Abstract
Background
Chest wall resection is a complicated treatment modality with significant morbidity. The purpose of this study is to report our experience with chest wall resections and reconstructions.
Methods
The records of all patients undergoing chest wall resection and reconstruction were reviewed. Diagnostic procedures, surgical indications, the location and size of the chest wall defect, performance of lung resection, the type of prosthesis, and postoperative complications were recorded.
Results
From 1997 to 2008, 162 patients underwent chest wall resection.113 (70%) of patients were male. Age of patients was 14 to 69 years. The most common indications for surgery were primary chest wall tumors. The most common localized chest wall mass has been seen in the anterior chest wall. Sternal resection was required in 22 patients, Lung resection in 15 patients, Rigid prosthetic reconstruction has been used in 20 patients and nonrigid prolene mesh and Marlex mesh in 40 patients. Mean intensive care unit stay was 8 days. In-hospital mortality was 3.7 % (six patients).
Conclusions
Chest wall resection and reconstruction with Bone cement sandwich with mesh can be performed as a safe and effective surgical procedure for major chest wall defects and respiratory failure is lower in prosthetic reconstruction patients than previously reported (6).
Keywords: chest wall tumor, chest wall resection, chest wall disease
Introduction
Early attempts for chest wall resection were limited by availability of suitable materials for reconstruction. Initial materials used for chest reconstruction included autogenous tissue such as fascia lata grafts, rib grafts, or large cutaneous grafts (1),(2). Since the first known chest wall resection in the18th century, improvements in surgical technique and anesthesia, critical care units, antibiotics, and the development and refinements in reconstraction techniques have allowed extensive chest wall resection to be performed with acceptable morbidity and mortality (3). After radical en bloc chest wall resection, skeletal reconstraction when appropriate and adequate skin coverage to preserve the reconstraction are the essential elements for successful management of the chest wall defects. In cases of chest wall tumors wide excision and reconstraction is very important because distant metastasis and local recurrence in incomplete resection is high leading to poor survival. Since 1980s, the use of prosthetic materials including polytetrafluoroethylene, polypropylene mesh, and polypropylene mesh–methylmethacrylate composites combined with the use of myocutaneous flaps has enabled successful reconstruction of even the largest chest wall defects (3). Although primary closure of muscle and skin after chest wall resection is attainable in most cases, many patients commonly require more sophisticated reconstructive soft-tissue and skin coverage. A variety of techniques including pedicled muscle transposition, free muscle flaps, and omental flaps have been used to provide adequate wound coverage, it allows quick healing, early rehabilitation, and better cosmesis (4). Respiratory complications continue to be the most frequent and are reported in 20% to 24% of the patients (3). The Purpose of this study is to retrospectively review our experience with chest wall resections and reconstruction and determine the postoperative complications
Patients and methods
It is a retrospective study performed on the available charts of 162 consecutive patients who underwent chest wall resection and reconstruction at Guilan University of Medical Sience Razi Hospital between1997 and 2008. All patients with more than two rib resections were included in the present series. Mean age was 40 years (range 20 to 70); 114 (70%) were men and 48 (30%) women. Prior to chest wall resections, all patients underwent pulmonary function tests. All patients received conventional chest roentgenography, which occasionally detects a defect or mass. For patients with a mass, a computed tomography (CT) scan or MRI of the chest was done to evaluate the extentension of the lesion and a tissue diag-nosis utilizing fine or core needle aspiration or incisional biopsy was attempted. In patients with suspected distant metastases, CAT Scans were used. Patients’ charts were retrospectively reviewed for age, sex, anatomic defect during the surgical resection, the number of ribs or the portion of sternum resected, and the surgical reconstruction technique. The in-hospital outcomes as morbidity and mortality, length of hospital stay (in the intensive care unit, and postoperative, hospital stay) was reviewed. For the infected chest wall requiring resection Vicry mesh was used in addition to muscle flap coverage. In Some instances where chest wall resection was done for radionecrosis only muscle flaps were used.
