Abstract
Background
To elucidate the differences in chemosensitivity to anticancer drugs between primary and metastatic lesions in non-small cell lung cancer (NSCLC) patients, we examined the in vitro chemosensitivities of surgically resected NSCLC tissues.
Methods
A total of 32 specimens were enrolled: 26 specimens of primary lesions paired with metastases in the lymph node, 3 specimens of primary lesions paired with metastases in the adrenal gland, and 3 specimens of primary lesions paired with metastases in the lung. The collagen gel droplet embedded culture drug test (CD-DST) was applied to examine the sensitivity of the tissues to anticancer drugs, including cisplatin, gemcitabine, vinorelbine, docetaxel and 5-fluorouracil.
Results
The degree of in vitro sensitivity to each anticancer drug varied between the primary and metastatic lesions. The sensitivity of the paired metastatic lesions was significantly lower than that of the primary lesions only for gemcitabine (P=0.029), vinorelbine (P=0.012), and docetaxel (P=0.009). The incidence of cases diagnosed as CD-DST-sensitive among the paired metastatic lesions was significantly lower than that for the primary lesions for vinorelbine (P=0.035) or docetaxel (P=0.022). The difference in the sensitivity to gemcitabine between the primary and paired non-lymphatic metastases was clearer than that between the primary lesion and paired lymph node metastases.
Conclusions
The sensitivities of the paired metastatic lesions to some anticancer drugs were significantly lower than those of the primary lesions. When performing chemotherapy based on CD-DST data using primary tumors from patients with postoperative recurrence, an appropriate regimen can be selected by carefully considering these differences.
Keywords: Non-small cell lung cancer (NSCLC), chemosensitivity test, collagen gel droplet embedded culture drug test (CD-DST), metastatic tissue, chemotherapy
Introduction
Lung cancer is a major cause of death worldwide. More than 70% of lung cancer patients die of systemic metastasis. Although chemotherapy modalities to fight this disease have been aggressively developed, they have failed to achieve satisfactory therapeutic effects and prognoses. Indeed, many advanced lung cancers are finally resistant to anticancer drugs, and the response rates of systemic metastatic disease are worse than those associated with induction chemotherapy (1)-(3). Also, primary lesions and their corresponding metastases frequently show significant differences in their sensitivity to chemotherapy, and similar differences are also seen among metastatic sites (4). Considering these observations together, it was suggested that the chemosensitivity of some tumors is strongly affected by the biological aggressiveness of the tumor, such as the metastatic potential of the tumor cells themselves, the metastatic route, and site-specific circumstances associated with the metastatic process (5)-(9). Thus, it is clinically important to analyze the heterogeneity of chemosensitivity to anticancer drugs within tumor tissue. In fact, such tumor heterogeneities of potential drug sensitivity was recently indicated in the patient-derived xenograft specimen treated experimentally with anticancer drugs (10), but the heterogeneity of tumor chemosensitivity in individual patients has been not sufficiently examined.
Recently, in vitro anticancer drug sensitivity tests using clinical specimens have been used to provide data for designing individualized chemotherapies. Several in vitro anticancer drug sensitivity tests have been developed for various types of malignant tumors, and these tests have been applied experimentally as well as clinically (11)-(14). The collagen gel droplet embedded culture drug test (CD-DST) is an in vitro anticancer drug sensitivity test (15)-(17) that has been used at our institute in chemotherapy for patients with non-small cell lung cancer (NSCLC) (18)-(20) as well as those with other thoracic tumors (21),(22). So far, this test has been used to assess surgically resected specimens from NSCLC primary lesions and to provide data regarding their sensitivity to anticancer drugs and has also been clinically applied to aid the development of individualized chemotherapies for NSCLC patients who have suffered postoperative recurrence (18),(20). In fact, good predictability was obtained when the test was used to aid the treatment of recurrent disease, and the accuracy of treatment response predictions based on the CD-DST data was as high as 70%, but this was still not satisfactory because these chemosensitivity data were obtained from primary NSCLC tissues, not systemic metastatic tissues (20).
