ca2+ can increase mitochondrial Ca2+ level and promote cell death (1)-(3). Therefore, careful control of calcium signaling is required for cell survival. The intracellular calcium concentration plays an important role in cell activities, regulated by release from endoplasmic reticulum stores or influx through a variety of Ca2+ ion channels (4). Voltage-gated (VGCC), receptor-gated (ROCC) and store-operated (SOCC) channels in the membrane, along with ryanodine receptors (RynR) and inositol triphosphate receptors (IP3R) at the ER store, provides fluxes of Ca2+ to the cytoplasm. Furthermore calcium pumps and ion exchangers are involved in the Ca2+ releasing too (5),(6). ATPases pumps transport Ca2+ against a concentration gradient, including the plasmalemmal Ca2+-ATPase (PMCA) in the plasma membrane which is responsible for the efflux to Ca2+ out of cells, and the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) which pump Ca2+ from cytoplasm into ER. Ca2+ exchangers such as Na+/Ca2+ exchanger are crucial in the transport of Ca2+ in neurons and cardiac cells (7),(8).
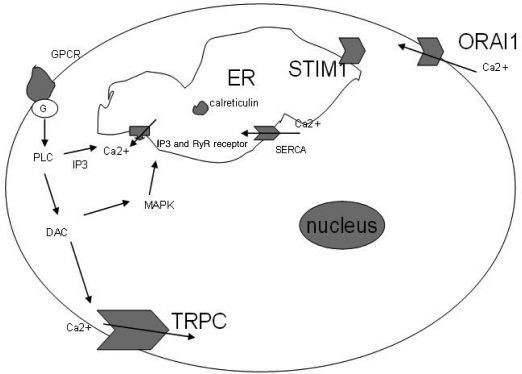
ER Ca2+-homeostasis is one of the most important apoptosis pathways. And Ca2+ is the crucial effector, so careful control of calcium in ER is important for the cell apoptosis. Figure 1 shows the releasing of Ca2+ from ER. Signaling pathway involved in the Ca2+ release from the ER are the PLC-IP3 and MAPK, activated by calcium-sensing G-protein-coupled receptor (GPCR). The key receptors regulating Ca2+ release from the ER are IP3R and RyR, and SERCA force calcium against the concentration gradient from the cytoplasm into ER. Furthermore Ca2+ modulation is performed by calreticulin in the ER (9),(10). Reducing of the Ca2+ in ER can result from Ca2+-influx from the extracellular space. SOCC in the membrane is activated by the emptying of the intracellular Ca2+-stores causing Ca2+ influx. This process is name by store-operated calcium entry (SOCE). SOCE plays a vital role in the cell function including emiocytosis, enzyme activity, cell cycle and apoptosis (11). The most popular channel in SOCE is calcium-release activated calcium (CRAC) channel. Stim1 as the ER Ca2+ sensor, the highly Ca2+-selective CRAC channel Orai1 and transient receptor potential (TRPC) as the effector of membrane, expressed in cells (12),(13). Moreover Stim1-Orai1 and Stim1-TRPC are important protein complexes in CRAC, and there maybe functional interactions among Orai1, TRPCs, and Stim1 in regualting cell proliferation and apoptosis (14)-(16).
Fig. 1. Calcium releasing from ER or influx from the extra cell.
Lung cancer is the most common malignant tumor in the world. Non-small cell lung cancer (NSCLC) is the majority of lung cancer, approximately 80% of total malignancies, with a 5-year survival of only 15%. The other 20% of total lung cancer is small cell lung cancer (SCLC). Here we focus on how Ca2+ might contribute to tumorigenesis and tumor growth in lung cancer.
