Abstract
Non-small cell lung cancer constitutes about 85% of all newly diagnosed cases of lung cancer and continues to be the leading cause of cancer-related deaths worldwide. Standard treatment for this devastating disease, such as systemic chemotherapy, has reached a plateau in effectiveness and comes with considerable toxicities. For all stages of disease fewer than 20% of patients are alive 5 years after diagnosis; for metastatic disease the median survival is less than one year. Until now, the success of active-specific immunotherapy for all tumor types has been sporadic and unpredictable. However, the active-specific stimulation of the host’s own immune system still holds great promise for achieving non-toxic and durable antitumor responses. Recently, sipuleucel-T (Provenge®; Dendreon Corp., Seattle, WA) was the first therapeutic cancer vaccine to receive market approval, in this case for advanced prostate cancer. Other phase III clinical trials using time-dependent endpoints, e.g. in melanoma and follicular lymphoma, have recently turned out positive. More sophisticated specific vaccines have now also been developed for lung cancer, which, for long, was not considered an immune-sensitive malignancy. This may explain why advances in active-specific immunotherapy for lung cancer lag behind similar efforts in renal cell cancer, melanoma or prostate cancer. However, various vaccines are now being evaluated in controlled phase III clinical trials, raising hopes that active-specific immunotherapy may become an additional effective therapy for patients with lung cancer. This article reviews the most prominent active-specific immunotherapeutic approaches using protein/peptide, whole tumor cells, and dendritic cells as vaccines for lung cancer.
Keywords: vaccine, lung cancer, NSCLC, immunotherapy
Introduction
For long, lung cancer was not considered an immune-sensitive malignancy. However, there is increasing evidence that both non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) can evoke specific humoral and cellular antitumor immune responses. With increasing knowledge about the link between the induced immune response and a resulting objective clinical response, lung cancer vaccines may hold great promise in sequence and/or combination with other anti-tumor treatment modalities such as surgery, chemotherapy and radiation. Lung cancer is still the deadliest cancer in the world and killed an estimated 157,300 in the US in 2010 including 15,000 to 20,000 “never smokers” (1),(2). Despite progress in the treatment of lung cancer over the past two decades, there are still few long-term survivors: only about 10% of all patients will ever be cured of this devastating disease.
The optimal chemotherapy regimens available (platinum-based regimens including paclitaxel, docetaxel, gemcitabine or vinorelbine) have demonstrated only limited activity; there are modest improvements in overall survival with median survival rates of less than 11 months and 1-year survival rates of 31% to 36%. Most large randomized trials assessing systemic chemotherapy for lung cancer were performed in patients with metastatic disease, who represent the majority of NSCLC patients (3)-(5). Systemic chemotherapy is also modestly effective in the adjuvant setting, however, there is considerable toxicity. In summary, systemic chemotherapy for lung cancer of all stages is given with palliative intent in metastatic disease and has only modest effects on survival.
In the search for novel therapeutic approaches, the active-specific stimulation of the host’s own immune system holds great promise for achieving non-toxic and durable antitumor responses. Recently, sipuleucel-T (Provenge®; Dendreon Corp., Seattle, WA) became the first therapeutic cancer vaccine to receive market approval in the US based on the prolongation of overall survival (OS) among men with metastatic castration-resistant prostate cancer (6). Driven by this outcome and by positive results of recent clinical trials in other tumor types, such as follicular lymphoma and metastatic melanoma (7)-(10), a positive momentum for active-specific immunotherapy has arisen. Now, also for lung cancer, more complex vaccines are available and promising results from early phase clinical trials have cleared the way for large randomized phase III trials. This paper reviews the most important vaccination approaches that have found their way into late stage clinical development for both early and advanced NSCLC.