In the present study, in order to elucidate the differences in the chemosensitivity to anticancer drugs between primary and metastatic lesions in individual NSCLC patients, we examined the in vitro chemosensitivities of surgically resected NSCLC tissues to representative anticancer drugs. In addition, the in vitro chemosensitivities of tumors at different metastatic sites was examined.
Patients and methods
Patients, tissue specimens, and CD-DST data acquisition
The primary lesions and paired metastatic NSCLC tissues used in the present study were collected in the following manner: between 2001 and 2009, 1790 patients underwent surgical treatment for lung cancer, and of them, the CD-DST was selectively performed in 597 NSCLC patients after they had provided informed consent. The tested specimens were primary lung cancer tissues, and in cases in which the tumor was locally advanced, 37 metastatic tissues, including 32 nodal and 5 pulmonary metastatic lesions were also tested at the same time. The paired specimens used for the test were obtained by surgical resection for postoperative recurrence, such as adrenal and pulmonary metastasis. In addition, the following specimens were excluded from the present analysis: patients undergoing neoadjuvant chemotherapy before primary surgery or chemotherapy before metastasectomy for recurrent disease, and patients whose CD-DST data were not obtained due to a technical issue. As a result, a total of 32 CD-DST datasets for primary and metastatic NSCLC lesions were enrolled: 26 CD-DST datasets for primary tumors paired with nodal metastatic lesions, 3 for primary tumors paired with adrenal gland metastatic lesions, and 3 for primary tumors paired with pulmonary metastatic lesions (2 displaying synchronism and 1 displaying asynchronism).
The clinicopathological data of the patients enrolled in the present study are summarized in Table 1. The pathological stage (p-stage) of the disease was based on the general guidelines of the Japan Lung Cancer Society (23). As described above, the patients were divided into two groups, those (n=26) with CD-DST data for primary and nodal metastatic lesions and those (n=6) with CD-DST data for primary and distant metastatic lesions. The former group consisted of 8 squamous cell carcinomas, 14 adenocarcinomas, 3 large cell carcinomas, and one adenosquamous cell carcinoma. The nodal specimens used for the test were obtained from dissected mediastinal or hilar lymph nodes while the metastases were histologically confirmed during surgery. The latter group consisted of 1 squamous cell carcinoma, 4 adenocarcinomas, and 1 large cell carcinoma. The metastatic lesions were diagnosed using intraoperative and postoperative histological examinations.
Table 1. Clinicopathological characteristics of the patients.
| No. of patients (%) | ||
| Patients with paired primary and nodal metastatic lesions (n=26) | ||
| Age | mean | 66 years-old |
| Gender | male / female | 18 (69%) / 8 (31%) |
| p-stage (23) | IIB / IIIA /IIIB / IV | 2 (8%) / 18 (69%) / 5(19%) / 1(4%) |
| Histology (23) | squamous / adeno / large / adenosquamous * | 8 (31%) /14 (54%) /3 (12%) /1 (4%) |
| Patients with paired primary and distant metastatic lesions (n=6) | ||
| Age | mean | 62 years-old |
| Gender | male / female | 5 (83%) / 1 (17%) |
| Distant site | lung / adrenal gland | 3 (50%) / 3 (50%) |
| Histology* | squamous / adeno / large | 1 (17%) / 4 (67%) / 1 (17%) |
* adeno: adenocarcinoma; squamous: squamous cell carcinoma; large: large cell carcinoma; adenosquamous: adenosquamous cell carcinoma
CD-DST was performed as described previously by Kobayashi et al. (15)-(17). In brief, each surgically obtained specimen was finely minced using a scalpel and digested in cell dispersion enzyme solution (EZ, Nitta Gelatin Inc., Osaka, Japan) for 2 hr. The dispersed cancer cells were then washed twice, collected by centrifugation at 250 g for 3 min, filtered through an 80 um nylon mesh, and then incubated in a collagen gel coated flask (CG-flask, Nitta Gelatin Inc.,) in a CO2 incubator at 37 °C for 24 hr. Only the viable cells adhering to the collagen gel were collected and suspended in the reconstructed type I collagen solution (Cellmatrix Type CD, Nitta Gelatin Inc.) at a final density of 1×105 cells/ml. Three drops of the collagen-cell mixture (30 ul/drop) were placed in each well of a 6-well multiplate and a 60 mm dish and allowed to gel at 37 °C in a CO2 incubator for 1 ho-ur. The final concentration was about 3×103 cells/collagen gel droplet. The culture medium was overlaid on each well, and the plate was incubated in a CO2 incubator at 37 °C overnight.
