Abstract
Objective
To evaluate the effect of noninvasive Bi-level Positive Airway Pressure (BiPAP) ventilation on the severe influenza A virus associated with pneumonia and acute respiratory failure (ARF).
Methods
Based on conventional therapy via face mask using BiPAP ventilator positive airway pressure ventilation in the treatment of severe pneumonia caused by influenza A (H1N1) virus with acute respiratory failure (ARF) in 18 cases, we observed and evaluated the therapeutic effects.
Results
PaO2 and SaO2 before and after treatment were (48.85 ± 12.15)mmHg, (68.56 ± 16.25) mmHg and (80 ± 6)%, (92 ± 5)%, respectively. The results were significantly different (P<0.05) before and after treatment. Endotracheal intubation rate was 25% (6/24) and case-fatality rate was 8.3% (2/24).
Conclusion
BiPAP ventilator airway pressure by face mask ventilation can reduce the rate of endotracheal intubation in the treatment of severe pneumonia caused by influenza A (H1N1) virus in acute respiratory failure. It could be an effective approach in the emergency treatment with clinical value.
Keywords: Bi-level positive airway pressure ventilation, severe influenza A (H1N1) virus, acute respiratory failure
Introduction
Influenza A H1N1 is a new type of global infectious disease (1),(2). The genetic sequence of Influenza A (H1N1) virus is different from the known human influenza virus (3)-(5). The crowd would be generally susceptible and vulnerable to its outbreak. Since the first case of H1N1 flu reported in Mexico (6), South America in March 2009, Influenza A H1N1 had extended rapidly to more than 30 countries and regions throughout the world, including China, the United States (7),(8), Canada, Japan (9), and Hong Kong. Particularly in winter, the patient number of outbreak was increasing, as well as the cases of pneumonia caused by this virus. The major pathological features of influenza A (H1N1) complicated with severe pneumonia are bronchial wall necrosis, neutrophil infiltration, diffuse alveolar damage with hyaline membrane disease, including diffused alveolar damage, bronchioles and perivascular lymphocyte infiltration, proliferative changes in airway and bronchiolitis obliterans (10). It may affect the ventilation and air exchange, and easily leads to hypoxia. Irreversible damage to important viscera, which is fetal, will be followed if without prompt therapy.
Non-invasive ventilation (NIV) refers to various methods of respiratory support that do not require tracheal intubation, such as continuous positive airway pressure ventilation (CPAP) and bi-level positive airway pressure (BiPAP). By contrast to CPAP wherein fixed pressure support is maintained throughout the respiratory cycle, BiPAP devices deliver bias-flow pressure-controlled air via a nasal, oral-nasal, or helmet mask. Bi-level positive airway pressure is generally applied in adults with chronic obstructive pulmonary diseases (COPD), obstructive sleep apnea, or acute respiratory distress syndrome (ARDS) (11). Non-invasive ventilation was introduced for use in children during the polio epidemic of the 1950s (the iron lung) in the form of negative pressure ventilation (12).
Maurizio et al reported that BiPAP showed up an antiarrhythmic effect in patients with acute respiratory failure caused by COPD in a randomized clinical trail. It was reported that the BiPAP can affect global cardiac performance through its effects on the determinants of cardiac function, i.e. heart rate, preload, contractility and afterload (13).
The routine therapies of influenza A (H1N1) include anti-virus treatment, glucocorticoids therapy, anti-infection treatment and nutrition support therapy. The drug susceptibility can be found in the U.S Centers of Diseases of Control and Prevention (CDC) and Chinese Ministry of Health (14).
In this study, we designed a new therapeutic approach by adding the non-invasive BiPAP based on the routine therapy strategy in 18 cases of severe influenza caused by H1N1 influenza virus pneumonia with acute respiratory failure (ARF) in single institution, and retrospectively evaluated the effects of this approach by lung function test, which might induce a new therapeutic direction on this disease.
