Abstract
Objective
Prospective randomized controlled study was conducted to explore the effects and safety of prophylactic use of noninvasive positive pressure ventilation (NPPV) in post-thoracic surgery (PTS) patients, especially on the lung re-expansion, lung function change and postoperative pulmonary complications (PPCs).
Methods
Fifty PTS patients met the inclusion criterion were enrolled in the study. All subjects were randomly divided into conventional treatment (control) group and NPPV group. NPPV group received intermittent NPPV therapy in first three days of PTS. BiPAP ventilator was used with S/T mode in the study. The average IPAP was (13±3.2)cmH2O (ranged from 7 to 18 cmH2O) and EPAP was 4cmH2O. Total ventilation time was (13.5±4.9) hours (ranged from 6.5 to 23 hours). PPCs rate, lung re-expansion, the volume of residual cavity, lung function and tolerance to NPPV were assessed with chest roentgenography, CT scan, lung function testing and clinical evaluation before and one week after surgery.
Results
1. There was no significant difference of total PPCs rate during hospitalization between the two groups (5/23 in NPPV group vs 6/27 in control group, P= 0.967). Multiple factorial logistic regression analysis showed that COPD was a risk factor for PPCs (B=1.705, P=0.027). 2. Compared with control group, NPPV therapy reduced inadequate lung expansion rate (3/23 vs 13/27, P=0.008) and volume of residual cavity calculated with CT scan [(31.9±71.7)ml vs (63.6±78.3)ml, P=0.02]. However, there were no significant difference in the change of lung function parameters after operation between the two groups (all P>0.05). No significant adverse effects of NPPV were found in the present study.
Conclusions
In the current study of prophylactic application of NPPV in post-thoracic surgery patients, the use of NPPV resulted in improved lung re-expansion, but had no significant effects on post-operative pulmonary complications and lung functions.
Keywords: noninvasive positive pressure ventilation, thoracic surgery, postoperative pulmonary complications
Introduction
Despite significant progress in surgical procedures and postoperative care, postoperative pulmonary complications (PPCs), including atelectasis, pulmonary infection, respiratory failure remain to be major clinical problems in post-thoracic surgery (PTS) patients. The overall rates of pulmonary complications after thoracic surgery are around 30%, ranging from 7% to 49% as reported in the literature (1). Pulmonary complications are the main causes of morbidity and mortality in PTS patients (2). The mechanisms related to PPCs include surgical trauma, pain, anesthesia, diaphragmatic dysfunction, airway obstruction, reduced lung function, poor lung expansion and secretion retention. Noninvasive positive pressure ventilation (NPPV) might have potential roles in reducing PPCs and facilitating lung re-expansion after PTS by positive pressure and inspiratory assistance. Recently, there are reports showing that NPPV might be effective in managing PPCs. NPPV is reported to be able to reduce intubation rate and mortality in PTS patients complicated with respiratory failure. It is unclear whether prophylactic use of NPPV in PTS patients can improve lung re-expansion and reduce the morbidity of pulmonary complications, which demands further clinical investigation. We hypothesized that NPPV may facilitate the expansion of the lung through positive airway pressure and assisted inspiration, which might be beneficial for the prevention and treatment of PPCs in PTS patients. Thus, this study was performed to explore the potential effects of prophylactic use of NPPV on the lung re-expansion, lung function change and PPCs.
Subjects and methods
Subjects
From August 2008 to January 2009, fifty PTS patients were enrolled in the study. The inclusion criteria were as follows:
(1) Subjects receiving non-emergency thoracic surgery;
(2) No contra-indications of NPPV;
(3) Agreed to sign an informed consent after full discussion of the procedure of NPPV before surgery.
Exclusion criteria included the following:
(1) Subjects refusing to participate or sign the informed consent.
(2) Any contra-indications of NPPV, such as unconsciousness, copious sputum, hemodynamic instability, etc.