Then, one of the anticancer drugs was added and incubated for 1 hr (gemcitabine) oor 24 hr (other drugs). After the removal of the medium containing the anticancer drug, each well was rinsed twice, overlaid with serum-free culture medium (PCN-1, Nitta Gelatin Inc.), and incubated for seven days. On the fourth day of the incubation, the medium was replaced. At the end of the incubation, neutral red was added to each well at a final concentration of 50 ug/ml, and the colonies in the collagen gel droplets were stained for three hr. The collagen droplets in the 60 mm dish were stained just before exposure (day 1). Thereafter, each collagen droplet was fixed with 10% neutral formalin buffer, washed in water, air dried, and quantified by image analysis. The growth rate of the controls was calculated as the total volume of the control group on day 7/total volume on day 1. The in vitro sensitivity was expressed as the T/C ratio (%), where T was the total volume of the treated group and C was the total volume of the control group. A T/C (%) of 50% or less to an anticancer drug was regarded demonstrating in vitro-sensitivity.
Anticancer drugs
The anticancer drugs tested in the CD-DST were 0.2 ug/ml cisplatin (CDDP), 0.1 ug/ml docetaxel (TXT), 0.05 ug/ml vinorelbine (VNR), 8.0 ug/ml gemcitabine (GEM), and 1.0 ug/ml 5-furuolouracil (5-Fu). The culture time was 1 hr for GEM, while it was 24 hr for the other drugs (15)-(18),(20).
Statistical analyses
Statistical analyses were performed using the paired T test or Fisher's exact probability test. The level of significance was set at 5%.
Results
Chemosensitivity of the primary tissues and paired metastatic lesions to each anticancer drug
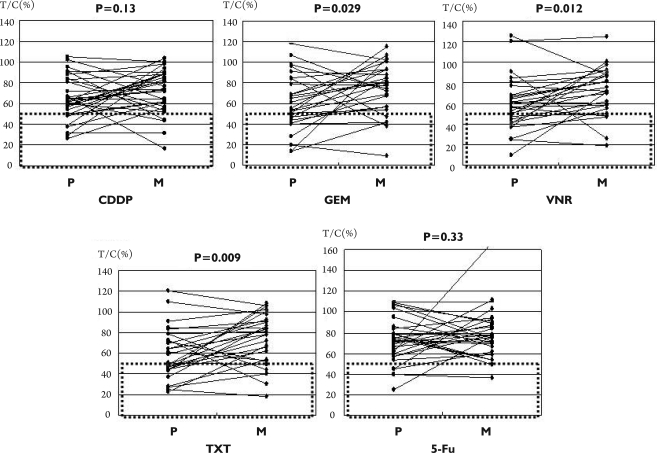
In vitro sensitivity data for the primary and paired metastatic tissues were obtained in all 32 patients for CDDP, but were only obtained in 29 patients for 5-FU, and in 28 patients for GEM, VNR, and TXT, because of technical problems or material deficiencies. Table 2 shows a summary of the chemosensitivity data of the primary (P) and paired metastatic lesions (M) for each anticancer drug. With regard to the T/C ratio (%) of each anticancer drug, the mean ratio in the primary tissue was generally lower than that in the paired metastatic lesion, especially for GEM (P=0.029), VNR (P=0.012), and TXT (P=0.009), in which the difference was significant, indicating that the paired metastatic lesions showed significantly less sensitivity to these drugs than the primary lesions. Fig 1 shows a comparison of the CD-DST data for the primary lesions and their paired metastatic lesions for each anticancer drug. For GEM, VNR, and TXT, although a minority of cases showed T/C ratios that were lower in the paired metastatic lesions than those in the primary tissues, the T/C ratios of the metastatic lesions were generally higher than those of the primary lesions. In contrast, for CDDP and 5-FU (Figure 1), no such observation was seen, as described in Table 2.
