Abstract
Background
Stage IV non-small cell lung cancer (NSCLC) is thought to uniformly carry a poor prognosis with a median survival of less than 1 year and 5-year survival of less than 5%. In patients with a low volume (i.e. single site) of distant disease, the prognosis is slightly more favorable than that of more advanced (i.e. multiple sites of metastases) disease. For those with limited metastases, we developed a paradigm of adding concurrent chemotherapy and radiotherapy to the primary tumor once the tumor demonstrated chemotherapy sensitivity.
Methods
Charts of patients from 1999-2006 with non-small cell lung cancer were reviewed to find those with a single extra-thoracic site of disease treated with combined modality therapy. We found nine patients of 640 who met these criteria. Initial treatment consisted of induction chemotherapy, except for brain metastases which were managed first (n=1). If patients experienced a response to chemotherapy without new metastases, the extra-thoracic site was treated for total control with curative dose chemoradiotherapy to the primary site. Survival, time to progression, and sites of progression were assessed.
Results
Median survival was 28 months (95% CI 18-50 mo) with median time to progression of 15 months (95% CI 8-24 mo). All except one patient progressed in the CNS, either with brain metastases (n=7) or leptomeningeal disease (n=1).
Conclusions
Such an approach offers the potential for enhanced quality and quantity of survival by incorporating aggressive RT for select patients without disease progression after induction chemotherapy. Patients tended to fail in the CNS, suggesting the importance of continued surveillance of the neuraxis or possibly prophylactic cranial irradiation. Future plans will correlate outcomes with molecular markers.
Keywords: oligometastatic, non-small cell lung cancer, chemoradiation
Introduction
Radiotherapy is used primarily for palliative purposes in stage IV NSCLC to allow timely and increased chemotherapy delivery. However, patients with single site metastases fare better than higher volume stage IV non-small cell lung cancer (NSCLC), suggesting that is could be worthwhile to reconsider this approach.
Several reviews of prognostic factors showed that the number of sites of metastatic disease impacts survival. Stanley et al showed that prognostic factors for NSCLC include performance status, weight loss, and extent of disease (1). Specifically, weight loss and performance status significantly affect survival (2). In a large analysis by the Southwest Oncology Group involving 2531 patients, a single metastatic site proved to be an independent predictor of improved survival (3). Likewise, another series confirmed that the presence of two or more extrathoracic metastatic organ sites was associated with shorter survival (4).
Overall survival for patients without brain metastases correlates with the number of metastatic sites (i.e. tumor burden). In patients with brain metastases, survival has correlated inversely with the volume of all metastases or the largest lesion (5). For cases with a solitary focus of metastatic disease, controversy exists regarding the optimal management of the primary thoracic NSCLC and the oligometastatic site. Based on the findings from larger data sets, we developed a paradigm of adding concurrent chemotherapy and radiotherapy to the primary tumor and synchronous, solitary metastatic focus once the tumor demonstrated chemotherapy sensitivity.
Methods and materials
Institutional Review Board approval for record review was obtained. A retrospective review of all patients with histological confirmation of non-small cell lung cancer treated at the Cancer Institute of New Jersey, Robert Wood Johnson Medical School-University of Medicine and Dentistry of New Jersey from 1999 to 2006 was conducted.
Review parameters included complete history and physical examination, routine laboratory tests including complete blood count and chemistry panel, and CT scan with intravenous contrast of the chest, abdomen, and pelvis as well as PET scan. Brain MRI was routinely used at our institution for patients with stage III or greater local disease or neurological symptoms or signs. A histological diagnosis of non-small cell lung carcinoma was required.
The eligibility criteria for inclusion of patient records in our study included: 1) Single site of extra-thoracic metastatic disease at the time of initial diagnosis, termed an oligometastatic site; 2) Primary diagnosis of non-small cell lung carcinoma treated with definitive combined modality treatment (CRT) consisting of chemotherapy (ChT) and radiation therapy (RT); 3) ECOG performance status of 0-2. Using these criteria, nine patients with minimal metastatic disease (single extra-thoracic site) who were treated with combined modality were identified.
Initial treatment consisted of induction ChT with 3-6 cycles of platinum based ChT, except for brain metastases, which were managed first (n=1). After initial ChT, the patient's disease was assessed for response. If the tumor showed evidence of response to chemotherapy and no new metastatic sites were seen, the oligometastatic site was treated, in addition to definitive CRT to the primary lung disease. Patients underwent follow-up imaging after treatment and were managed with additional ChT and palliative RT as necessary.