Material and method
Patient basic data
18 cases of influenza A (H1N1) complicated with severe pneumonia in our study are diagnosed etiologically, the throat swab specimens were detected by RT-PCR. And they were sent to pneumology department and infectious disease department in NO.1 Hospital of Jilin University ( Jilin, China).
Among the 18 cases, there were 10 male patients and 8 female cases, the average age was 28.73±9.24 (16 to 57), and all got community-acquired pneumonia (CAP). 2 cases were complicated with basic diseases (11.1%), while 1 case was with the bronchial asthma. And there is 1 case got CAP after the organ transplant. 2 cases were pregnant (11.1%). The BMIs in 12 of the 18 cases were all above 30 (66.7%).
Comprehensive treatment strategy
According to the standard procedure of Ministry of Health (14),(15), the comprehensive treatment of viral pneumonia is adapted, including: (1) Anti-virus treatment: each of the 18 patients was given anti-virus therapy under the regimen of Oseltamivir 75mg or 150mg, bid, for oral use; (2) Glucocorticoids: 16 cases were treated with glucocorticoids (88.9%) under Methylprednisolone 0.5-1mg/Kg.d and other 2 cases are not; (3) Anti-infection treatment: the routine anti-infection drug was Carbapenems Antibiotic at the beginning, and then was adjusted according to the patients’ condition; (4) Nutrition support was used on all the 18 cases; (5) Mechanical ventilation: Based on the conventional treatment above, we applied nasal mask positive airway pressure. We chose the appropriate masks (including closed type and nasal type) according to the factors below: mouth coverage, secretion amount and the surface shape&size (BIPAP Synchrony ventilator S/T self-trigger respiratory mode, Wellkang LLC, USA). The primary parameters setting were: respiratory frequency was 18/min; the IPAP increased gradually from 6 cmH2O (1 cmH2O=0.098kPa) to 12∼20 cmH2O in 30 minutes; the EPAP was 4 cmH2O and the pressure range was 4 to 12 cmH2O, which adjusted to the IPAP synchronously with oxygen flow of 5 L/min. After 6 hours’ ventilation, we reexamined the blood and gas, then analyzed and recorded the clinical condition. When the worse conditions, for example, suffocating or disturbance of consciousness, hypotension, that is systolic pressure is less than 90mmHg (1mmHg=0.133kPa), PaO2 decreases to 40mmHg, and arterial pH≤7.25, happened, the patient would be given endotracheal intubation mechanical ventilation treatment.
We monitored and recorded the blood and gas before the treatment for 6 hours every 24 hours after their treatment. If PaO2/FiO2>200 or increased by >100 compared to before the treatment, it was believed that the air exchange had been improved (16). If it could last until the ventilation ending, it was believed that the air exchange improved constantly.
CT scanning
We scanned all the patients 3 days later after the therapy to identify the change in lung parenchyma, which could further prove the effectiveness.
Statistics processing
We analyzed the data with SPSS13.0 software. The data is represented by -x±s, and paired t test is applied. The difference has statistical significance if P<0.05.
Results
Blood gas analysis
After the treatment of 18 cases, 12/18 had been statistically improved (Table 1). In these 12 cases, the expiratory dyspnes had been lessened, respiratory frequency and heart rate had been decreased, with PaO2and PaO2/FiO2 increased. The efficiency rate was 66.7%. Endotracheal intubation was applied in other 6 cases, among whom, 4 survived and 2 died. Ratio of the application of endotracheal intubation was 33.3 % (6/18) and fatality rate was 11.1% (2/18). Among the 18 cases, facial compression hyperemia was found in 2 cases, eye infections in 1 case, oropharyngeal dry in 4, phlegm obstacles in 4 and gaseous distention in 6 (Table 2).