Study design and experimental procedure
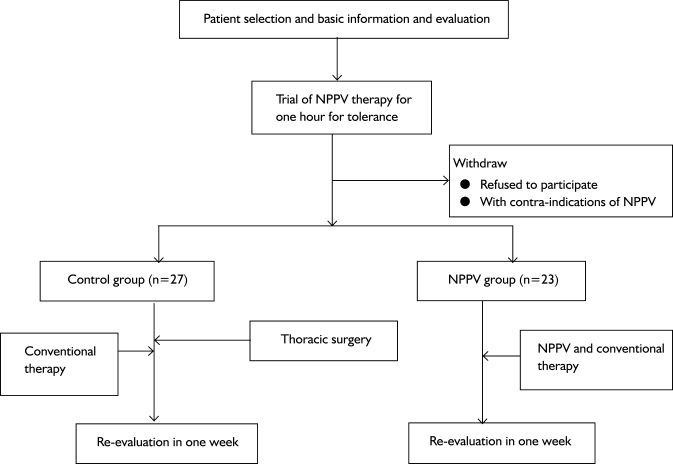
This was a prospective controlled study. All the subjects eligible for the study were randomized to receive conventional treatment or additional NPPV therapy. The experimental procedures are shown in figure 1.
Fig 1. Flow chart of the experimental procedure.
Note: NPPV: noninvasive positive ventilation
NPPV treatment procedure
Respironics BiPAP Synchrony ventilator was employed for the study. The procedure of NPPV application is in accordance with the “Consensus Report on Clinical Application of Noninvasive Positive Pressure Ventilation” (3). All involved subject received adaptation trial of NPPV treatment for 1 to 2 hours before operation. After operation, NPPV was initiated once the patient was consciousness and able to cough. S/T mode was used with mean IPAP (13 ± 3.2)cmH2O (ranging from 7cmH2O to 18cm H2O), EPAP 4cmH2O. The total duration of NPPV was (13.5 ± 4.9) hours (ranging from 6.5 to 23 hours). Nasal mask was used in 21 (91%) patients and facial mask in 2 (9%) patients.
Observation and evaluation
All the subjects were evaluated before operation, during NPPV treatment and one week after operation. Methods used for evaluation included chest roentgenography, CT scan, lung function testing and clinical evaluation. The main criteria for assessment are as following:
(1) Acute respiratory failure: clinical presentation of breathlessness and arterial blood gas analysis showing PaO2<8KPa (60mmHg) and/or PaCO2>6.67KPa (50mmHg).
(2) Postoperative pneumonia: Determined according to the criteria of “hospital-acquired pneumonia diagnosis and treatment guidelines" by Chinese Society of Respiratory Disease in 1998.
(3) Wheezing: Episode of wheezing together with breathlessness as well as wheezing on auscultation.
(4) Atelectasis: Based on chest roentgenography as reported by radiologist.
(5) ARDS: The diagnosis of ARDS was based on acute onset of respiratory distress, PaO2/ FiO2≤200mmHg and chest roentgenography showing bilateral wide spread shadow of infiltration after elimination of cardiogenic pulmonary edema and major pulmonary atelectasis.
(6) Pulmonary embolism: Patients with un-explained breathlessness and elevated D-dimer would receive CT pulmonary angiography to confirm or exclude the diagnosis of pulmonary embolism.
(7) Poor lung expansion: Evaluated according to the appearance on chest roentgenography as reported by radiologist.
(8) Change of pulmonary function and lung volume: Spirometer and bodybox (MicroQuark, COSMED Co. Italian) was employed for evaluating pulmonary function before and one week after operation.
(9) Evaluation of the volume of residual cavity: multiple sliced computerized tomography scan and “EmphylxJLaunche” software were used for computer-aided calculation of the volume of residual cavity after operation.
(10) Observation of adverse effects of NPPV: gastric distention, dry mouth, pleural air leak, nasal skin erosion and claustrophobia were checked and recorded during NPPV therapy.
Statistics
SPSS11.0 software package was used for statistical analysis. Qualitative data was expressed as percentage and Chi-Square test was employed for analysis. Quantitative data was expressed as mean ± standard deviation and analyzed with student T test for group comparison and one way Anova for repeated measurement. Multiple linear correlation test was used to assess the associated factors for post-operation change. Multiple factorial analyses were conducted with logistic regression. The primary endpoints were lung re-expansion and post-surgery lung function change, and the second endpoints were PPCs and safety of NPPV.