Statistics/endpoints
Survival, time to progression and sites of progression were assessed. Survival was defined as time from onset of treatment to death. Time to progression was defined as time from onset of treatment to first event, including failure at any site, local or distant or death. Survival data were calculated using the Kaplan-Meier product limit method. Survival estimates were obtained using SAS version 9.3.
Results
From January 1999 to December 2006, of 640 NSCLC patients, we identified 9 patients with oligometastatic NSCLC who were evaluated and treated according to our algorithm. Patient characteristics and treatment details are shown in Table 1. Sites of oligometastatic disease included bone (n=3), pericardial fluid (n=2), lymph nodes (retroauricular lymph node n=1, axilla n=1), brain (n=1), and adrenal gland (n=1). This group of retrospectively reviewed patients is highly selected with excellent performance statuses, minimal weight loss (n=6), and initial response to chemotherapy treatment at a tertiary care center.
Table 1. Patient Characteristics and Treatment Details.
| Characteristics | |
| Age at Original Diagnosis, years | |
| Median (range) | 55 (24-72) |
| Gender, number | |
| Male | 5 |
| Female | 4 |
| ECOG Performance Status at Diagnosis, number (%) | |
| 0 | 7 |
| 1 | 2 |
| Histology, number | |
| Adenocarcinoma | 3 |
| Poorly Differentiated Carcinoma | 6 |
| Local Stage of Primary Lung Cancer | |
| T2 N2-3 | 2 |
| T4 Nx-N3 | 7 |
| Radiation Dose to Primary Lung Cancer (n=9) | |
| Median (range) (cGy) | 6400 (5600-7200) |
| Initial PET Staging | |
| Yes | 8 |
| No | 1 |
| Weight loss | |
| Insignificant (0-3% of baseline body weight) | 6 |
| Significant (5-15%) | 3 |
Patients received induction chemotherapy consisting of carboplatin-paclitaxel (n=4), cisplatin-docetaxel (n=2), gemcitabine-carboplatin-paclitaxel (n=2), carboplatin-paclitaxel-bevacizumab (n=1). A total of 3 to 6 cycles of induction chemotherapy were delivered (3 cycles n=2; 4 cycles n=4; 5 cycles n=1, 6 cycles n=2)
Consolidative chemotherapy, after completion of CRT was delivered in 2 patients. 7 patients underwent additional chemotherapy combinations for disease progression. Of these 7 patients, they received one additional systemic combination therapy (n=2); two additional lines of systemic therapy (n=1), three lines (n=2), four lines (n=1), and five lines (n=1).
CRT to the primary lung disease was delivered to a median dose of 64 Gy (range 56-72 Gy) with a median daily dose of 2 Gy (range 1.8-2 Gy) with conformal 3D CT-based treatment. Oligometastatic sites were treated to a median dose of 40 Gy (range 30-60 Gy) with a median daily dose of 2 Gy (range 1.8-3 Gy). For the two patients with pericardial fluid, they received cardiac irradiation to 40 Gy. Concurrent chemotherapy regimens used during radiation therapy consisted of carboplatin/paclitaxel (n=7) for the majority of patients in addition to cisplatin/etoposide (n=1), and one patient received no concurrent chemotherapy during radiation therapy.
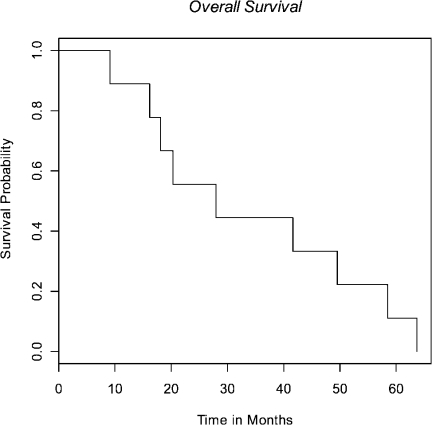
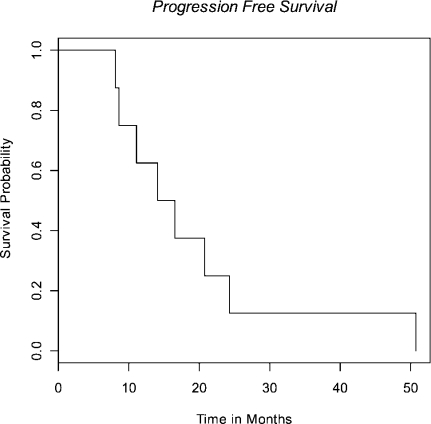
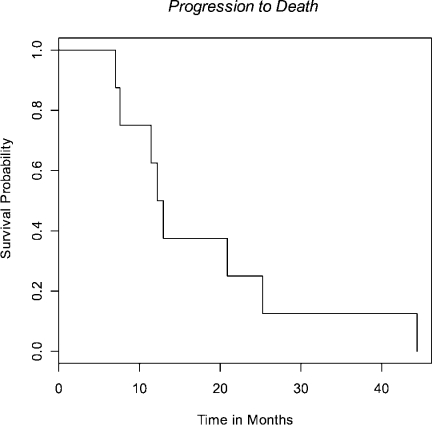
Median survival was 28 months (95% CI 18-50 mo) (Fig 1) with median time to progression of 15 months (95% CI 8-24 mo) (Fig 2). All patients progressed in the CNS, either with brain metastases (n=7) or leptomeningeal disease (n=1), with the exception of local progression in one patient (n=1). Median time from failure to death was 13 months (95% CI 8-25 mo) (Fig 3).