Results
During 11 years duration, 162 patients underwent chest wall resection .113 (70%) of patients were male. Age of patients was 14 to 69 years. (Median age of patients was 40 years). The most common indications for surgery were primary chest wall tumors (120patients, 74%) (Table 1). Ten patients (6%) underwent concomitant lung surgery and chest wall resection. The median number of ribs resected was 3 (range, 2 to 6). The anterior and lateral ribs were the most commonly resected ribs (62 pa-tients, 37%; A total of 22 patients underwent sternal resection; the most common was an Upper sternal resection in 10 patients. Six patients (3.7%) required total sternectomy for chondrosarcoma (Table 2). Immediate closure and repair was performed in all of the patients. Primary repair of the soft tissue and skin was performed in 86 patients (53%) and synthetic materials were used for chest wall integrity reconstruction in 76 patients (Prolene mesh, Marlex mesh, Bone cement sandwich with mesh). Sixtheen patients (8%) underwent pedicled muscle flap transposition. The three most common muscle groups utilized were latissimus flap, pectoralis flap and Latismos dorsi (Table 3). Bone and soft tissue sarcoma was the most commonly foung histological chest was tumor. The overall length of stay in the intensive care unit in rigid reconstraction patients was 5 to 16 days (median 8 days) and for non-rigid patients was 3 to 8 (median 5 days) and the postoperative in hospital length of stay in rigid reconstraction patients was 16 days (range 6 to 42). And for non-rigid patients was 6 to 10 (median 7 days).
Tab 1: Indiacations for Chest Wall Resection.
| Primary Chest wall tumor | 120 (74%) |
| Pectus (Sever) | 12 (7%) |
| Chest wall infections | 12 (7%) |
| Lung cancer | 10 (6%) |
| Radiation necrosis | 4 (2%) |
| Sternal Cleft | 2 (1%) |
| Metastatic chest wall tumor | 2 (1%) |
Tab 2: Chest Wall Resection: Anatomic Defects.
| Ribdefects | |
| Anterior rib resection | 62 (37%) |
| Anterolateral rib resectin | 20 (12%) |
| Posterior rib resectin | 20 (12%) |
| Posterolateral rib resectin | 18 (11%) |
| Sternal defects | |
| Upper sternal resection | 10 (16%) |
| Lower sternal resection | 6 (3.7%) |
| Total sternectomyin | 6 (3.7%) |
Tab 3: Chest Wall Reconstruction with Autogenous and Synthetic Materials.
| Number of Patients | |
| Primary chest wall closure | 86 (53%) |
| Prosthetic replacements | 60 (37%) |
| Prolene mesh | 30 (18%) |
| Marlex mesh | 10 (6%) |
| Mesh/methylmethacrylate composite | 20 (12%) |
| Autogenous replacements | |
| Latissimus muscle | 8 (4%) |
| Pectoralis muscle | 6 (3%) |
| Serratus muscle | 2 (1%) |
Six of 162 patients (3.7%) died during their hospital stay due to multiplesystem organ failure, including 2 of the 18 patients who underwent rigid prosthetic reconstruction and 4 of the patients undergoing concomitant lung surgery and chest wall resection. We have shown a successful repair of a patient with lung cancer and chest wall invasion requiring en bloc removal of the entire forequarter, the third to eight ribs, and the involved chest wall. The defect was repaired with a double fold of prolen mesh and Bone cement sandwich (Fig. 1, 2, 3, 4). Twenty-Eight of 76 patients (36 %) had complications during their hospital stay (Table 4). The most common complications were atelectasis (8 patients, 10%), Atrial fibrillation (5 patients, 6 %) and Pneumonia (4 patients, 5 %). Factors associated with postoperative complications were type of prosthesis, the location of the lesion, the need for sternal resection, medical comorbidities, and lung resection and size of the chest wall defect.
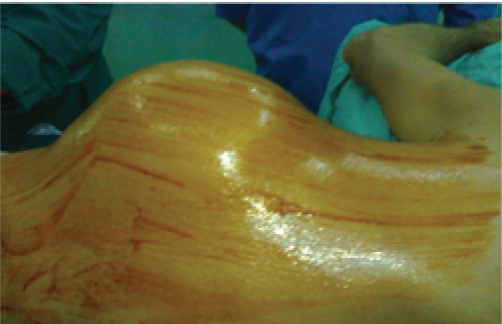
Fig 1. Huge Chest wall Chonderosarcom.
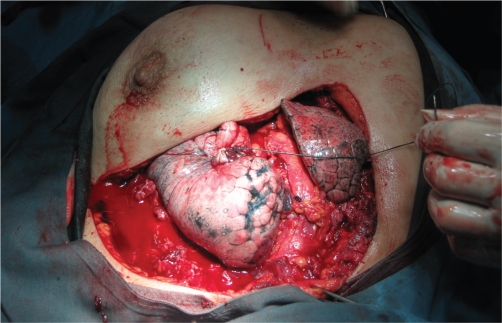
Fig 2. Chest wall defect after resection of Chonderosarcom.