Table 1. The changes of PaO2/FiO2, PaCO2, heart rate, and respiratory frequency during NIV treatment (n=12).
| PaO2/Fi O2 | PaO2(mmHg) | Heart rate (b/min) | Respiratory frequency(T/M) | |
| Before Treatment | 110±30 | 52±10 | 125±18 | 32±6 |
| After Treatment | 200±28* | 88±9* | 92±11* | 22±5* |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
* The data of "before the treatment" means the last data of blood-gas analysis before the first time to take the BiPAP ventilator
Table 2. Complications (n=18).
| Complication | Numbers | Ratio |
| Facial compression hyperemia | 2 | 11.1% |
| Eye infections | 1 | 5.5% |
| Oropharyngeal dry | 4 | 22.2% |
| Phlegm obstacles | 4 | 22.2% |
| Gaseous distentin | 6 | 33.3% |
CT scanning analysis
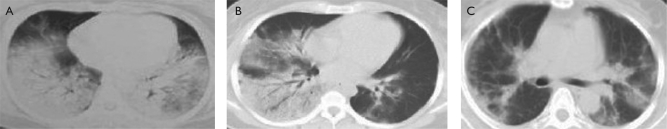
After 3 days of therapy, we scanned the lung with Computed Tomography (CT). We found partly absorption of consolidation in 15 cases (83.3%), while thickening interlobular septa in 12 cases (66.7%) (Fig 1b). After 7 days of therapy, we scanned the lung again with CT. We found significantly absorption of consolidation of 14 cases (77.8%), while fiber rope formation in the disease lesion in 16 cases (88.9%) (Fig 1c).
Fig 1. Representative CT scanning result. (A) Before the therapy, (B) 3 days after the therapy, (C) 7 days after the therapy.
Discussion
Bi-level positive airway pressure ventilation (BiPAP) is a new type of respiration support mode, which has been used in ARF caused by COPD or paediatric oncology, heart failure caused by sleep apnea and other respiratory diseases (12)-(14). BiPAP ventilator enables patients’ spontaneous breath by Bi-level positive airway pressure, therefore, it has the characteristic of spontaneous breath and breath control simultaneously.
On one hand, the operating principle of BiPAP ventilator S and S/L modes is PSV. That is because the respiratory frequency and ventilation VT mainly depend on the patients’ self-regulation, which is closer to physiological status than other modes (13). Therefore, it can lessen the influence of volume damage on hemodynamic and the counteraction between the ventilator and patient. On the other hand, the ventilator provides non-invasive Bi-level positive airway pressure, so it reduces respiratory workload and oxygen consumption, meanwhile increases the alveolar ventilation and reopens the atelectatic lung resulting in improving the ventilation-flow ratio (14).
The pathological symptoms of influenza A (H1N1) complicated by severe pneumonia are lung tissue oedema, inflammatory exudation, partly sinking and collapsing alveolus and lung consolidated. Hyoxemia is mainly caused by arterial and venous blood diffluency and the imbalance of ventilation-flow ratio. The application of BiPAP ventilator can raise pulmonary oxygen pressure by reducing the pulmonary workload and activating the collapsing alveolus (17).
This study illustrated that BiPAP ventilator had improved the clinical status of the patients enrolled in the study. Respiratory frequency and heart rate had fallen (P<0.05). PaO2 rised (P<0.05). 66.7% of the patients didn’t experience intubation and related complicated diseases. The costs of medical care had decreased.
The result of this study had proved the application of BiPAP ventilator could improve respiratory status of patients with H1N1 influenza virus pneumonia and acute respiratory failure. It could significantly improve arterial blood gas analysis status of the patient without negative influence on haemodynamics. What’s more, in the study we found that the application of BiPAP ventilator at the early stage could reduce intubation and related complicated diseases. That is because when acute respiratory failure happens, the capillary alveolar membrane permeability increases, which leads to the pulmonary edema (11),(14). Most patients have little purulent secretion in our study, which provides suitable conditions for NPPV treatment. NPPV reduces the pulmonary effusion and edema status through positive pressure, which improves oxygenation. The incidence rate of ventilator related pneumonia and lung injury is lower than that of invasive ventilation (18). Our data showed that the BiPAP could have a significant effect on the treatment of the influenza H1N1 virus pneumonia with acute respiratory failure patients, especially on emergency situations. Further large samples randomized controlled trials are needed to evaluate whether the death rate of patients could be reduced.