Therapeutic lung cancer vaccines
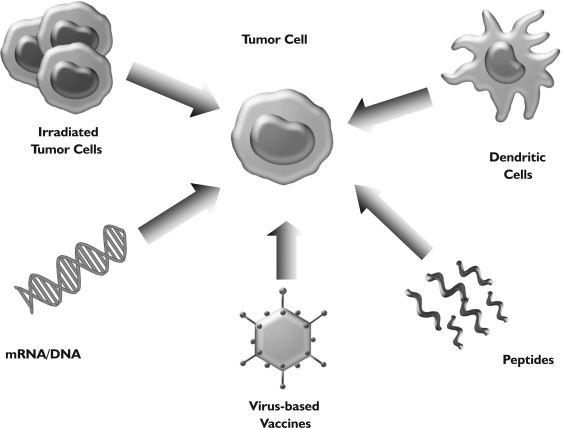
In contrast to prophylactic vaccination, which is employed against infectious diseases or cancers associated with viral infection (cervical cancer, hepatocellular carcinoma), the only relevant vaccination strategy for cancer patients must be therapeutic. Generally, cancer vaccines incorporate a source of tumor antigens combined with some type of “adjuvant”. Vaccine adjuvants are components that potentiate the immune response to an antigen and/or modulate it towards the desired immune responses (also referred to as “immune potentiators” or “immunomodulators”). Sources of tumor-associated antigens include whole autologous or allogeneic tumor cells, lysates of tumor cells, defined proteins, specific peptide epitopes or mRNA/DNA encoding for relevant antigens (Fig 1). Most likely due to the heterogeneous histology of lung cancers, the relevant immunologically dominant antigens are not known; however, there has been progress in this area (e.g. MUC-1, MAGE-3). Therefore, the use of autologous tumor cells might be particularly suitable for vaccination strategies in lung cancer, because no prior knowledge of specific tumor antigens is necessary and the induced immunity may not be confined to a single, specific antigen that could be downregulated by the tumor. However, using defined antigens such as e.g. specific peptide epitopes, may allow for easier monitoring of the resulting immune response and may facilitate the understanding of the interplay between a systemic immune response and anti-tumor activity of a specific lung cancer vaccine. Figure 2 illustrates the proposed mechanism of action of therapeutic cancer vaccines in order to induce a systemic immune response against relevant tumor rejection antigens. The following paragraphs review the most prominent vaccination approaches in lung cancer ranging from non-specific immune stimulation to combination therapies.
Fig 1. Therapeutic vaccination in lung cancer. Various approaches are currently being used to vaccinate patients: inactivated, whole (autologous or allogeneic) tumor cells, formulations of relevant tumor antigens (mRNA/DNA, proteins, peptides), dendritic cells or virus-based vaccines.
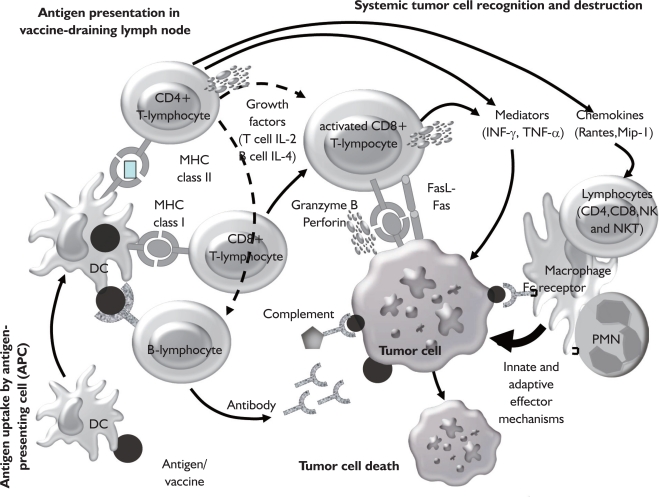
Fig 2. Induction of an immune response by antigen vaccination. Active immunization occurs following administration of tumor antigens, which are processed by antigen-presenting cells resulting in activation of immune effector cells such as T lymphocytes and B lymphocytes. Effector cells fight the tumor through several different effector pathways such as antibodies, cytokines or direct cellular interaction (Fas/Fas ligand, perforin/granzymes). Ideally this results in immunologic memory with long-lasting immunity against the tumor. Stimulation of the innate immune system is also known to have an important role in the evolution of an adaptive immune response (e.g. a rapid burst of inflammatory cytokines leads to activation of DC and macrophages). However, tumor-specific T cells also secrete chemokines (e.g. RANTES, MIP-1) that attract cells of the innate immune system to the tumor site. This mechanism may further contribute to the tumoricidal activitiy exhibited during T cell-mediated tumor regression. (TCR: T cell receptor, MHC: major histocompatibility complex, DC: dendritic cell, PMN: polymorphonuclear neutrophil, NK: natural killer cell, NKT: NK T cell, RANTES: regulated upon activation, normal T cell expressed, and secreted, MIP-1: macrophage inflammatory protein 1).
Non-specific vaccine approaches
The best known non-specific vaccine approach for NSCLC is a preparation of killed Mycobacterium vaccae (SRL172) in combination with systemic chemotherapy. With this strategy, the non-specific immune stimulation is supposed to enhance recognition of tumor antigens that are released by chemotherapy-induced tumor destruction. After a small phase II study with 20 previously untreated NSCLC patients produced promising results (11), a phase III randomized trial was initiated. With almost 200 advanced NSCLC patients in the vaccination cohort, this trial failed to demonstrate a survival improvement for vaccination and chemotherapy compared to chemotherapy (mitomycin-C, vinblastine and cisplatin or carboplatin) alone (12). Improved quality of life was found in patients randomized to vaccine plus chemotherapy and retrospective analyses revealed a significant increase in survival in adenocarcinoma patients that received more than 2 injections of SRL172. However, only 30 patients (14%) received one or more SRL172 injections after the treatment phase, i.e. 63% of patients did not receive further injections after the end of the 15-week treatment phase (12),(13). Here, the natural history of different NSCLC histologies, i.e. less aggressive tumors enable patients to receive more treatment without documented progressive disease, may contribute to differences in these retrospective analyses.