Calcium channels in cancer
Previous data suggest that carcinogenic stimuli cause local increase in the Ca2+ concentration leading to activation of proto-oncogenes and to inactivation of tumor-suppressor genes, which lead to the manifestation of a malignant phenotype. Tumor cell proliferation maybe stimulated by persistant increase of Ca2+, in contrary the transitory fulminic increase of Ca2+ induce the activation of mitochondrial apoptotic pathway (17). As described above, local increases in Ca2+ concentration can be caused by efflux from the ER or influx from the extra cell through Ca2+ channels. Most reports show the Ca2+ channels increase in the malignant tumors, and the correlations between these channels (VGCC, ROCC and SOCC) and tumor have been addressed widely. Moreover, SOCE induced by SOCC is mostly investigated in the malignant tumor now. T-type Ca2+ channels play an important role in controlling cell growth. Similarly the mRNA and protein expression of TRPC famliy are found increasing in the cell lines of breast, prostate and liver cancer, therein TRPC1 and TRPC6 are most popular (18)-(21). Furthermore, tumor cells growth could be inhibited by silencing these Ca2+ channels genes expression. Signal pathway activation, i.e., GPCR-PLC-IP3 or GPCR-PLC-DAG, are improtant for SOCE induced by TRPC, which lead to the increase of calcium concetration activating calcium binding proteins and nuclear transcription factors, causing tumor cell proliferation (22).
Stim1 and Orai1 are essential for tumor cell migration and proliferation in vitro and vivo. In breast cancer, reduction of Orai1 or Stim1 by RNA interference in highly metastatic human breast cancer cells or treatment with a pharmacological inhitor of SOCC decreased tumor metastasis in animal models (23). In liver cancer, it’s reported that TRPC6, Stim1 and Orai1 regulate tumor migration and proliferation together (24). SOCE amplitude could be reduced by Stim1 and Orai1 knockdowns, suggesting possible cooperation between these proteins and TRPC6 in controlling tumor proliferation and apoptosis. However the mechanism is still unknown.
L-type calcium channel (LTCC) is widely studied in VGCC. It was shown that colon cancer cells expressed LTCCmRNA, comprising an alph-1D and a beta-3 subunit. The selective calcium channel agonist could dose-dependently increase intracellular Ca2+ levels and the level of apoptosis in colon cancer cells. On the contrary, the inhibitor of calcium channels could abolish completely the above results (25). However Berchtold had the contrary report in B-lymphoma and breast cancer cells (26). The inhibitor of LTCC reduced the level of calcium-dependent NF-κ B in tumor cells which expressing LTCC subunit Cav1.3 gene, causing the decrease of calcium influx and the increase of apoptosis in tumor cells. The difference of the above results may be due to the different subunits of LTCC expressed by tumor cells, determining Ca2+ to participate in proliferation or apoptosis in tumor cells.
Calcium channels and lung cancer
Lambert Eaton myasthenic syndrome (LEMS) is usually associated with SCLC. VGCC (P/Q subtype) antibodies are often found in these patients, which play a pathogenic role in LEMS. Monstad et al showed that VGCC antibodies were seen in a proportion of SCLC patients, thus similar immunoreaction maybe exist in SCLC. But the VGCC antibodies do not correlate with the prognosis of the SCLC (27). There are a few researches about calcium channels in NSCLC. Report by Carlisle, et al. showed that nicotine could activate LTCC inducing the increase of Ca2+ influx in 273T NSCLC cell line, which inhibited by the inhibitor of nicotinic acetylcholine receptor or PI3K (28). A study performed in NSCLC cell lines found that overexpression of CACNA2D2 gene (a subunit of the Ca2+-channel complex) induced apoptosis in H1299, H358, H460 and A549 cell lines through elevating intracellular free Ca2+ level (29).
Calcium-binding proteins and lung cancer
Ca2+ appears to exert mitosis or apoptosis of cells as a secondary messenger or signal transducer determined by its location, intracellular concentration and so on. Ca2+ store, releasing and uptake in all cells except muscle cell are regulated by ER. After IP3 binding with IP3 receptor of ER, Ca2+ channels are open associated with the increase of intracellular Ca2+ concentration. Then Ca2+-dependent proteins or Ca2+-binding proteins are stimulated exerting cell biological effects. A number of Ca2+-binding proteins have been characterized as having properties, to play a role as putative intracellular Ca2+ receptors.