Table 2. Comparison of in vitro sensitivity data produced by the CD-DST between primary and paired metastatic lesions in patients with NSCLC.
| CDDP (P) | CDDP (M) | GEM (P) | GEM (M) | VNR (P) | VNR (M) | TXT (P) | TXT (M) | 5-FU (P) | 5-FU (M) | |
| Number | 32 | 32 | 28 | 28 | 28 | 28 | 28 | 28 | 29 | 29 |
| T/C ratio (%) | ||||||||||
| Mean | 64.3 | 72.0 | 61.3 | 75.2 | 58.7 | 72.8 | 59.6 | 74.1 | 73.1 | 79.0 |
| S.D. | 20.7 | 21.0 | 26.9 | 24.8 | 25.9 | 22.9 | 24.5 | 24.3 | 21.4 | 24.6 |
| Median | 62 | 74 | 58 | 79 | 56 | 74 | 55 | 79 | 73 | 77 |
| p value (P vs M)* | 0.13 | 0.029 | 0.012 | 0.009 | 0.33 | |||||
| Mean | ||||||||||
| Number of sensitive cases (%) | 7 (22%) | 4 (13%) | 9 (32%) | 5 (18%) | 11 (39%) | 4 (14%) | 13 (46%) | 5 (18%) | 4 (14%) | 2 (7%) |
| P value (P vs M)** | 0.32 | 0.21 | 0.035 | 0.022 | 0.39 | |||||
(P): Primary lesions, (M): Paired metastatic lesions; *: Paired T test; **: Fisher's exact probability test.
Figure 1. Comparison of the in vitro chemosensitivities (T/C ratio, %) of primary lesions and their paired metastatic NSCLC lesions.
P: primary lesions, M: paired metastatic lesions. The cases surrounded with dotted-line frames were diagnosed as being in vitro-sensitive. The P value for each anticancer drug was calculated using the Paired T test. Fig 1. shows a comparison of CD-DST data between each primary lesion and its paired metastatic lesion for each anticancer drug. For GEM, VNR, and TXT, the T/C ratio of the metastatic lesions was significantly higher than that of the primary lesions. In contrast, for CDDP and 5-FU, no significant differences in chemosensitivity were observed.
Regarding the diagnosis of CD-DST, whether the lesion was in vitro-sensitive or –resistant based on this chemosensitivity test, the frequency of in vitro-sensitive cases was generally lower for the metastatic lesions than the primary tissues, especially for VNR (P=0.035) and TXT (P=0.022), and the difference was significant according to Fisher's exact probability test (Table 2).