GVAX
The genetic modification of autologous tumor cells to secrete immunomodulatory cytokines has been shown to induce antitumor immunity in a number of preclinical models. Of these cytokines, GM-CSF has demonstrated the greatest induction of antitumor immunity (14). Two early phase clinical trials using GM-CSF-secreting, autologous tumor cells (GVAX) in patients with NSCLC have revealed encouraging preliminary results. Salgia and coworkers reported on safety and feasibility of this approach in 33 advanced NSCLC patients; the most common toxicities were local injection site reactions and flu-like symptoms. A mixed response in one patient and long recurrence-free intervals in two other patients following isolated metastasectomy were observed (15). In another phase I/II trial involving patients with early-stage (n=10) and advanced-stage (n=33) NSCLC, using the GVAX platform, autologous tumor cells were transduced with GM-CSF through an adenoviral vector (Ad-GM) and administered as a vaccine (16). Seventy-eight percent of patients developed antibody reactivity against allogeneic NSCLC cell lines. Three durable complete responses were observed. Interestingly, two of these responses were seen in patients with bronchoalveolar carcinoma. Subset analyses in this trial demonstrated a correlation between the amount of GM-CSF secreted by the vaccine and survival.
In an effort to have high GM-CSF secretion at a constant level, autologous NSCLC cells were mixed with an allogeneic GM-CSF-secreting cell line (K562 cells) (“bystander” GVAX). The Phase I/II trial of this vaccine failed, however, to produce objective tumor responses in 49 patients despite significant increased GM-CSF secretion (17). While the reason for the failure remains unknown, the significant increase in GM-CSF secretion by the “bystander” GVAX (25-fold higher than the autologous vaccine) may have had a negative effect. In this context, Serafini and coworkers have reported, that tumor vaccines that secrete high levels of GM-CSF induce myeloid suppressor cells that, in turn, inhibit anti-tumor immunity (18). Currently, there are no ongoing clinical studies in lung cancer using the GVAX vaccine platform.
MUC1 vaccines
Mucin-1 (MUC1) is a highly glycosylated type 1 transmembrane protein with a molecular weight of 200 kD that is expressed on the cell surface of many common adenocarcinomas, including lung cancer. Because of its involvement in cell-cell interaction between malignant and endothelial cells, MUC1-targeted strategies may be useful in preventing metastatic spread of tumor cells in addition to mediating direct anti-tumor effects. MUC1 has been targeted using a variety of approaches including dendritic cells (DC), recombinant vaccinia virus-expressing MUC1, MUC1 peptide, antibody-based strategies, and liposo-mal delivery of MUC1 (19),(20).
A phase I study using a vaccine consisting of a modified vaccinia virus (Ankara) expressing human MUC1, which also contains a coding sequence for human IL-2 (MVA-MUC1-IL2), reported a safe toxicity profile and some clinical activity (19). A subsequent multicenter phase II trial of this vaccine, called TG4010, investigated the combination of TG4010 with first-line chemotherapy in patients with stage IIIB/IV NSCLC (21). TG4010 was either combined upfront with cisplatin/vinorelbine or administered as single-agent until disease progression and then followed by TG4010 and cisplatin/vinorelbine. Sixty-five patients were enrolled, 44 in the combination arm and 21 in the sequential arm. In the combination arm, the objective response rate was 29.5%. In the sequential arm, two patients experienced stable disease lasting more than 6 months with single-agent TG4010; in the subsequent combination part, one complete and one partial response were observed in 14 patients (14.3%). The 1-year survival rates were 53% and 60% for the combination and sequential groups, respectively. TG4010 was well tolerated with mild to moderate injection site reactions and constitutional symptoms being the most frequent adverse events. In a subsequent phase IIB study, patients were treated with cisplatin/gemcitabine with or without TG4010 administered weekly for 6 weeks and then every 3 weeks until disease progression. Seventy-four patients were included in each treatment arm. The primary endpoint of this study was met with progression-free survival rates at 6 months of 44% in the combination arm and 35% in the chemotherapy alone group. Response rates were 43% and 27%, respectively. Interestingly, patients with normal levels of lymphocytes and an activated natural killer cell phenotype at baseline experienced a longer survival for TG4010 plus chemotherapy but not for chemotherapy alone (22). A pivotal phase IIB/III study of TG4010 in combination with first-line therapy in patients with advanced MUC1 expressing NSCLC and with normal levels of activated natural killer cells is expected to start in Q4/2010.