The reaction to Ca2+ in cells lies on Ca2+-binding proteins and Ca2+/CaM -dependent kinase. CaM has a vital role in transferring signal out of cells into intracellular biological effects as the predominant receptor of Ca2+. CaM is a small, heat and acid-stable protein which exists as a monomer and presents four similar but distinguishable Ca2+-binding domains allowing interacting with different poteins (30). It was found that the CaM level of lung cancers was significantly higher than that of host lungs, benign lung diseases and normal lungs and significantly correlated with the histopathological grading and TNM staging of lung cancers. Moreover, there was a significant positive correlation between the cellular DNA content and tissue CaM level in lung cancers. So it’s believed that CaM plays an important role in the proliferation of lung cancer cells (31). CaMII phosphorylation could activate all kinds of kinases or transcription factors regulating tumor proliferation and apoptosis. Death – associated protein kinase (DAPK) is one of these kinases, which involved in DNA damage-induced apoptosis and showed low level in the early stage of NSCLC. Thus DAPK is crucial in the progression of NSCLC (32). Camp-regulatory element-binding protein (CREB) is a key transcription factor in NSCLC, which can be activated through phosphorylation by a number of kinases including Ca2+/CaM-dependent kinases. CREB is overexpressed and constitutively phosphorylated in NSCLC, and appears to play a direct role indisease pathogenesis and prognosis (33).
Calcineurin (CaN) is serine/threonine protein kinase regulated by Ca2+/CaM, which exert biological effects through dephosphorylation. Maxeine, et al demonstrated that nuclear factor of activated T cells c2 (NFATc2) mediated by CaN expressed low level of mRNA in NSCLC, furthermore more and large tumors were developed and T cell immunity decreased in NFATc2 (-/-) mice (34). Mitochondrial stress can cause resistance to apoptosis in cancer. Both insulin and insulin-like growth factor-1 receptor (IGF1R) are increased in response to mitochondrial stress. CaN is activated as part of this stress signaling. In A549 lung cancer cell line, inhibiting CaN expression using inhibitor or small interference RNA could inhibit significantly the IGF1R pathway which is important in tumor cell proliferation and reduced apoptosis (35).
ER stress can induce cell apoptosis, which is one of the most important pathways of apoptosis in vivo. The imbalance of the ER Ca2+ homeostasis results in ER stress and cell apoptosis. Calreticulin is important Ca2+-binding protein in ER. The cell is more sensitive to apoptosis while the protein is overexpressed. Recent investigation suggests that in the SCLC (H1339) and NSCLC (HCC) cell lines the ER Ca2+-content was reduced and correlated with a decreased level of calreticulin compared to NHBE cell line, which could lead to reduced apoptosis in cells (36).
Calcium and chemoresistance
Chemotherapy often leads to encouraging reponses in lung cancer. But, in the course of the treatment, resistance to chemotherapy ultimately limits the life expectancy of the patient. Intracellular calcium concentration may play a role in the development of chemoresistance. Altered Ca2+ homeostasis of cell is correlated with cisplatin or Taxol resistance in NSCLC cell lines (A549 and EPLC) or SCLC cell line (H1339). The Ca2+ content of the ER is decreased with the low level of SERCA expression in chemoresistant lung cancer cell lines. Thus a reduced Ca2+ content of the ER maybe induce chemoresistance in lung cancer (37),(38).
Multi drug resistance (MDR) is a process where malignant cells become resistant to structurally diverse chemotherapeutic agents exposure to a single type of cytotoxic drug. Certain cell lines have been associated with a decrease of drug accumulation due to enhanced efflux of drugs, which is attributed to the overexpression of the P-gp (39). Calcium channel and calmodulin antagonist could reverse the drug resistance due to MDR. It has been suggested that the antagonist may have pharmacological effects like calmodulin or protein kinase C inhibition causing P-gp primary structure or post translational modification and changing of functional state of P-gp (30).
Calcium, hypoxia and lung cancer
Calcium channels are open induced by hypoxia with the increase of Ca2+ influx. During the progression of malignant tumor, with the tumor size increasing hypoxia can occur in the local region. Thus hypoxia has a vital role in tumor angiogenesis, metastasis, apoptosis and chemoresistance. Hypoxia inducible factor 1 (HIF-1) is important protein involved in regulation of the transcriptional of a variety of genes related to oxygen homeostasis and hypoxia, which is crucial in the occurrence and development of NSCLC (40),(41). The mutation of PI3K or PTEN is one of the most important mechanisms related to HIF-1α activation under normoxic condition. However, HIF-1 proteins could be stimulated through MAPK pathway no matter hypoxia or not, and increasing of Ca2+ influx and calmodulin would act upstream of ERK in the hypoxia signal transduction pathway leading to enchanced HIF-1 transcriptional activity (42),(43). In NSCLC, induced by hypoxia, Zhang et al. reported that nicotine increased HIF-1α and VEGF expression in A549 cell line, which could be inhibited after blocked by Ca2+/calmodulin inhibitor (44).