Chemosensitivity analysis based on the metastatic route
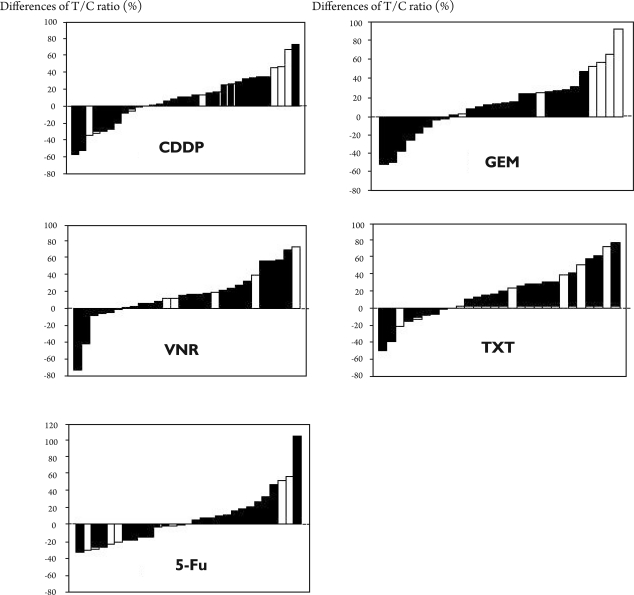
As described in Patients and Methods, 6 metastatic specimens were obtained from non-nodal metastases (3 from the adrenal gland and 3 from the lung). Then, the difference in chemosensitivity between the primary lesions and their paired metastatic lesions was analyzed according to the metastatic route, namely lymphatic (n=26) versus non-lymphatic (n=6). Figure 2 shows waterfall plots of each anticancer drug reflecting the differences in the T/C ratio (%) between the primary and metastatic lesions. The cases associated with a non-lymphatic route (white column) were widely distributed in these plots, and in the GEM group, 4 cases (67%) showed at least a 30% greater number of in vitro resistant metastatic lesions than primary tumors. Also, three cases (50%) showed similar observations for CDDP.
Figure 2. Differences in the T/C ratio (%) between the primary lesions and their paired metastatic lesions.
Differences in the T/C ratio (%) between the primary lesions and their paired metastatic lesions are shown by waterfall plots. The black columns show the difference between primary lesions and their paired lymph node lesion. The white columns show the difference between primary lesions and their paired non-lymphatic lesions. Although the cases involving a non-lymphatic route (white column) were widely distributed, 4 cases (67%) treated with GEM showed a 30% or greater increase in in vitro resistance in the paired metastatic lesions compared with the primary tissue. Three cases (50%) showed similar results for CDDP.
Discussion
For the past 20 years, we have investigated the technical development of the CD-DST and have presented experimental and clinical results that aid the selection of individualized chemotherapy for patients, especially for those with lung cancer (15)-(17). For example, we reported that the CD-DST displayed clinical significance for some “old generation” anticancer drugs in NSCLC patients (18). In this report, the chemotherapeutic effects of treatment for postoperative recurrence were analyzed in comparison with the CD-DST data obtained by surgery. CDDP-based combined chemotherapy yielded a good response more frequently with in vitro-sensitive regimens than with non-in vitro-sensitive regimens; and consequently, the CD-DST results for CDDP and CBDCA, a key drug for chemotherapy, correlated with the clinical response. Next, we showed the similar results for some “new generation” anticancer drugs, which were thought to have stronger therapeutic effects than the “old generation” drugs. In fact, so good responses to the recurrent tumors were obtained by chemotherapy even with a single agent regimen such as GEM, TXT, and VNR, when diagnosed as in vitro- sensitive (20).
In addition to our series (18),(20), there have been several reports regarding the clinical application of in vitro sensitivity tests for the treatment of lung cancer patients. Kawamura et al. (19) described the survival benefit of CD-DST-based chemotherapy for patients with stage IV lung cancer. Yoshimasu et al. (24) also reported the usefulness of another in vitro chemosensitivity test, the histoculture drug response assay (HDRA), for treating postoperative recurrence in lung cancer patients. Recently, Tanahashi et al. (25) reported the clinical application of the HDRA for postoperative adjuvant chemotherapy in lung cancer patients and demonstrated that overall survival was prolonged by treatment using an HDRA-sensitive regimen. In addition, there have also been some promising reports regarding other novel chemosensitivity tests for the treatment of patients with NSCLC (10),(26). In particular, such an in vivo test system as patient-derived xenograft model described by Dong et al. (10) was newly promising for predicting drug sensitivities. Thus, it is considered that these chemosensitivity tests may be clinically applicable for sensitivity test-guided, individualized treatment of cancer patients. However, it has been well recognized that there are some limitations to apply these in vitro tests in clinical practice enough. In fact, there are still some technical problems of primary culture failure, anticancer drug level, bacterial contamination, measurement only for cancer cells, and so on. Anyway, these tests including CD-DST (15)-(17) have been developed while overcoming such technical problems step by step.