Results
A total of 50 cases were enrolled, with 27 cases in control group (male: 20 and female: 7) and 23 cases in NPPV group (male: 17 and female: 6). The underline diseases included malignant tumor in 30 cases (60%), COPD and bulla in 11 cases (20%), benign lung tumor in 3 cases (6%), organized pneumonia in 3 cases (6%), bronchiectasis in 2 cases (4%), esophageal carcinoma in 1 case (2%), and pericardial cyst in 1 case (2%). There were no significant difference between the control group and NPPV group in average age [(55 ± 12.9) vs (59.1 ± 9.7) yrs], body mass index [(22.4 ± 3.4) vs (22.5 ± 4.1) KG/M2], and smoking index [(18.5 ± 23.2) vs (24.4 ± 24.9) package*year] (all P> 0.05).
The baseline lung function parameters in control and NPPV group were comparable, with FEV1 [(2.3±0.77) vs (1.95±0.8L)], FEV1 as percentage of prediction [(78.5±25.1) vs (76±29%)], FVC [(3.09±0.72) vs (2.85±0.86L)], FVC as percentage of prediction [(91.3±16.6) vs (89.5±18.4%)], and FEV1/FVC [(73.3±13.2) vs (68.3±17.3%)], (all P>0.05).
Tolerance to NPPV treatment was good in 13 cases (57%), general in 7 (30%) cases, and poor in 3 (13%)
cases among the 23 cases in NPPV group. Three cases with poor tolerance to NPPV were due to gastric distention in 1 case (4.5%), claustrophobia in 1 case (4.5%) and unwilling to use NPPV in 1 case (4.5%).
Impact on lung re-expansion and volume of residual cavity after operation
The volume of residual cavity after operation calculated with CT scan at one week after operation was significantly smaller in NPPV group than control group [(31.9 ± 71.7) vs (63.6 ± 78.3) ml, P= 0.02].
Poor re-expansion of the lung was observed in 3 cases in NPPV group and 13 cases in the control group, showing statistically significant difference between the two groups (P= 0.008). Logistic multiple factorial analysis with above mentioned confounding factors showed that “NPPV application” was the only significant protective factor of poor re-expansion (B=-1.772, P=0.015).
The impact on lung functions
At one week after operation, the changes of lung function before and after operation were shown to be similar between NPPV and control group (Table 1).
Table 1. Comparison of lung function change after operation between two groups.
| Δ FEV1 | Δ FEV1%pred | Δ FVC | Δ FVC%pred | Δ FEV1/FVC | Δ TLC | Δ RV | Δ FRC | |
| Control group | 0.58±0.7 | 17.9±23.8 | 0.93±0.63 | 28.9±14.7 | 4.36±19.57 | 1.15±0.83 | 0.39±0.59 | 0.5±0.43 |
| NPPV group | 0.39±0.57 | 17.4±20.4 | 0.59±0.61 | 22.3±14.3 | 3±12.8 | 1.31±1.2 | 0.78±1.23 | 0.55±1.19 |
| P value | 0.313 | 0.943 | 0.064 | 0.124 | 0.332 | 0.579 | 0.327 | 0.291 |
PPCs and recovery during hospitalization
During hospitalization, total PPCs occurred in 5 cases in NPPV group and 6 cases in control group (P= 0.967). According to subgroup analysis, there was no significant difference in atelectasis (2 cases in NPPV group and 1 in control group, P>0.05), wheezing (1 case in NPPV group and 2 in control group, P>0.05), pneumonia (1 case in NPPV group and 2 in control group, P>0.05) and acute respiratory failure (1 case in NPPV group and 1 in control group, P>0.05).
Multiple factorial analysis with logistic regression was performed with above mentioned confounding factors including NPPV application, gender, age, smoking status, body mass index, operation procedure, anesthesia time, bleeding volume during operation, baseline FEV1, FVC, FEV1/FVC ratio, COPD, hypertension and diabetes. It was observed that COPD was the only significant risk factor for PPCs (P= 0.027) and baseline FVC was potential protective factor of PPCs (B =-1.121, P=0.058).
