Abstract
Heart-lung transplantation is reserved for selected patients who have end-stage cardiac and pulmonary disease. Gastroparesis, which is associated with aspiration, is commonly seen after transplantation. Here, we present a case in which gastric contents aspiration leads to Pseudomonas aeruginosa pulmonary infection, 11 months after the heart lung transplantation. We strongly recommend that early diagnosis and aggressive management of post-transplantation gastroparesis is essential to prevent lung allograft injury and pulmonary infections.
Keywords: heart-lung transplantation, gastroparesis, Pseudomonas aeruginosa, pulmonary infection
Introduction
Heart-lung and lung transplantation has become an acceptable treatment option for end-stage lung disease, and the number of transplants being performed in the world is increasing. Among the possible complications, gastroparesis is a frequent complication of lung or heart-lung transplantation that may lead to micro aspiration into the lung allograft (1). Gastroparesis also leads to poor absorption of immunosuppressant medications and aspiration pneumonia (1).
The respiratory infections have been reported as one of the complications of gastroparesis and it remains an important cause of postoperative mortality, but the microbiological consequences have rarely been evaluated. In the case reported here, pseudomonas aeruginosa infection of the lungs led to catastrophic consequences 11 months after the transplant.
Case report
A 29-year-old man with primary pulmonary hyper-tension underwent heart-lung transplantation on June 8, 2005 (2). He had no reflux disease or gastroparesis prior to transplantation. The patient was extubated on postoperative day 2. Perioperative antibiotics were administered routinely as infection prophylaxis. The patient received post-operative immunosuppression with cyclosporine A, mycophenolate mofetil and prednisone. By postoperative day 8, he complained of early satiety, epigastric pain, belching and bloating. A gastric scintigraphy revealed an estimated gastric emptying half-life of 390 minutes (normal, 45-100 min). At the time of scintigraphy, he was not taking any medications affecting the study (these medications were stopped 4 days before scintigraphy), except for the immunosuppressive scheme. Conservative treatment was started that is administration of promotility agents (cisapride and metoclopramide), high-dose proton pump inhibitors and frequent small meals with the patient in an upright position. But this did not significantly attenuate his symptoms which resulted in a 5-kg weight loss within the first 2 months (from 65 kg to 60 kg).
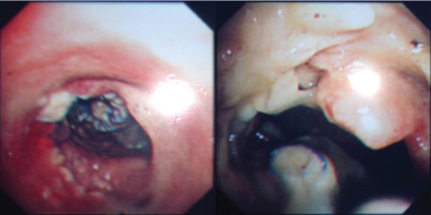
Within the next 9 months, recurrent bouts of vomiting led to 2 episodes of aspiration requiring hospitalization, and he continued to experience bloating, early satiety, belching and sporadic bucking. After 11 months, he presented with sudden onset shortness of breath, fever (temperature 39.3°C) and productive cough associated with greenish sputum. Laboratory tests yielded white blood cells 6830 mm(3) (85% neutrophils, 3.7% lymphocytes, 10.7% monocyte). In emergency room, patient was found to be in respiratory distress leading to emergent intubation to maintain the saturations. New radiographic infiltrates appeared in pulmonary parenchyma and some purulent bronchial secretion was extracted by consecutive endotracheal suction on admission. The culture yielded Pseudomonas aeruginosa. This is the sign of pneumonia (3). Bronchoscopy also revealed some mucus pluggings and food particles at the entrances to the left main bronchi, which also yielded Pseudomonas aeruginosa after culture (Fig. 1). The patient (weight 55 kg) was treated with imipenim/cilastin (1 g three times a day). However, his condition deteriorated rapidly and he died 1 week later. No postmortem examination could be performed.
Fig 1. Bronchoscopy revealed some mucus pluggings and food particles in trachea and at the entrances to the left main bronchi, which yielded Pseudomonas aeruginosa after culture.
Discussion
Gastroparesis in the heart-lung recipient can cause the undesirable symptoms and several respiratory distress due to aspiration pneumonia (4). The mechanism of posttransplant gastroparesis is thought to be multifactorial (5).
Gastroparesis might predispose to microaspiration and pulmonary infections (4), which was main cause in our case. Frequent nausea, vomiting, and gastroesophageal reflux can lead to aspiration pneumonitis. Laryngeal exposure to acid and aspiration of gastric contents may lead to severe respiratory disorders. The mechanism involved in the complication of bacterial pneumonia in patients with aspiration of gastric contents, however, remains uncertain. The effect of this aspiration is generally thought to be caused by gastric acid because the gastric contents introduced into the lungs can cause severe pulmonary injury, which is indistinguishable from that caused by the aspiration of an equivalent amount of gastric acid (6).