The extracellular core peptide of MUC1 is targeted by a liposomal vaccine named L-BLP25 (Stimuvax). In addition to the MUC1 peptide, L-BLP25 consists of a monophosphoryl lipid as an adjuvant and three other lipids to facilitate delivery and uptake of the vaccine components by immune cells. A phase I study with 17 patients suffering from NSCLC stages IIIB and IV evaluated the safety and immunogenicity of L-BLP25 (23). Two patients developed clinically insignificant grade 3 lymphopenia and non-hematological adverse events were mild and self-limiting. No objective tumor responses were observed, however, 5 out of 12 evaluable patients developed a MUC1-specific T cell response. Based on the excellent overall safety profile and the observation of a median OS was 14.6 months in the higher of two dose cohorts, a multicenter phase IIB study investigating the vaccine in NSCLC patients with stages IIIB and IV disease was conducted. All patients had stable disease or a clinical response after standard first-line chemotherapy, after which patients were either vaccinated with L-BLP25 or received best supportive care. Although OS did not reach statistical significance, the survival of patients in the vaccine arm with stage IIIB (locoregional disease) was improved at 3 years compared to stage IIIB patients with malignant pleural effusion and stage IV patients with 48.6% and 26.7%, respectively (24),(25). Very recently, the safety and clinical activity of a new formulation of BLP25, which will be used in the phase III program, were confirmed (26). An international, randomized, multicenter phase III trial called START (Stimulating Targeted Antigenic Response to NSCLC) for unresectable stage III NSCLC patients with stable disease or better following first-line chemoradiation is currently underway. This trial will enroll 1,322 patients and OS will be the main endpoint.
MAGE-A3 protein vaccine
The melanoma-associated antigen (MAGE)-A gene family is of particular interest as target for active-specific immunotherapy since – as a so-called cancer testis antigen – it is expressed on cancer cells but not normal tissue. MAGE-A3 is expressed in about 30% - 50% of lung cancers depending on stage and histological subtype and may be associated with poor prognosis (27). The successful induction of humoral and cellular immune responses in patients with NSCLC following vaccination with MAGE-3 with and without adjuvant chemotherapy was reported in 2004 (28). Seventeen patients with no evidence of disease following surgical resection were enrolled. Nine patients received 300µg of the MAGE-3 protein alone, whereas 8 patients were treated with MAGE-3 combined with the adjuvant AS02B. In the first cohort (no adjuvant), only one patient showed a MAGE-3-specific CD4+ T cell response. In contrast, 4 patients in the second cohort (MAGE-3 plus adjuvant) developed a CD4+ T cell response against the MAGE-3 DP4-peptide. Based on these results, a multinational phase II trial investigating the therapeutic efficacy of the MAGE-3 vaccine in patients with resected MAGE-3-positive stage IB/II NSCLC was performed. In this placebo-controlled study, 182 patients (122 stage IB and 60 stage II MAGE-3-positive NSCLC) were vaccinated five times at 3-week intervals following surgical resection of their tumors. Final results presented at the 2007 ASCO annual meeting (29) revealed a 27% disease-free survival improvement for vaccinated compared to placebo-treated patients. No significant toxicities were observed. Based on these results a large international, multicenter phase III study (MAGRIT), in which 2,270 resected MAGE-A3-positive patients will be randomized to either vaccine or placebo, with disease-free survival being the primary endpoint, is currently enrolling patients (30).
Belagenpumatucel (Lucanix)
Elevated levels of transforming growth factor (TGF)-β2 are frequently linked to immunosuppression (counteracting NK cell activity and suppressing dendritic cells) in lung cancer patients and may be inversely correlated with prognosis in NSCLC (31). Belagenpumatucel-L (Lucanix; NovaRx Corp., San Diego, USA) is a non-viral, gene-modified allogeneic vaccine with potential immunostimulatory and antineoplastic activities. It is prepared by transfecting 4 different NSCLC cell lines with a plasmid containing a TGF-β2 antisense transgene, expanding the cells, and then irradiating them. Upon administration, this agent may elicit a specific T cell response against host NSCLC cells. The vaccine’s immunogenicity may be potentiated by suppression of tumor TGF-β2 production by the antisense RNA expressed by the vaccine plasmid TGF-β2 antisense transgene.
Belagenpumatucel-L was first evaluated in a phase II clinical trial involving 75 patients with NSCLC stages II-IV (32). Patients received intradermal vaccine injections at 3 different doses monthly or every other month up to a maximum of 16 injections. Treatments were well tolerated with no severe side effects. A dose-related improvement in survival was noted in patients who received at least 2.5 x 107 cells/injection. A 15% partial response rate was achieved in the 61 late-stage (IIIB and IV) assessable patients. Within the same group of patients an increase in cytokine production (interferon gamma, IL-6, IL-4) was observed among clinical responders who also displayed an elevated antibody response to vaccine HLA antigens.