Nevertheless, some complications of BiPAP shown in our study has to be noticed, such as eye and face skin damage, oropharyngeal dry, phlegm difficulties and gaseous distention. Based on our experience, choosing appropriate masks with better tissue compatibility, applying humidifying device, choosing appropriate pressure, guiding the nasal respiration and avoidance of mouth respiration are the effective approaches to deal with the complications. However, gaseous distention is also one of the complications, and the gastrointestinal decompression should be given in time.
In conclusion, BiPAP ventilator airway pressure by face mask ventilation can reduce the rate of endotracheal intubation in the treatment of severe pneumonia caused by influenza A (H1N1) virus in acute respiratory failure. It could be an effective approach in the emergency treatment and has an important application value.
Footnotes
No potential conflict of interest.
References
- 1.Centers for Disease Control and Prevention (CDC) Update: infections with a swine-origin influenza A (H1N1) virus-United States and other countries, April 28, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:431–3. [PubMed] [Google Scholar]
- 2.Naffakh N, van der Werf SV. April 2009: an outbreak of swine-origin influenza A(H1N1) virus with evidence for human-to-human transmission. Microbes Infect. 2009;11:725–8. doi: 10.1016/j.micinf.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S. et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 4.Trifonov V, Khiabanian H, Greenbaum B, Rabadan R. The origin of the recent swine influenza A (H1N1) virus infecting humans. Euro Surveill. 2009;14:19193. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:433–5. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection -Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–70. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Swine-origin influenza A (H1N1) virus infections in a school-New York City, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:470–2. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children -Southern California March-April 2009. MMWR Morb Morta Wkly Rep. 2009;58:400–2. [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) Human infection with new influenza A (H1N1) virus: clinical observations from a school-associated outbreak in Kobe, Japan, May 2009. Wkly Epidemiol Rec. 2009;84:237–44. [PubMed] [Google Scholar]
- 10.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–43. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiller O, Schonfeld T, Yaniv I, Stein J, Kadmon G, Nahum E. Bi-level positive airway pressure ventilation in pediatric oncology patients with acute respiratory failure. J Intensive Care Med. 24:383–8. doi: 10.1177/0885066609344956. [DOI] [PubMed] [Google Scholar]
- 12.Aschenbrenner R, Donhardt A, Foth K. Permanent artificial respiration in the iron lung; report on experiences with 105 respirator paralyzed poliomyelitis patients from 1947 to 1952. Munch Med Wochenschr. 1953;95:748–51. [PubMed] [Google Scholar]
- 13.Marvisi M, Brianti M, Marani G, Turrini G, Zambrelli P, Ajolfi C, et al. Acute antiarrhythmic effects of bi-level positive airway pressure ventilation in patients with acute respiratory failure caused by chronic obstructive pulmonary disease: a randomized clinical trial. Respiration. 2004;71:152–8. doi: 10.1159/000076676. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:433–5. [PubMed] [Google Scholar]
- 15.Hui DS, Lee N, Chan PK. Clinical Management of Pandemic (H1N1) Infection. Chest. 2010;137:916–25. doi: 10.1378/chest.09-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meduri GU, Turner RE, Abou-Shala N, Wunderink R, Tolley E. Noninvasive positive pressure ventilation via face mask. First-line intervention in patients with acute hypercapnic and hypoxemic respiratory failure. Chest. 1996;109:179–93. doi: 10.1378/chest.109.1.179. [DOI] [PubMed] [Google Scholar]
- 17.Elliott MW. Non-invasive ventilation for acute respiratory disease? British Medical Bulletin. 2004;72:83–9. doi: 10.1093/bmb/ldh042. [DOI] [PubMed] [Google Scholar]
- 18.Burns KE, Adhikari NK, Keenan SP, Meade M. Use of non-invasive ventilation to wean critically ill adults off invasive ventilation: meta-analysis and systematic review. BMJ. 2009;338:b1574. doi: 10.1136/bmj.b1574. [DOI] [PMC free article] [PubMed] [Google Scholar]