Fig 1. Kaplan-Meier survival estimate from date of treatment initiation.
Fig 2. Progression-free survival.
Fig 3. Time from progression to death.
Discussion
Based on the suggestion that oligometastatic NSCLC patients fare better, we employed aggressive radiotherapy in chemosensitive disease. We took advantage of the fact that patients with oligometastatic NSCLC tend to have better outcomes when compared to patients with a higher burden of metastatic disease. Our review suggests that we identified a select population who achieve a much better outcome using a more aggressive approach with induction chemotherapy followed by concurrent thoracic CRT and radiation to the solitary synchronous site of oligometastasis. Our approach differs by focusing on patients with chemosensitive disease. The median survival in this series exceeds those of previous reports, approaching that of locally advanced NSCLC. We identified at least five patients whose disease did not respond to initial chemotherapy. For those with chemosensitive disease, this approach is highly effective. As we apply this in a formal prospective protocol, we will be better able to collect the ratios of patients who do not achieve chemotherapy response.
One arena of this approach that has received some attention is the group with single site CNS metastases. Multiple reports have focused on treatment of synchronous NSCLC brain metastases with neurosurgery combined and thoracic surgery for the primary lung cancer (Table 2).
Table 2. Series of resection for both lung cancer and solitary brain metastasis.
| Author | Patients (n) | Years of Study | 1, 2, 5-year Survival (%) |
| Bonnette (29) | 103 | 1985-1998 | 56, 28, 11 |
| Billing (8) | 28 | 1975-1997 | 64, 54, 21 |
| Burt (6) | 185 (Sync, Meta) | 1974-1989 | 55, 27, 18 |
| Magilligan (7) | 41 | 1960-1985 | 55, 31, 21 |
| Rossi (30) | 40 (Sync, Meta) | Not specified | 35,25,12.5 |
Sync: synchronous; Meta: metachronous solitary metastases
Burt et al and Magilligan et al suggested that complete resection of the primary tumor was a more important prognostic factor than surgical stage (6),(7). However, Billing et al found that the presence of thoracic lymph node metastases significantly affected 5-year survival (8). In patients with evidence of pathological lymph node metastases, 5-year survival was 0% versus 35% in those without lymph node involvement.
Series incorporating stereotactic radiosurgery (SRS) for solitary brain metastases from NSCLC demonstrate comparable survival rates to surgery. Flannery et al reported a median survival of 33 and 9 months and 5-year survival of 13% and 8% for metachronous and synchronous brain metastases, respectively (9). SRS provides good local control and can be considered a reasonable alternative to craniotomy in patients who are neurologically stable (10). The optimal management of a solitary brain metastasis is controversial and some argue that local control rates are inferior with SRS compared to surgery (11). Others favor the addition of whole brain RT to SRS for improved local control (12),(13). In spite of these reports, the determinants of a patient's outcome in the setting of brain metastases include performance status, age, control of extracranial disease, and quantity (number and volume) of brain metastases (14),(15).
Hu et al evaluated 84 patients with solitary synchronous brain metastases treated either with neurosurgery or SRS. Thoracic disease was managed either with RT or surgery. Thoracic stage was found to influence survival, with stage I patients experiencing an unexpectedly favorable outcome (median survival of 27 months), which was comparable to stage I disease without brain metastases, warranting aggressive treatment of the lung (11). Flannery also showed that definitive thoracic RT significantly affected overall survival in the setting of synchronous, solitary brain metastases treated with RS, with a 5-year overall survival of 21% (16).