Based on these data, a placebo-controlled phase III clinical trial, called STOP, has been initiated; an estimated 700 patients with stage III/IV NSCLC following front-line chemotherapy will be recruited. Overall survival will be the primary endpoint.
Epidermal growth factor vaccine (EGF-rP64K/Montanide ISA 51)
To induce a systemic immune response against growth factors and/or their receptors seems an obvious strategy in fighting cancer because they are known to augment tumor cell proliferation and invasion and have been shown to be overexpressed in many solid malignancies where the overexpression has been associated with a more aggressive course of disease and poor survival (33). C-erb B-1 and c-erb B-2 are the two growth factor receptor families that have been studied most extensively. C-erb B-1 is better known under the name HER1 or epidermal growth factor receptor (EGFR). An active immunotherapeutic strategy was evaluated using a vaccine that consists of human recombinant EGF linked to a recombinant carrier protein from Neisseria meningitides and is administered with Montanide ISA 51 (34). Two pilot clinical trials have been conducted to assess safety and immunogenicity of the vaccine and to optimize the adjuvant and treatment schedule. Pooled data from both trials revealed that inclusion of Montanide ISA 51 lead to a greater percentage of antibody responses which, in turn, correlated with better survival when compared to patients who did not develop a good antibody response (35). Another phase I trial evaluated the vaccine in 43 patients with advanced NSCLC following front-line therapy (36). No major adverse events were recorded. Thirty-nine percent of the patients developed a robust antibody response against EGF with a median OS of 8.23 months in all vaccinated patients. A pooled analysis of all these early phase trials confirmed the survival benefit for the 83 patients who had received the vaccine (37), which formed the basis for a randomized (best supportive care (BSC) as comparator) phase II clinical trial in 80 advanced NSCLC patients, who had received first-line chemotherapy (38),(39). The median OS was 6.47 months in the treatment arm compared to 5.33 months in the BSC group. Median OS for patients who developed a robust antibody response against EGF was 11.7 months. A significant positive correlation was found between antibody titer, EGF-EGFR binding inhibition, immunodominance of anti-EGF antibodies, and survival. Currently, a phase II/III trial is on its way in Malaysia looking to recruit 230 patients with advanced NSCLC after first-line therapy.
Dendritic cell-based vaccines
Dendritic cells (DC) pulsed with different antigens have been used to vaccinate lung cancer patients. In a phase I/II clinical trial, 16 patients with NSCLC stages IA to IIIB were vaccinated with DC loaded with apoptotic bodies of an allogeneic NSCLC cell line overexpressing HER2/neu, MAGE-2 and other tumor antigens, after receiving treatment with surgery, chemoradiation or multimodality therapy (40). The autologous DC were matured using DC/T cell-derived Maturation Factor (DCTCMF). Six patients showed an antigen-specific response following vaccination, however, the clinical outcome failed to show a clear correlation with the induced immune responses. In a continuation study in 14 NSCLC patients (stages I, II and III) using an immature DC vaccine, Hirschowitz and co-workers reported immunological responses in 4/7 stage III unresectable, and 6/7 stage I/II surgically resected patients. One of seven resected patients recurred and 4/7 stage III patients progressed; 3/5 patients with progressive disease showed no immunological response (41). Recently, the same group reported early clinical results using the DC vaccine 1650-G (which incorporates the same antigens as in the earlier studies) in combination with granulocyte/macrophage-colony stimulating factor (GM-CSF) (42).
Another DC-based vaccination approach used s.c./intradermal injections of ex vivo generated dendritic cells modified with a recombinant fowlpox vector encoding carcinoembryonic antigen (CEA) and a triad of costimulatory molecules (rF-CEA(6D)-TRICOM) (43). Although only very few lung cancer patients were treated with this vaccine, potent anti-CEA immune responses were observed. Further studies using DC-based vaccines in NSCLC are currently underway.
Other vaccination strategies
Additional vaccines for lung cancer are currently being tested in clinical trials, but a review including all strategies in clinical development would go beyond the scope of this paper [earlier phase clinical data recently reviewed in (44)]. However, we believe a few approaches should be mentioned in brief because of their promising early clinical results:
IDM-2101 vaccine (previously EP-2101), a multi-peptide vaccine containing 10 lung cancer epitopes (e.g. p53, CEA, HER2/neu, MAGE-2/3) (45)
α(1,3)-Galactosyltransferase (agal), a vaccine made out of 3 irradiated lung cancer cell lines gene-modified to express xenotransplantation antigens by retroviral transduction with the murine α-gal gene (46)
B7.1 vaccine, an allogeneic whole cell-based vaccine expressing the co-stimulatory molecule B7.1 (CD80) (47)
Anti-idiotype vaccine using the monoclonal antibody racotumomab (formerly known as 1E10), a vaccine targeting a N-glycosyl (NGc) ganglioside (48).