Interestingly, we must also pay particular attention to the fact that most of these analyses are based on sensitivity data obtained from primary, not metastatic, lesions. In other words, it is possible that these data do not reflect the characteristics of all tumor tissues in a particular patient. Since chemosensitivity data could not be obtained for all sites, chemotherapy was performed based on the data of the most representative primary site in patients with NSCLC (18),(20),(24). However, the prediction of chemotherapeutic effects using sensitivity tests was not always satisfactory in our series (18),(20) or those of others (19),(24). Unfortunately, the reason for these problems is unclear. From this standpoint, this study is extremely important for elucidating the cause of predictive failure.
According to the present study, although a minority of patients had metastatic lesions that were more in vitro-sensitive than the primary lesion, some anticancer drugs, for example, TXT, VNR, and GEM, showed significantly less in vitro-sensitivity in metastatic lesions than in primary lesions, and then, these differences resulted in a lower incidence of in vitro-sensitive cases for TXT and VNR. In contrast, only small differences in sensitivity were detected between the primary and metastatic lesions for CDDP and 5-Fu. Furukawa et al. (27) reported similar results for various anticancer drugs using specimens from primary breast cancer lesions and their paired nodal metastatic tumors by the HDRA. Interestingly, they also showed that the sensitivity of the metastatic nodal lesions to CDDP was not different from that of the primary lesions. Therefore, when performing individualized chemotherapy based on CD-DST data using primary tumor specimens, false-sensitive regimens, including some anticancer drugs that display in vitro-sensitivity, e.g., TXT and VNR, might be selected.
Surprisingly, the effectiveness of some anticancer drugs depended on the metastatic route or site. GEM and CDDP showed a trend towards reduced sensitivity in non-lymphatic metastatic lesions compared with lymph node metastases, although the observation was not subjected to statistical analysis because of the small number of samples. This trend might be closely associated with our previously reported result (20); i.e., in CD-DST-based chemotherapy for recurrent disease, the predictive accuracy of CD-DST data was highest for lymph node recurrence. In contrast, among patients with pleural or bone metastasis, few such associations between CD-DST data and response were observed, despite the fact that we performed chemotherapy with an in vitro- sensitive regimen (20). In addition, a similar tendency was also found in a recent report: Tanahashi et al. (25) described that the incidence of postoperative lymph node recurrence was low in lung cancer patients undergoing adjuvant chemotherapy involving an in vitro-sensitive regimen. Thus, for some anticancer drugs, the metastatic route or site may also influence the predictive performance of CD-DST.
A few reports have demonstrated clear differences in the in vitro or in vivo chemosensitivity of primary lesions and their paired metastatic lesions using human tumor tissues (10). Also, several biomarkers associated with chemosensitivity have been aggressively developed (28),(29), but although the chemosensitivity heterogeneity of tumor tissues has been studied (10), no studies comparing tumors according to the site of the lesion have been performed. Besides, there are few studies comparing these tests. Inaba et al. (30) previously reported an in vitro-in vivo correlation of CD-DST, and such comparison studies may be also necessary in the future. Anyway, in this study, the sensitivity of tumors to anticancer drugs showed surprisingly variation among the tumor tissues in individual patients. In particular, it was interesting that these differences were closely related to the type of anticancer drug used and the metastatic route/site. Based on these observations, when performing CD-DST-based chemotherapy for NSCLC patients, especially those with postoperative recurrent disease, an appropriate regimen should be selected after carefully considering these differences. Further analysis is required to establish a promising strategy for CD-DST-guided chemotherapy for patients with NSCLC.
Acknowledgments
The authors thank Ms. Eri Yoneima for her valuable technical assistance.
Footnotes
No potential conflict of interest.