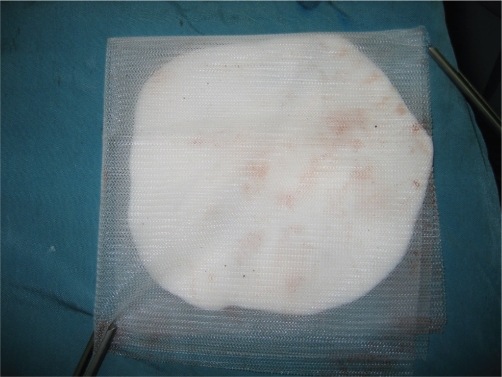
Fig 3. Bone cement sandwich with prolene mesh.
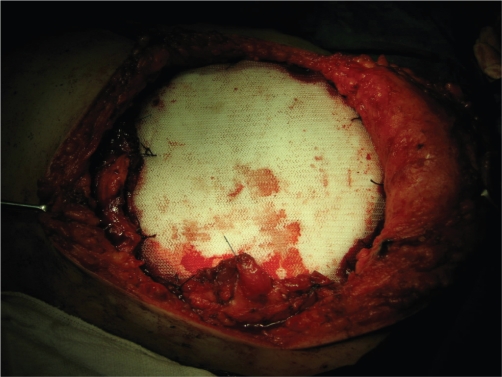
Fig 4. Chest wall reconstraction with bone cement sandwich with prolene mesh.
Tab 4: Surgical Complications.
|
Number of Patients |
||
| Rigid reconstraction | Non rigid | |
| Atelectasia | 4 (22%) | 4 (6%) |
| Pneumonia | 2 (11%) | 2 (3%) |
| Atrial fibrillation | 2 (11%) | 3 (5%) |
| Wound infection | 1 (5%) | 2 (3%) |
| Acute reapiratory failure | 2 (11%) | _ |
| Flap seroma | _ | 2 (3%) |
| Flap hematoma | _ | 2 (3%) |
| Prolong air Leak | 1 (5%) | 1 (1.7%) |
Discussion
In the treatment of patients requiring chest wall resection, three tenets of surgical resection should be main Tained (5). First, a sufficient amount of tissue must be resected to dispose of all devitalized tissue. Second, in segments of large chest wall resections a replacement must be found to restore the rigid chest wall to prevent physiologic flail. Third, healthy soft – tissue coverage is essential to seal the pleural space, to protect the viscera and great vessels, and to prevent infection. Although there is controversy as to which chest wall lesions should be reconstructed but, generally, lesions less than 5 cm in size in any location, and those up to 10 cm in size posteriorly do not need reconstruction for functional reasons (3). Posterior defects in proximity to the tip of the scapula, larger lesions likely to produce paradoxical chest wall motion and most anterior defects require reconstruction (6).
A basic tenet prior to the initiation of chest wall reconstruction is an appropriate and thorough chest wall resection that leaves healthy, viable margins to which materials and tissues used in a reconstruction may be anchored securely (4).
For patients in whom combined pulmonary and chest wall resection may be required we agree with Pairolero, that if the mediastinal lymph nodes are not positive, an en bloc resection is warranted as the 5-year mortality is more associated with the extent of the pulmonary cancer than with the extent of chest wall resection (7). In contrast Magdeleinat and colleagues do not consider N2 disease a contraindication to en bloc resection and have recently reported an actuarial 5-year survival after complete en bloc resection of lung cancer invading the chest wall (8). Chape and colleagues found that only histologic differentiation (well versus poorly differentiated) and the depth of chest wall invasion (parietal pleura versus other) were independent predictors of long-term survival in multivariate analyses (9).
Persisting or recurring chest wall involvement with breast carcinoma after local excision and radiation therapy may require chest wall resection to achieve local control (10),(11). Chest wall recurrences were found in 1% to 2% of stage I and in 10% to 12% of stage II breast carcinomas surgically treated with an extremely variable disease-free interval (11). In current series we have 4 cases of non-healing radiation-induced ulcers who underwent chest wall resection and reconstruction.