Other strategies attempt to manipulate the host in order to improve the results of vaccination (49). The combination of immunotherapy and chemotherapy has been shown to augment the immune response in both preclinical and human trials. Recent preclinical studies suggest an even higher therapeutic efficacy of cancer vaccines if the host was made lymphopenic and reconstituted with autologous peripheral blood mononuclear cells (PBMC) prior to vaccination (49). Dudley et al combined the adoptive transfer of both CD4+ and CD8+ tumor-infiltrating lymphocytes (in this case without vaccination) in patients with metastatic melanoma with a non-myeloablative chemotherapy regimen and observed impressive clinical response rates (50),(51). Currently, clinical trials are ongoing that aim to transfer parts of this strategy to the treatment of other solid tumors such as lung cancer combining preparative chemotherapy with adoptive transfer of peripheral blood T cells and vaccination. In this strategy, patients receive immunomodulatory doses of cyclophosphamide and fludarabine and a reinfusion of autologous PBMC prior to therapeutic vaccination with irradiated autologous tumor cells in combination with the continuous infusion of GM-CSF at the vaccination site (52).
Table 1 summarizes recent positive clinical phase III trials using therapeutic cancer vaccines and currently ongoing randomized phase III trials in NSCLC including the clinicaltrials.gov identifier as a reference.
Table 1. Recent positive clinical phase III trials using therapeutic cancer vaccines (upper panel) and currently ongoing randomized phase III trials in NSCLC (lower panel).
| Vaccine | Target/Approach | Patients/Treatment | Results (vs control) | Trial/Reference |
| Provenge® (Sipuleucel-T) | Dendritic cells + prostatic acid phosphatase (PAP) | •Metastatic, androgen-independent prostatic adenocarcinoma (n=512) | •Median survival 25.8 months vs 21.7 months | IMPACT/(6) |
| •Co-administration with GM-CSF | •31.7% 3-year survival rate vs 23.0 % | |||
| BiovaxID | Autologous tumor cells fused with murine/human heterohybridoma to produce idiotype (then conjugated to KLH) | •follicular lymphoma in complete remission for at least 6 months following PACE CTX | •Of 177 randomized patients, 117 maintained CR ≥ 6 months after PACE CTX | BV 301/(7) |
| •Co-administration with GM-CSF | •Median time to relapse: 44.2 months vs 30.6 months | |||
| Gp100 antigen + Montanide ISA | Modified gp100:209-217 (210M) peptide | •HLA-A0201 positive, locally advanced stage III or IV cutaneous melanoma | •Objective response rate 22.1% vs 9.7% | CCCGHS-NCI-T98-0085/(8) |
| •Vaccine followed by high dose IL-2 vs IL2 alone | •PFS 2.9 months vs 1.6 months | |||
| Stimuvax® (L-BLP 25) | MUC1 | •Unresectable stage III NSCLC | Pending | START/NCT00409188 |
| •SD or better after first line RCT | ||||
| •Estimated enrollment: 1322 | ||||
| •Primary endpoint: survival | ||||
| recMAGE-A3 + AS15 | MAGE-A3 | •Resectable stages IB-IIIA NSCLC | Pending | MAGRIT/NCT00480025 (30) |
| •MAGE-A3-expression on tumor | ||||
| •Estimated enrollment: 2270 | ||||
| •Primary endpoint: DFS | ||||
| EGF-rP64K + Montanide ISA51 | EGF | •NSCLC stage IIIB/IV | Pending | NCT00516685 |
| •Estimated enrollment: 230 | ||||
| •Primary endpoint: OS | ||||
| Lucanix® (Belagenpumatucel-L) | Allogeneic cell lines + TGFβ antisense | •NSCLC stages III/IV | Pending | STOP/NCT00676507 |
| •SD or better following front-line platinum-based CTX | ||||
| •Estimated enrollment: 700 | ||||
| •Primary endpoint: OS |
GM-CSF: granulocyte/macrophage-colony stimulating factor; KLH: keyhole limpet hemocyanin; PACE: prednisone, doxorubicin, cyclophosphamide, etoposide; CTX: chemotherapy; HLA: human leukocyte antigen; IL-2: interleukin-2; DFS: disease-free survival; OS: overall survival
Conclusion
Historically, lung cancer has been regarded as a non-immunogenic cancer. Hypotheses as to why active-specific immunotherapeutic approaches to NSCLC have yielded disappointing results range from ineffective priming of tumor-specific T lymphocytes to physical or functional disabling of immune effector cells by primary host and/or tumor-related mechanisms. However, there is increasing evidence that NSCLC and SCLC can evoke specific humoral and cellular antitumor immune responses. Facilitated methodology for characterizing antigen profiles in lung cancer may lead to customized immunotherapy for this disease. Additionally, once molecular analysis is able to determine more accurately which individuals are at the highest risk of relapse after surgical resection, immunotherapy trials may be more efficiently conducted in a defined population with minimal residual disease in which immunotherapy will be most likely to provide therapeutic benefit. Finally, immunotherapeutic approaches in the treatment of lung cancer will be used in concert with standard treatment modalities or in combination with multiple immunotherapeutic agents rather than as single-agent strategies.