Solitary adrenal metastases treated with adrenalectomy have revealed long-term survival rates of greater than 5 years (17), 6 and 14 years (18), and 9 years after bilateral adrenalectomy for bilateral metastases (19). It is clear that chemotherapy alone for solitary adrenal metastases portends a worse outcome, with median survivals of 9 months compared to 31 months with surgery and chemotherapy (20). In a multi-institutional review, Porte et al showed median survival of 11 months for both synchronous and metachronous adrenal metastases with 3 patients surviving more than 5 years (21). Approaches incorporating both chemotherapy and surgery for patients with synchronous solitary metastases, with 3 cycles of induction chemotherapy followed by resection of all disease sites followed by consolidative chemotherapy showed a median survival of 11 months (22).
Two series incorporated chemoradiation therapy for synchronous oligometastatic NSCLC. Khan et al showed a median survival of 20 months for those treated with thoracic CRT and either surgery followed by RT or stereotactic radiosurgery alone to the metastatic focus (1-2 sites permitted) (23). A report from Japan of two patients both with synchronous brain metastases received chemotherapy on day 1 with whole brain RT, and on day 29 with thoracic RT, with survivals of at least 37 and 53 months (24).
The incidence of oligometastatic NSCLC is quite low, as Ambrogi et al report a 2% incidence of patients who were able to undergo resection of both the primary lung tumor and metastatic focus (25). Although 5 year-survival was 56%, all patients with N2 disease died, patients with more advanced thoracic disease would not have undergone thoracic surgery.
The advent of PET scanning may change the detection rate of true solitary synchronous metastases. Most of the previously mentioned studies did not uniformly incorporate PET scanning. Analysis of 1,509 patients who underwent PET scan detected only 10 patients who satisfied the criteria for synchronous hematogenous solitary metastasis (26). Median overall survival was 26 months. It is possible that many of the “solitary” metastases detected were truly multiple at the time of diagnosis but could not be detected at initial staging in prior studies. This could upstage oligometastatic patients, making outcomes worse or potentially could treat patients definitively when in fact oligometastatic disease is present, contributing to the variety of outcomes mentioned above.
Conclusion
Single modality therapy in NSCLC, except for stage I disease, is unlikely to produce long-term overall survival. Combined RT and chemotherapy has shown considerable advantage in stage III NSCLC (27),(28). In the case of oligometastatic NSCLC, CRT is a unique approach if we can select the appropriate chemosensitive patients. With the advancement of stereotactic body radiosurgery and improved thoracic radiation therapy techniques, this paradigm is particularly appealing.
It is clear that aggressive management of both the primary and oligometastatic site offer the potential for enhanced quality and quantity of survival. We advocate an aggressive RT approach after the use of induction chemotherapy, except in the setting of brain metastases, which should be treated first. Given the abundance of single institution data resulting in prolonged survival rates, a prospective phase II multi-institutional clinical trial would be appropriate for this group of patients. The North Central Cancer Treatment Group (NCCTG) initiated a trial, N0724 to address this topic. Patients with 3 or fewer metastases were to receive chemotherapy and then were to be randomized to either RT to all residual cancer or no RT. However accrual has been slow, possibly due either to the rarity of this scenario or to the no RT arm, since the addition of RT or resection of the metastases is known to result in relatively favorable outcomes. As we learn more about molecular markers, we believe that we can achieve even better outcomes with the application of individually selected therapy in oligometastatic NSCLC.
Footnotes
Presented at the American Society of Radiation Oncology (ASTRO) 50th Annual Meeting 2008, Boston, MA, USA.