References
- 1.Date H, Kiura K, Ueoka H, Tabata M, Aoe M, Andou A, et al. Preoperative induction chemotherapy with cisplatin and irinotecan for pathological N(2) non-small cell lung cancer. Br J Cancer. 2002;86:530–3. doi: 10.1038/sj.bjc.6600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–23. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 3.Akamine S, Nakamura Y, Oka T, Soda H, Taniguchi H, Fukuda M, et al. Induction chemotherapy with cisplatin, vinorelbine, and mitomycin-C followed by surgery for patients with pathologic N2 non-small-cell lung cancer. Clin Lung Cancer. 2008;9:44–50. doi: 10.3816/clc.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 4.Kern DH. Heterogeneity of drug resistance in human breast and ovarian cancers. Cancer J Sci Am. 1998;4:41–5. [PubMed] [Google Scholar]
- 5.Slack NH, Bross ID. The influence of site of metastasis on tumour growth and response to chemotherapy. Br J Cancer. 1975;32:78–86. doi: 10.1038/bjc.1975.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamby C, Vestlev PM, Mouridsen HT. Site-specific effect of chemotherapy in patients with breast cancer. Acta Oncol. 1992;31:225–9. doi: 10.3109/02841869209088907. [DOI] [PubMed] [Google Scholar]
- 7.Nicolson GL. Paracrine and autocrine growth mechanisms in tumor metastasis to specific sites with particular emphasis on brain and lung metastasis. Cancer Metasatasis Rev. 2006;12:325–43. doi: 10.1007/BF00665961. [DOI] [PubMed] [Google Scholar]
- 8.Wu J. Apoptosis and angiogenesis: two promising tumor markers in breast cancer (review) Anticancer Res. 1996;16:2233–9. [PubMed] [Google Scholar]
- 9.Glinsky GV, Glinsky VV, Ivanova AB, Hueser CJ. Apoptosis and metastasis: increased apoptosis resistance of metastatic cancer cells is associated with the profound deficiency of apoptosis execution mechanisms. Cancer Lett. 1997;115:185–93. doi: 10.1016/s0304-3835(97)04738-1. [DOI] [PubMed] [Google Scholar]
- 10.Dong X, Guan J, English JC, Flint J, Yee J, Evans K, et al. Patient-derived first generation xenografts of non-small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy. Clin Cancer Res. 2010;16:1442–51. doi: 10.1158/1078-0432.CCR-09-2878. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman RM. In vitro sensitivity assays in cancer: a review, analysis, and prognosis. J Clin Lab Anal. 1991;5:133–43. doi: 10.1002/jcla.1860050211. [DOI] [PubMed] [Google Scholar]
- 12.Kondo T, Imamura T, Ichihashi H. In vitro test for sensitivity of tumor to carcinostatic agents. Gann. 1966;57:113–21. [PubMed] [Google Scholar]
- 13.Bertelsen CA, Sondak VK, Mann BD, Korn EL, Kern DH. Chemosensitivity testing of human solid tumors. A review of 1582 assays with 258 clinical correlations. Cancer. 1984;53:1240–5. doi: 10.1002/1097-0142(19840315)53:6<1240::aid-cncr2820530604>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Shaw GL, Gazdar AF, Phelps R, Steinberg SM, Linnoila RI, Johnson BE, et al. Correlation of in vitro drug sensitivity testing results with response to chemotherapy and survival: comparison of non-small cell lung cancer and small cell lung cancer. J Cell Biochem Suppl. 1996;24:173–85. doi: 10.1002/jcb.240630513. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Higashiyama M, Minamigawa K, Tanisaka K, Takano T, Yokouchi H, et al. Examination of in vitro chemosensitivity test using collagen gel droplet culture method with colorimetric endpoint quantification. Jpn J Cancer Res. 2001;92:203–10. doi: 10.1111/j.1349-7006.2001.tb01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi H. Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness. Recent Results Cancer Res. 2003;161:48–61. doi: 10.1007/978-3-642-19022-3_5. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H. Collagen gel droplet culture method to examine in vitro chemosensitivity. Methods Mol Med. 2005;110:59–67. doi: 10.1385/1-59259-869-2:059. [DOI] [PubMed] [Google Scholar]