No matter whether hypoxia or normoxia exists, NF-κ B is the vital transcriptional factor in the progress of tumor development. Under normoxic condition, the expression of LTCC subunit Cav1.3 mRNA is increased in B-cell lymphoma and breast cancer cell lines. Moreover CaM-dependent NF-κ B is activated with the increasing of Ca2+ influx (26).ER stress or overload (accumulation of proteins in the ER membrane) can lead to efflux of Ca2+ from ER through IP3R or RyR activating NF-κ B pathway (45). However now there is no similar research under hypoxic condition. In liver and brain cells, NF-κ B links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α in vivo and vitro (46). But the above result is still unidentified in lung tissues, so we believe it is possible that CaM-dependent NF-κ B is activated to hypoxic condition by the increase channels of Ca2+ and play a vital role in regulating HIF-1α or other downstream genes which may be enhanced by nicotine.
Conclusion
Ca2+ regulates various cellular processes by activating or inhibiting cellular signaling pathways and Ca2+-regulated proteins, and it deserves to do further researches in lung cancer. Since tumorigenesis and tumor growth in lung cancer are complicated and multiple factor resulted, the role of Ca2+ in lung cancer cells is complicated too, which is determined by its location and combined proteins, moreover different subtypes of Ca2+ channel may play a various role in it.
Footnotes
No potential conflict of interest.
References
- 1.Lipskaia L, Hulot, Lopre AM Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflugers Arch. 2009;457:673–85. doi: 10.1007/s00424-007-0428-7. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–3. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 3.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Bil. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 5.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–22. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 7.Lytton J, Li XF, Dong H, Kraev A. K+-dependent Na+/Ca2+ exchangers in the brain. Ann N Y Acad Sci. 2002;976:382–93. doi: 10.1111/j.1749-6632.2002.tb04765.x. [DOI] [PubMed] [Google Scholar]
- 8.Hryshko LV. Tissue-specific modes of Na/Ca exchanger regulation. Ann N Y Acad Sci. 2002;976:166–75. doi: 10.1111/j.1749-6632.2002.tb04738.x. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE. Calcium signalling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 12.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–99. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. Stim1 is Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–9. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Y, Erxleben C, Yildirim E. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104:4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Natl Acad Sci USA. 2008;105:2895–900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffe LF. A calcium-based theory of carcinogenesis. Adv Cancer Res. 2005;94:231–63. doi: 10.1016/S0065-230X(05)94006-2. [DOI] [PubMed] [Google Scholar]
- 18.Guilbert A, Dhennin-Duthille I, Hiani YE, Haren N, H Khorsi, Sevestre H, et al. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer. 2008;8:125. doi: 10.1186/1471-2407-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EI Hiani Y, Lehen’kyi V, Ouadid-Ahidouch HA, Ahidouch A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch Biochem Biophys. 2009;486:58–63. doi: 10.1016/j.abb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Pigozzi D, Ducret T, Tajeddine N, Gala JL, Tombal B, Gailly P. Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium. 2006;39:401–5. doi: 10.1016/j.ceca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.EI Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatoloty. 2008;47:2068–77. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 22.Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen’kyi V, et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–47. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastsis. Cancer Cell. 2009;15:124–34. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 24.EI Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Gapiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068–77. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 25.Zawadzki A, Liu Q, Wang Y, Melander A, Jeppsson B, Thorlacius H. Verapamil inhibits L-type calcium channed mediated apoptosis in human colon cancer cells. Dis Colon Rectum. 2008;51:1696–702. doi: 10.1007/s10350-008-9372-7. [DOI] [PubMed] [Google Scholar]
- 26.Berchtold CM, Chen KS, Miyamoto S, Gould MN. Perillyl alcohol inhibits a calcium-dependent constitutive nuclear factor-kappaB pathway. Cancer Res. 2005;65:8558–66. doi: 10.1158/0008-5472.CAN-04-4072. [DOI] [PubMed] [Google Scholar]