Acknowledgments
Christian H. Poehlein, Hauke Winter and Dominik Rüttinger were Chiles Foundation Visiting Fellows. The authors represent the Munich NSCLC Vaccine Study Group.
Footnotes
This work was supported by the Chiles Foundation, Portland, Oregon, USA and the Walter-Schulz-Foundation, Munich, Germany.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Henley S J, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst. 2006;98:691–9. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 3.Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–24. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–91. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Schuster SJ, Neelapu SS, Gause BL, Muggia FM, Gockerman JP, Sotomayor EM, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. J Clin Oncol (Meeting Abstracts) 2009;s27:2. [Google Scholar]
- 8.Schwartzentruber DJ, Lawson D, Richards J, Conry RM, Miller D, Triesman J, et al. A phase III multi-institutional randomized study of immunization with the gp100:209-217 (210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol (Meeting Abstracts) 2009;s27:CRA9011. [Google Scholar]
- 9.Rüttinger D, Winter H, van den Engel NK, Hatz R, Jauch K-W, Fox BA, et al. Immunotherapy of cancer: key findings and commentary on the third Tegernsee conference. Oncologist. 2010;15:112–8. doi: 10.1634/theoncologist.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse MA, Whelan M. A year of successful cancer vaccines points to a path forward. Curr Opin Mol Ther. 2010;12:11–3. [PubMed] [Google Scholar]
- 11.O’Brien ME, Saini A, Smith IE, Webb A, Gregory K, Mendes R, et al. A randomized phase II study of SRL172 (Mycobacterium vaccae) combined with chemotherapy in patients with advanced inoperable non-small-cell lung cancer and mesothelioma. Br J Cancer. 2000;83:853–7. doi: 10.1054/bjoc.2000.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien ME, Anderson H, Kaukel E, O’Byrne K, Pawlicki M, Von Pawel J, et al. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann Oncol. 2004;15:906–14. doi: 10.1093/annonc/mdh220. [DOI] [PubMed] [Google Scholar]
- 13.Stanford JL, Stanford CA, O’Brien ME, Grange JM. Successful immunotherapy with mycobacterium vaccae in the treatment of adenocarcinoma of the lung. Eur J Cancer. 2008;44:224–7. doi: 10.1016/j.ejca.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Dranoff G, Jaffee E, Lazenbay A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21:624–30. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 16.Nemunaitis J, Sterman D, Jablons D, Smith JW 2nd, Fox B, Maples P, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:326–31. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 17.Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, et al. Phase I/II trial of autologous tumour mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–62. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 18.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 19.Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5:690–9. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 20.Kontani K, Taguchi O, Ozaki Y, Hanaoka J, Sawai S, Inoue S, et al. Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int J Mol Med. 2003;12:493–502. [PubMed] [Google Scholar]
- 21.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Lévy E, et al. A phase II study of Tg4010 (Mva-Muc1-IL2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J Thorac Oncol. 2008;3:735–44. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 22.Acres B, Quoix E, Ramlau R, Lacoste G, Marie Bastien B, Tavernaro A, et al. Biomarkers associated with clinical outcome in advanced non-small cell lung cancer patients treated with TG4010. J Clin Oncol (Meeting Abstracts) 2009;s27:3027. [Google Scholar]
- 23.Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, et al. Phase I study of the BLP25 (MUC1 Peptide) liposomale vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer. 2001;3:49–57. doi: 10.3816/clc.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 24.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulières D, et al. Randomized Phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–81. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 25.Butts C, Maksymiuk A, Goss G, Soulieres D, Marshall E, Cormier Y, et al. A mulit-centre phase IIB randomized controlled study of BLP25 liposome vaccine (L-BLP25 or Stimuvax) for active specific immunotherapy of non-small cell lung cancer (NSCLC): updated survival analysis: B1-01. J Thor Oncol. 2007;2:s332–3. [Google Scholar]
- 26.Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K, Maksymiuk A, et al. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer. 2010;11:391–5. doi: 10.3816/CLC.2010.n.101. [DOI] [PubMed] [Google Scholar]