Pleural air leak associated with application of NPPV
Significant pleural air leak occurred in 4 cases (17%) in NPPV group and in 3 cases in control group (11%). There was no statistically significant difference between two groups (P=0.692), suggesting that NPPV did not have significant effect on pleural air leak after thoracic operation.
Discussion
Postoperative pulmonary complications (PPCs) and lung function recovery are important issues in post-thoracic surgery patients. PPCs are still common clinical problems, which prolong duration of hospitalization, increase the economic burden and mortality. Currently available procedures to prevent and treat PPCs include improving surgical procedure, applying appropriate anesthesia, increasing the lung volume during breathing and strengthening patient's education. In all of above procedures, increasing the lung volume with deep breathing training, incentive spirometry and intermittent positive pressure breathing are now generally recognized as effective. Lung function recovery is important regarding the patient’s functional recovery. Apart from the operation procedure itself, lung re-expansion and residual cavity in the pleural space are important factors for lung function recovery.
Noninvasive positive pressure ventilation (NPPV) provides continuous positive airway pressure and increases tidal volume. The positive end-expiratory pressure can increase the end expiratory lung volume and keep the distal small airway open. Thus, in theory, NPPV might be effective in preventing PPCs and facilitating lung re-expansion in post-thoracic patients.
There have been reports in the literature on the effect of NPPV on the PPCs in post-thoracic surgery patients with inconsistent results. Perrin studied whether prophylactic use of NPPV administered pre-and postoperatively may reduce the postoperative pulmonary function impairment in thoracic surgery (4). Thirty-two patients with a preoperative FEV(1) <70% of the predicted value scheduled for elective lobectomy related to lung cancer were involved in the study. Patients received standard treatment without (control group, n=18) or with NPPV (study group, n=14) during 7 days at home before surgery, and during 3 days postoperatively. It was shown that short term after surgery (two to one day), PaO(2), FVC and FEV(1) values were significantly better in the NPPV group. However, atelectasis rate was slightly, but not significantly lower in NPPV group than in the control group (14.2% vs 38.9%, P>0.05), perhaps due to small number of cases (32 cases) involved in the study. Stock used continuous positive airway pressure ventilation in patients of upper abdominal surgery within 72 hours after surgery, resulting in slight lower, but not statistically significant atelectasis rate than in the incentive spirometry group and conventional cough and deep breathing (23% vs 41%) (5). Kindgen used CPAP prophylacticly in thoracoabdominal aortic surgery patients with high risk of postoperative pulmonary complications, and CPAP significantly reduced the incidence of PPCs effectively (28% vs 96%) (6). This study is characterized by major surgical trauma, long surgery time (an average of 272 minutes), a large amount of blood loss (an average of 4179ml) and high PPCs rate in control group (96%), which might be the explanation for more obvious effect of NPPV in the study.
In our study, NPPV was initiated on the first day after surgery in a group of patients with baseline lung function close to normal. So, the main outcomes should be lung re-expansion and lung function recovery. Although the overall lung function change was similar between the two groups, the lung re-expansion was better in NPPV group. In our study, CT scan was used for calculating the volume of residual cavity, which is more accurate than the assessment with roentgenography, providing stronger evidence that NPPV facilitates the lung re-expansion after thoracic surgery. As for the negative effects on post-operation lung function change, the potential explanation could be that the small sample size in our study and many related confounders make the study not powerful to detect the change. In addition, in the study, we failed to show any beneficial effect of NPPV on the rate of PPCs. There were several reasons responsible for that. First, the baseline lung function of the subjects was normal or close to normal, so that the overall PPCs rate was low. Secondly, all patients involved in the study received minimally invasive video-assisted thoracic surgery (VATS), which would further reduce the PPCs rate. It has been reported that AVTS surgery has lower PPCs rate, ranged from 3.6% (8) -10.9% (9) than conventional surgery generally, ranged from 7%-49% (1). Thirdly, the sample size of the study was small.
The logistic regression analysis of PPCs showed that the underline disease of COPD was a risk factor for PPCs, which is consistent with reports in literature (10),(11). Further stratified analysis also showed that, COPD patients had a slightly higher, but not significant poor lung re-expansion rate than those in lung tumor group (54.5% vs 25.8%, P= 0.136). These results suggested that prophylactic use of NPPV might be more valuable in thoracic surgery patients with underline COPD.