- 18.Higashiyama M, Kodama K, Yokouchi H, Takami K, Nakagawa H, Imamura F, et al. Cisplatin-based chemotherapy for postoperative recurrence in non-small cell lung cancer patients: relation of the in vitro chemosensitive test to clinical response. Oncol Rep. 2001;8:279–83. doi: 10.3892/or.8.2.279. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura M, Gika M, Abiko T, Inoue Y, Oyama T, Izumi Y, et al. Clinical evaluation of chemosensitivity testing for patients with unresectable non-small cell lung cancer (NSCLC) using collagen gel droplet embedded culture drug sensitivity test (CD-DST) Cancer Chemother Pharmacol. 2007;59:507–13. doi: 10.1007/s00280-006-0292-8. [DOI] [PubMed] [Google Scholar]
- 20.Higashiyama M, Oda K, Okami J, Maeda J, Kodama K, Imamura F, et al. Prediction of chemotherapeutic effect on postoperative recurrence by in vitro anticancer drug sensitivity testing in non-small cell lung cancer patients. Lung Cancer. 2010;68:472–7. doi: 10.1016/j.lungcan.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Noda T, Higashiyama M, Oda K, Higaki N, Takami K, Okami J, et al. Mucoepidermoid carcinoma of the thymus treated by multimodality therapy: a case report. Ann Thorac Cardiovasc Surg. 2006;12:273–8. [PubMed] [Google Scholar]
- 22.Higashiyama M, Oda K, Okami J, Maeda J, Kodama K, Takami K, et al. In vitro-chemosensitivity test using the collagen gel droplet embedded culture drug test (CD-DST) for malignant pleural mesothelioma: possibility of clinical application. Ann Thorac Cardiovasc Surg. 2008;14:355–62. [PubMed] [Google Scholar]
- 23.The Japan Lung Cancer Society . 6th ed. Tokyo, Japan: Kanehara-Shuppan; 2003. General rule for clinical and pathological record of lung cancer. [Google Scholar]
- 24.Yoshimasu T, Oura S, Hirai I, Tamaki T, Kokawa Y, Hata K, et al. Data acquisition for the histoculture drug response assay in lung cancer. J Thorac Cardiovasc Surg. 2007;133:303–8. doi: 10.1016/j.jtcvs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Tanahashi M, Niwa H, Yukiue H, Suzuki E, Haneda H, Yoshii N. Adjuvant chemotherapy based on the in vitro hisotculture drug response assay for non-small cell lung cancer improves survival. J Thorac Oncol. 2010;5:1376–81. doi: 10.1097/JTO.0b013e3181e7d035. [DOI] [PubMed] [Google Scholar]
- 26.Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, et al. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007;109:1829–35. doi: 10.1002/cncr.22601. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa T, Kubota T, Tanino H, Oura S, Yuasa S, Murate H, et al. Chemosensitivity of breast cancer lymph node metastasis compared to the primary tumor from individual patients tested in the histoculture drug response assay. Anticancer Res. 2000;20:3657–8. [PubMed] [Google Scholar]
- 28.Sekine I, Minna JD, Nishio K, Tamura T, Saijo N. A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with lung cancer. J Thorac Oncol. 2007;2:845–53. [PubMed] [Google Scholar]
- 29.Glaysher S, Yiannakis D, Gabriel FG, Johnson P, Polak ME, Knight LA, et al. Resistance gene expression determines the in vitro chemosensitivity of non-small cell lung cancer (NSCLC) BMC Cancer. 2009;9:300. doi: 10.1186/1471-2407-9-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inaba M, Tashiro T, Sato S, Ohnishi Y, Tanisaka K, Kobayashi H, et al. In vitro-in vivo correlation in anticancer drug sensitivity test using AUC-based concentrations and collagen gel droplet-embedded culture. Oncology. 1996;53:250–7. doi: 10.1159/000227569. [DOI] [PubMed] [Google Scholar]