- 27.Sienel W, Varwerk C, Linder A, Kaiser D, Teschner M, Delire M, et al. Melanoma associated antigen (MAGE)-A3 expression in stages I and II non-small cell lung cancer: results of a multi-center study. Eur J Cardiothorac Surg. 2004;25:131–4. doi: 10.1016/j.ejcts.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172:3289–96. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 29.Vansteenkiste J, Zielinski M, Linder A, Dahabre J, Esteban E, Malinowski W, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol (Meeting Abstracts) 2007;s25:7554. [Google Scholar]
- 30.Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer. 2009;10:371–4. doi: 10.3816/CLC.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 31.Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-ß1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999;86:1712–9. [PubMed] [Google Scholar]
- 32.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24:4721–30. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 33.Reissmann PT, Koga H, Figlin RA, Holmes EC, Slamon DJ. Amplification and overexpression of the cyclin D1 and epidermal growth factor receptor genes in non-small cell lung cancer. Lung Cancer Study Group. J Cancer Res Clin Oncol. 1999;125:61–70. doi: 10.1007/s004320050243. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez G, Crombet T, Catala M, Mirabal V, Hernández JC, González Y, et al. A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: report of a pilot clinical trial. Ann Oncol. 1998;9:431–5. doi: 10.1023/a:1008261031034. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14:461–6. doi: 10.1093/annonc/mdg102. [DOI] [PubMed] [Google Scholar]
- 36.Ramos TC, Vinageras E, Ferrer MC, Verdecia BG, Rupalé IL, Pérez LM, et al. Treatment of NSCLC patients with an EGF-based cancer vaccine: report of a Phase I trial. Cancer Biol Ther. 2006;5:145–9. doi: 10.4161/cbt.5.2.2334. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic vaccination with epidermal growth factor (EGF) in advanced lung cancer: Analysis of pooled data from three clinical trials. Hum Vaccin. 2007;3:8–13. doi: 10.4161/hv.3.1.3537. [DOI] [PubMed] [Google Scholar]
- 38.Garcia B, Neninger E, de la Torre A, Leonard I, Martínez R, Viada C, et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res. 2008;14:840–6. doi: 10.1158/1078-0432.CCR-07-1050. [DOI] [PubMed] [Google Scholar]
- 39.Neninger Vinageras E, de la Torre A, Osorio Rodriquez M, Catalá Ferrer M, Bravo I, Mendoza del Pino M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:1452–8. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 40.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J, et al. Autologous dendritic cell vaccines for non-small cell lung cancer. J Clin Oncol. 2004;22:2808–15. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 41.Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007;57:365–72. doi: 10.1016/j.lungcan.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschowitz EA, Mullins A, Prajapati D, Baeker T, Kloecker G, Foody T, et al. Pilot study of 1650-G: A Simplified Cellular Vaccine for Lung Cancer. J Thorac Oncol. 2011;6:169–73. doi: 10.1097/JTO.0b013e3181fb5c22. [DOI] [PubMed] [Google Scholar]
- 43.Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–24. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 44.Kelly RJ, Gulley JL, Giaccone G. Targeting the immune system in non-small-cell lung cancer: bridging the gap between promising concept and therapeutic reality. Clin Lung Cancer. 2010;11:228–37. doi: 10.3816/CLC.2010.n.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barve M, Bender J, Senzer N, Cunningham C, Greco FA, McCune D, et al. Induction of immune responses and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26:4418–25. doi: 10.1200/JCO.2008.16.6462. [DOI] [PubMed] [Google Scholar]
- 46.Morris JC, Vahanian N, Janik JE, Moses L, Tennant L, Pittaluga S, et al. Phase I study of an antitumor vaccination using α-(1,3) galactosyltransferase expressing allogeneic tumor cells in patients with refractory or recurrent non-small cell lung cancer (NSCLC) J Clin Oncol (Meeting Abstracts) 2005;s23:2586. [Google Scholar]
- 47.Raez LE, Cassileth PA, Schlesselmann JJ, Sridhar K, Padmanabhan S, Fisher EZ, et al. Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:2800–7. doi: 10.1200/JCO.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 48.Neninger E, Diaz RM, de la Torre A, Rives R, Díaz A, Saurez G, et al. Active immunotherapy with 1E10 anti-idiotype vaccine in patients with small cell lung cancer: report of a phase I trial. Cancer Biol Ther. 2007;6:145–50. doi: 10.4161/cbt.6.2.3574. [DOI] [PubMed] [Google Scholar]
- 49.Ma J, Poehlein CH, Jensen SM, LaCelle MG, Moudgil TM, Rüttinger D, et al. Manipulating the host response to autologous tumor vaccines. Dev Biol (Basel) 2004;116:93–107. [PubMed] [Google Scholar]
- 50.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rüttinger D, van den Engel NK, Winter H, Schlemmer M, Pohla H, Grützner S, et al. Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response. J Transl Med. 2007;5:43. doi: 10.1186/1479-5876-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]