- 27.Monstad SE, Drivsholm L, Storstein A, Aarseth JH, Haugen M, Lang B, et al. Hu and voltage-gated calcium channel (VGCC) antibodies related to the prognosis of small-cell lung cancer. J Clin Oncol. 2004;22:762–4. doi: 10.1200/JCO.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Carlisle DL, Liu X, Hopkins TM, Swick MC, Dhir R, Siegfried JM. Nicotine activates cell-signaling pathways through muscle-type and neufonal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2007;20:629–41. doi: 10.1016/j.pupt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Carboni GL, Gao B, Nishizaki M, Xu K, Minna JD, Roth JA, et al. CACNA2D2-mediated apoptosis in NSCLC cells is associated with alterations of the intracellular calcium signalling and disruption of mitochondria membrane integrity. Oncogene. 2003;22:615–26. doi: 10.1038/sj.onc.1206134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayur YC, Jagadeesh S, Thimmaiah KN. Targeting calmodulin in reversing multi drug resistance in cancer cells. Mini-Reviews in Medicinal Chemistry. 2006;6:1383–9. doi: 10.2174/138955706778993021. [DOI] [PubMed] [Google Scholar]
- 31.Liu GX, Sheng HF, Wu S. A study on the levels of calodulin and DNA in human lung cancer cells. Br J Cancer. 1996;73:899–901. doi: 10.1038/bjc.1996.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo AL, Thiagalingam A, Pan H, Califano J, Cheng KH, Ponte JF, et al. Differential DNA hypermethylation of critical genes mediates the stage-specific tobacco smoke-induced neoplastic progression of lung cancer. Clin Cancer Res. 2005;11:2466–70. doi: 10.1158/1078-0432.CCR-04-1962. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto KM, Franck DA. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res. 2009;15:2583–7. doi: 10.1158/1078-0432.CCR-08-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxeiner JH, Karwot R, Sauer K, Scholtes P, Boross I, Koslowski M, et al. A key regulatory role of the transcription factor NFATc2 in bronchial adenocarcinoma via CD8+ T lymphocytes. Cancer Res. 2009;69:3069–76. doi: 10.1158/0008-5472.CAN-08-1678. [DOI] [PubMed] [Google Scholar]
- 35.Guha M, Srinivasan S, Biswas G, Avadhani NG. Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J Biol Chem. 2007;282:14536–46. doi: 10.1074/jbc.M611693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergner A, Kellner J, Tufman A, Huber RM. Endoplasmic reticulum Ca2+-homeostasis is altered in small and non-small cell lung cancer cell lines. J Exp Clin Cancer Res. 2009;28:25. doi: 10.1186/1756-9966-28-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padar S, van Breemen C, Thomas DW, Uchizono JA, Livesey JC, Rahimian R. Differential regulation of calcium homeostasis in adenocarcinoma cell line A549 and its Taxol-resistant subclone. Br J Pharmacol. 2004;142:305–16. doi: 10.1038/sj.bjp.0705755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrodl K, Oelmez H, Edelmann M, Huber RM, Bergner A. Altered Ca2+-homeostasis of cisplatin – treated and low level resistant non-small-cell and small-cell lung cancer cells. Cell Oncol. 2009;31:301–15. doi: 10.3233/CLO-2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inaba M, Johnson RK. Uptake and retention of adriamycin and daunorubicin by sensitive and anthracycline-resistant sublines of P388 leukemia. Biochem Pharmacol. 1978;27:2123–30. doi: 10.1016/0006-2952(78)90284-8. [DOI] [PubMed] [Google Scholar]
- 40.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumors and survival. Br J Cancer. 2001;85:881–90. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau SK, Boutros PC, Pintilie M, Blackhall FH, Zhu CQ, Strumpf D, et al. Three-gene prognostic classifier for early-stage non small cell lung cancer. J Clin Oncol. 2007;25:5562–9. doi: 10.1200/JCO.2007.12.0352. [DOI] [PubMed] [Google Scholar]
- 42.Mottet D, Michel G, Renard P, Ninane N, Raes M, Michiels C. ERK and calcium in activation of HIF-1. Ann N Y Acad Sci. 2002;973:448–53. doi: 10.1111/j.1749-6632.2002.tb04681.x. [DOI] [PubMed] [Google Scholar]
- 43.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–8. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, Tang X, Zhang ZF, et al. Nicotine induces hypoxia-inducible factor-1 alpha expression in human lung cancer cells via nicotinic acetylchline acetylcholine receptor-mediated signaling pathways. Clin Cancer Res. 2007;13:4686–94. doi: 10.1158/1078-0432.CCR-06-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–7. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 46.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]