The safety and tolerance of NPPV in post-thoracic patients have been studied in several studies. Our study results are consistent with those reported in literature proving that NPPV is safe and well tolerated in post-thoracic patients. The positive airway pressure in the study has no adverse effect on pleural air leak after thoracic surgery.
In summary, the current study has shown that prophylactic application of NPPV in post-thoracic surgery patients resulting in improved lung re-expansion, but no significant effects on postoperative pulmonary complications and lung functions. The potential role of NPPV in post-thoracic surgery deserves further large sample, multi-center study, especially in subjects with high risk of post-operation pulmonary complication, such as COPD patients.
Footnotes
No potential conflict of interest.
References
- 1.Stéphan F, Boucheseiche S, Hollande J, Flahault A, Cheffi A, Bazelly B, et al. Pulmonary complications following lung resection: A comprehensive analysis of incidence and possible risk factors. Chest. 2000;118:1263–70. doi: 10.1378/chest.118.5.1263. [DOI] [PubMed] [Google Scholar]
- 2.Auriant I, Jallot A, Hervé P, Cerrina J, Le Roy Ladurie F, Fournier JL, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am j Repir Crit Care Med. 2001;164:1231–5. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- 3.Consensus report on clinical application of noninvasive positive pressure ventilation. Chinese Journal of Tuberculosis and Respiratory Diseases. 2009;32:86–98. [Google Scholar]
- 4.Perrin C, Jullien V, Vénissac N, Berthier F, Padovani B, Guillot F, et al. Prophylactic use of noninvasive ventilation in patients undergoing lung resection surgery. Respir Med. 2007;101:1572–8. doi: 10.1016/j.rmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest. 1985;87:151–7. doi: 10.1378/chest.87.2.151. [DOI] [PubMed] [Google Scholar]
- 6.Kindgen-Milles D, Müller E, Buhl R, Böhner H, Ritter D, Sandmann W, et al. Nasal-continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest. 2005;128:821–8. doi: 10.1378/chest.128.2.821. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo SM, Park BJ, Wilton AS, Seshan VE, Bains MS, Downey RJ, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–6. doi: 10.1016/j.athoracsur.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 8.Inderbitzi RGC, Grillet MP. Risk and hazards of video-thoracoscopic surgery: a collective review. Eur J Cardiothorac Surg. 1996;10:483–9. doi: 10.1016/s1010-7940(96)80412-x. [DOI] [PubMed] [Google Scholar]
- 9.Jancovici R, Lang-Lazdunski L, Pons F, Cador L, Dujon A, Dahan M, et al. Complications of video-assisted thoracic surgery: a five-year experience. Ann Thorac Surg. 1996;61:533–7. doi: 10.1016/0003-4975(95)01060-2. [DOI] [PubMed] [Google Scholar]
- 10.Dancewicz M, Kowalewski J, Peplinski J. Factors associated with perioperative complications after pneumonectomy for primary carcinoma of the lung. Interact CardioVasc Thorac Surg. 2006;5:97–100. doi: 10.1510/icvts.2005.118125. [DOI] [PubMed] [Google Scholar]
- 11.Algar FJ, Alvarez A, Salvatierra A, Baamonde C, Aranda JL, López-Pujol FJ. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur J Cardiothorac Surg. 2003;23:201–8. doi: 10.1016/s1010-7940(02)00719-4. [DOI] [PubMed] [Google Scholar]
- 12.Lindner KH, Lotz P, Ahnefeld FW. Continuous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest. 1987;92:66–70. doi: 10.1378/chest.92.1.66. [DOI] [PubMed] [Google Scholar]
- 13.Matte P, Jacquet L, Van Dyck M, Goenen M. Effects of conventional physiotherapy, continuous positive airway pressure and noninvasive ventilatory support with bilevel positive airway pressure after coronary artery bypass grafting. Acta Anaesthesiol Scand. 2000;44:75–81. doi: 10.1034/j.1399-6576.2000.440114.x. [DOI] [PubMed] [Google Scholar]