References
- 1.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 2.Pater JL, Loeb M. Nonanatomic prognostic factors in carcinoma of the lung: a multivariate analysis. Cancer. 1982;50:326–31. doi: 10.1002/1097-0142(19820715)50:2<326::aid-cncr2820500227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell JP, Kris MG, Gralla RJ, Groshen S, Trust A, Fiore JJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol. 1986;4:1604–14. doi: 10.1200/JCO.1986.4.11.1604. [DOI] [PubMed] [Google Scholar]
- 5.Oh Y, Taylor S, Bekele BN, Debnam JM, Allen PK, Suki D, et al. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer. 2009;115:2930–8. doi: 10.1002/cncr.24333. [DOI] [PubMed] [Google Scholar]
- 6.Burt M, Wronski M, Arbit E, Galicich JH. Resection of brain metastases from non-small-cell lung carcinoma. Results of therapy. Memorial Sloan-Kettering Cancer Center Thoracic Surgical Staff. J Thorac Cardiovasc Surg. 1992;103:410–1. discussion. [PubMed] [Google Scholar]
- 7.Magilligan DJ, Jr, Duvernoy C, Malik G, Lewis JW, Jr, Knighton R, Ausman JI. Surgical approach to lung cancer with solitary cerebral metastasis: twenty-five years' experience. Ann Thorac Surg. 1986;42:360–4. doi: 10.1016/s0003-4975(10)60536-x. [DOI] [PubMed] [Google Scholar]
- 8.Billing PS, Miller DL, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg. 2001;122:548–53. doi: 10.1067/mtc.2001.116201. [DOI] [PubMed] [Google Scholar]
- 9.Flannery TW, Suntharalingam M, Kwok Y, Koffman BH, Amin PP, Chin LS, et al. Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer. 2003;42:327–33. doi: 10.1016/s0169-5002(03)00357-x. [DOI] [PubMed] [Google Scholar]
- 10.Simonova G, Liscak R, Novotny J, Jr, Novotný J. Solitary brain metastases treated with the Leksell gamma knife: prognostic factors for patients. Radiother Oncol. 2000;57:207–13. doi: 10.1016/s0167-8140(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 11.Hu C, Chang EL, Hassenbusch SJ, 3rd, Allen PK, Woo SY, Mahajan A, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106:1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 12.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 13.Chidel MA, Suh JH, Greskovich JF, Kupelian PA, Barnett GH. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Investig. 1999;7:313–9. doi: 10.1002/(SICI)1520-6823(1999)7:5<313::AID-ROI7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 16.Flannery TW, Suntharalingam M, Regine WF, Chin LS, Krasna MJ, Shehata MK, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys. 2008;72:19–23. doi: 10.1016/j.ijrobp.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 17.de Perrot M, Licker M, Robert JH, Spiliopoulos A. Long-term survival after surgical resections of bronchogenic carcinoma and adrenal metastasis. Ann Thorac Surg. 1999;68:1084–5. doi: 10.1016/s0003-4975(99)00654-2. [DOI] [PubMed] [Google Scholar]
- 18.Twomey P, Montgomery C, Clark O. Successful treatment of adrenal metastases from large-cell carcinoma of the lung. JAMA. 1982;248:581–3. [PubMed] [Google Scholar]
- 19.Urschel JD, Finley RK, Takita H. Long-term survival after bilateral adrenalectomy for metastatic lung cancer: a case report. Chest. 1997;112:848–50. doi: 10.1378/chest.112.3.848. [DOI] [PubMed] [Google Scholar]
- 20.Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996;62:1614–6. doi: 10.1016/s0003-4975(96)00611-x. [DOI] [PubMed] [Google Scholar]
- 21.Porte H, Siat J, Guibert B, Lepimpec-Barthes F, Jancovici R, Bernard A, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg. 2001;71:981–5. doi: 10.1016/s0003-4975(00)02509-1. [DOI] [PubMed] [Google Scholar]
- 22.Downey RJ, Ng KK, Kris MG, Bains MS, Miller VA, Heelan R, et al. A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer. 2002;38:193–7. doi: 10.1016/s0169-5002(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 23.Khan AJ, Mehta PS, Zusag TW, Bonomi PD, Penfield Faber L, Shott S, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC) Radiother Oncol. 2006;81:163–7. doi: 10.1016/j.radonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Harita S, Mizuta A, Kuyama S, Kikuchi T. Long-term survival following concurrent chemoradiotherapy in patients with non-small-cell lung cancer with concomitant brain metastases only. Int J Clin Oncol. 2005;10:63–8. doi: 10.1007/s10147-004-0436-y. [DOI] [PubMed] [Google Scholar]
- 25.Ambrogi V, Tonini G, Mineo TC. Prolonged survival after extracranial metastasectomy from synchronous resectable lung cancer. Ann Surg Oncol. 2001;8:663–6. doi: 10.1007/s10434-001-0663-7. [DOI] [PubMed] [Google Scholar]
- 26.De Pas TM, de Braud F, Catalano G, Putzu C, Veronesi G, Leo F, et al. Oligometastatic non-small cell lung cancer: a multidisciplinary approach in the positron emission tomographic scan era. Ann Thorac Surg. 2007;83:231–4. doi: 10.1016/j.athoracsur.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–9. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 28.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 29.Bonnette P, Puyo P, Gabriel C, Giudicelli R, Regnard JF, Riquet M, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119:1469–75. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 30.Rossi NP, Zavala DC, VanGilder JC. A combined surgical approach to non-oat-cell pulmonary carcinoma with single cerebral metastasis. Respiration. 1987;51:170–8. doi: 10.1159/000195199. [DOI] [PubMed] [Google Scholar]