Bacterial pneumonia is the result of infection of the lower respiratory tract by microorganisms of the oral and pharyngeal regions. Pseudomonas aeruginosa is an opportunistic pathogen which colonizes at oropharynx and is one of the potential pathogens isolated from sputum samples from patients with respiratory aspiration of gastric contents (7). Bacterial adherence to the airway epithelium is the first step of bronchopulmonary infections. Acid aspiration can worsen bacterial infection by a mechanism involving bacterial cell adherence to the airway epithelium. Ramphal et al. Ramphal et al previously reported Pseudomonas aeruginosa can adhere efficiently to acid-injured tracheal epithelium, but not to uninjured epithelium (8). The authors suggested that acid injury could expose binding sites or change the surface structure of the cell membrane, thus permitting adherence of a nonmucoid strain of Pseudomonas aeruginosa by pili and subsequently leading to bacterial pneumonia (9). In our case, respiratory aspiration of gastric contents induced airway epithelial injury and enhanced Pseudomonas aeruginosa adherence to the acid-injured epithelium, which led to the subsequent development of bacterial pneumonia. Further, this case was further complicated by the essential immunosuppressive therapy.
Gastrointestinal complications may be an important factor of respiratory infections or mortality during the peri-operative period (10) and the consequences of such complication are well described in the literatures but the microbiological consequences have rarely been evaluated. The main finding in this case is Pseudomonas aeruginosa pulmonary infection in the heart-lung transplant recipient with the complication of gastroparesis. However, no further attempts were made to obtain an etiologic diagnosis. The diagnosis of Pseudomonas aeruginosa pneumonia was not clearly proven. Nevertheless, colonization with Pseudomonas aeruginosa was considered clinically significant and antibiotic therapy was initiated by the clinician (11).
Heart-lung recipients with gastroparesis are at increased risk for aspiration pneumonitis. In order to avoid the morbidity of gastroparesis in this subset of fragile patients, it may be prudent to perform a prophylactic management, including surgery. Alternatively, a high suspicion of gastroparesis should be maintained early in the postoperative course.
References
- 1.Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602–7. doi: 10.1378/chest.108.6.1602. [DOI] [PubMed] [Google Scholar]
- 2.Sha J, Yan Z, Zheng L. Pneumonia in a heart-lung recipient after airway stent placement. Thorac Cardiovasc Surg. 2007;55:332–4. doi: 10.1055/s-2006-955962. [DOI] [PubMed] [Google Scholar]
- 3.Mylotte JM, Goodnough S, Naughton BJ. Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51:17–23. doi: 10.1034/j.1601-5215.2002.51004.x. [DOI] [PubMed] [Google Scholar]
- 4.Reid KR, McKenzie FN, Menkis AH, Novick RJ, Pflugfelder PW, Kostuk WJ, et al. Importance of chronic aspiration in recipients of heart-lung transplants. Lancet. 1990;336:206–8. doi: 10.1016/0140-6736(90)91734-r. [DOI] [PubMed] [Google Scholar]
- 5.Morgan KG, Szurszewski JH. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980;301:229–42. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St John RC, Mizer LA, Kindt GC, Weisbrode SE, Moore SA, Dorinsky PM. Acid aspiration-induced acute lung injury causes leukocyte-dependent systemic organ injury. Am J Physiol. 1993;74:1994–2003. doi: 10.1152/jappl.1993.74.4.1994. [DOI] [PubMed] [Google Scholar]
- 7.Bynum LJ, Pierce AK. Pulmonary aspiration of gastric contents. Am Rev Resp Dis. 1976;114:1129–36. doi: 10.1164/arrd.1976.114.6.1129. [DOI] [PubMed] [Google Scholar]
- 8.Ramphal R, Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983;41:345–51. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramphal R, Sadoff JC, Pyle M, Silipigni JD. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984;44:38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustine SM, Yeo CJ, Buchman TG, Achuff SC, Baumgartner WA. Gastrointestinal complications in heart and in heart-lung transplant patients. J Heart Lung Transplant. 1991;10:547–55. [PubMed] [Google Scholar]
- 11.Lehto JT, Koskinen PK, Anttila VJ, Lautenschlager I, Lemström K, Sipponen J, et al. Bronchoscopy in the diagnosis and surveillance of respiratory infections in lung and heart-lung transplant recipients. Transplant Int. 2005;18:562–71. doi: 10.1111/j.1432-2277.2005.00089.x. [DOI] [PubMed] [Google Scholar]