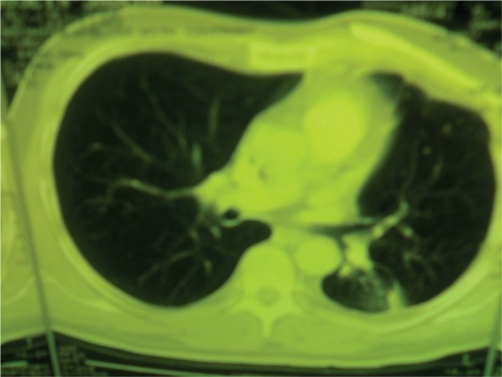
With modern surgical technique a wide range of reconstructive options are at the surgeon's disposal and hence it is imperative that the appropriate procedure be selected in a given patient. For small defects (less than 5 cm) or those located posteriorly under the scapula above the fourth rib (after resection of Pancoast tumors) the skeletal component can be ignored and the defect is uausally closed with only soft tissue. For patients undergoing large chest wall defects or pulmonary collapse, stabilization of the chest wall defect may be indicated. LeRoux and Shama have set forth the ideal characteristics of a prosthetic material (12): A. rigidity to abolish paradoxical chest motion, B. inertness allow in-growth of fibrous tissue and decrease the likelihood of infection, c. malleability so that it can be fashioned to the appropriate shape at the time of operation, D. radiolucency to allow radiographic follow-up of the underlying problem (Fig. 5).
Fig 5. Radiolucecy in CT–scan of a patient after reconstraction.
Historically, bone, cartilage, metal sheets, super-structures with autogenous rib graft, fascia lata, Teflon, and numerous other substances were used with minimal success (13).
In cases where structural integrity is necessary for preventing chest wall collapse, methyl methacrylate sandwich, silicone, Teflon, or acrylic materials have been utilized (5). The use of a rigid prosthesis using a PPM-methylmethacrylate “sandwich” was developed by some of the authors and has since been widely adopted (1),(3),(14),(15). It is our practice to use this rigid prosthesis for those defects likely to produce a flail segment, including large anterior or lateral lesions, or the area of the sternum in which some protection of the underlying organs is essential. This technique provides rigid repair that can be tailored to any size, shape, or contour of chest wall defect. The present report represents a good experience with the use of this method of reconstruction. While the importance of rigidity in chest wall reconstruction is still unclear, observations of chest wall trauma provide the significance of paradoxic motion of the chest wall. However, this respiratory uncoordinated motion is seen in almost every major resection of the chest wall but it is not associated with pulmonary insufficiency, which is seen with its traumatic counterpart, flail chest. We have commonly used Bone cement sandwich with prolene mesh with excellent physiologic and aesthetic success. Reports of transposition of the latissimus dorsi muscle for chest wall coverage had been described in 1896 by Tansini (18). The numerous advances in chest reconstruction over the years with the introduction of muscle and musculo-cutaneous flaps have made them the mainstay in chest wall reconstruction (19). The thoracic trunk is well suited for vascularized coverage given the many local muscle flaps (eg, latissimus dorsi, pectoralis major, rectus abdominis, trapezius, or deltoid muscles) or greater omentum (used alone or in combination as potions for wound coverage) (20). Respiratory complications including pneumonia, acute respiratory distress syndrome, and atelectasis are by far the most common and have been reported to be as high as 24% (4). Wound complications such as infection, dehiscence, flap loss, and hematoma are reported to occur in 8% to 20% (3),(4),(14). Our experience demonstrates that respiratory complications occurred in 18% of 76 patients and included pneumonia, atelectasis, respiratory failure (adult respiratory distress syndrome) and Prolong air leak. Tthis frequency of complications is far less than in other series (3), (4). Our experience reiterates the fact that respiratory complications will remain a significant problem. Lardinois and coworkers recently reported their experience with 26 patients having chest wall resection and reconstruction with mesh/methylmethacrylate (MMM). The authors report that there was no 30-day mortality and nearly all patients had a satisfactory cosmetic and functional outcome. (15). The prostheses were imaged with cine-magnetic resonance imaging and found to exhibit no paradoxic chest wall motion in any patient (15). In our series factors associated with postoperative complications and mortality were sternal resection, concominant lung resction and patient pulmonary capability, size of the chest wall resection as others (15). In conclusion: The key to a successful outcome in these complex cases is the coordinated effort by the surgical teams. The frequency of respiratory complications can be decreased by routine use of a rigid prosthesis for reconstruction.
Footnotes
Supporting Foundations: None
References
- 1.Carbone M, Pastorino U. Surgical treatment of chest wall tumors. World J Surg. 2001;25:218–30. doi: 10.1007/s002680020022. [DOI] [PubMed] [Google Scholar]
- 2.Arnold PG, Pairolero PC. Chest wall reconstructionan account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–8. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Deschamps C, Tirnaksiz BM, Darbandi R. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg. 1999;117:588–92. doi: 10.1016/s0022-5223(99)70339-9. [DOI] [PubMed] [Google Scholar]
- 4.Mansour KA, Thourani VH, Losken A, Reeves JG, Miller JI Jr, Carlson GW, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg. 2002;73:1720–5;discussion 1725-6. doi: 10.1016/s0003-4975(02)03527-0. [DOI] [PubMed] [Google Scholar]
- 5.Graeber GM, Langenfeld J. London: BC Decker; 1998. Chest wall resection and reconstruction; pp. 175–85. In: Franco KL, Putman JR, editors. Advanced therapy in thoracic surgery. [Google Scholar]
- 6.Weyant MJ, Bains MS, Venkatraman E, Downey RJ, Park BJ, Flores RM, et al. Results of Chest Wall Resection and Reconstruction With and Without Rigid Prosthesis. Ann Thorac Surg. 2006;81:279–285. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Pairolero PC. Extend resections for lung cancer. How far is too far? Eur J Cardiothorac Surg. 1999;16:S48–50. doi: 10.1016/s1010-7940(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 8.Magdeleinat P, Alifano M, Benbrahem C. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg. 2001;71:1094–9. doi: 10.1016/s0003-4975(00)02666-7. [DOI] [PubMed] [Google Scholar]
- 9.Chapelier A, Fadel E, Macchiarini P. Factors affecting long-term survival after en-bloc resection of lung cancer invading the chest wall. Eur J Cardiothorac Surg. 2000;18:513–8. doi: 10.1016/s1010-7940(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BO, Burt ME. Chest wall neoplasms and their management. Ann Thorac Surg. 1994;58:1774–81. doi: 10.1016/0003-4975(94)91691-8. [DOI] [PubMed] [Google Scholar]
- 11.Picciocchi A, Granone P, Cardillo G, Margaritora S, Benzoni C, D'ugo D. Prosthetic reconstruction of the chest wall. Int Surg. 1993;78:221–4. [PubMed] [Google Scholar]
- 12.LeRoux BT, Shama DM. Resection of tumors of the chest wall. Curr Probl Surg. 1983;20:345–86. doi: 10.1016/s0011-3840(83)80007-0. [DOI] [PubMed] [Google Scholar]
- 13.McCormack -PM. Use of prosthetic materials in chest-wall reconstruction. Surg Clin North Am. 1989;69:965–76. doi: 10.1016/s0039-6109(16)44932-7. [DOI] [PubMed] [Google Scholar]
- 14.McKenna Jr RJ, Mountain CF, McMurtrey MJ, Larson D, Stiles QR. Current techniques for chest wall reconstructionexpanded possibilities for treatment. Ann Thorac Surg. 1988;46:508–12. doi: 10.1016/s0003-4975(10)64686-3. [DOI] [PubMed] [Google Scholar]
- 15.Lardinois D, Müller M, Furrer M, Banic A, Gugger M, Krueger T, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg. 2000;69:919–23. doi: 10.1016/s0003-4975(99)01422-8. [DOI] [PubMed] [Google Scholar]
- 16.McCormack P, Bains M, Martini N, Burt M, Kaiser LR. Methods of skeletal reconstruction following resection of lung carcinomas invading the chest wall. Surg Clin North Am. 1987;67:979–86. doi: 10.1016/s0039-6109(16)44336-7. [DOI] [PubMed] [Google Scholar]
- 17.Tansini I. Nuovo processo per amputations della mammella per cancro. Reform Med. 1896;12:3–5. [Google Scholar]
- 18.Brown R, Fleming W, Jurkiewicz M. An island flap of the pectoralis major muscle. Br J Plast Surg. 1977;30:161–5. doi: 10.1016/0007-1226(77)90015-7. [DOI] [PubMed] [Google Scholar]
- 19.Cohen M, Ramasastry SS. Reconstruction of complex chest wall defects. Am J Surg. 1996;172:35–40. doi: 10.1016/S0002-9610(96)00058-X. [DOI] [PubMed] [Google Scholar]
- 20.Hultman CS, Culbertson JH, Jones GE, Losken A, Kumar AV, Carlson GW, et al. Thoracic reconstruction with the omentum: indications, complica-tions, and results. Ann Plast Surg. 2001;46:242–9. doi: 10.1097/00000637-200103000-00007. [DOI] [PubMed] [Google Scholar]