Abstract
Background
Identifying predictors of the course of depressive symptoms from pregnancy through postpartum is important to inform clinical interventions.
Methods
This longitudinal study investigated predictors of recovery from prenatal elevated depressive symptoms in the postpartum period. Forty-one pregnant women completed demographic, interpersonal, and psychosocial self-report assessment measures at 32 weeks of gestation and again 12 weeks postpartum.
Results
Of those with elevated depressive symptoms, defined as a Beck Depression Inventory-II (BDI-II) score ≥10, at the prenatal baseline, 39% (n=16) recovered to nonelevated symptom levels postpartum, whereas 61% (n=25) experienced sustained elevated symptoms. Women who recovered evidenced significantly lower baseline depression severity and more frequent engagement in physical activity and cohabitated with a romantic partner. In multiparous women (n=25), history of past postpartum depression (PPD) differentiated between those with transient and those with persisting symptoms, although history of lifetime depression did not. None of the additional demographic, interpersonal, or psychosocial variables investigated differentiated between groups. Logistic regression analysis showed prenatal depression severity and exercise frequency as predictors of recovery postpartum.
Conclusions
Results suggest most women will not experience spontaneous recovery. Women with prenatal heightened symptom severity and previous experiences with PPD are acutely vulnerable to experience sustained symptoms. In contrast, having a cohabitating partner and engagement in prenatal exercise predicted symptom improvement. Physical exercise may be an important clinical recommendation, as it may improve mood. Given the small sample size, these results are preliminary. Implications and future research recommendations are discussed.
Introduction
Depression at various levels of severity around the time of childbearing has been found to be common relative to other pregnancy complications. A meta-analysis found as many as 7% of women may experience episodes of major depression, and nearly 20% confront major or minor depression within the first 3 months after delivery.1 Prenatal depressive symptoms (PD) have been identified as a primary predictive factor of postpartum depressive symptoms (PPD),2 yet despite considerable associations between PD and PPD, approximately two thirds of women with PD or prenatal anxiety appear to recover before the postpartum period.3,4 Recognizing predictors of recovery may permit a better understanding of the development, course, and consequences of PD and improve differentiation between those at low vs. high risk for the persistence of symptoms into the postpartum period. Moreover, in the case of modifiable factors, care providers can tailor interventions to incorporate improvement-related variables.
Longitudinal studies investigating factors associated with PD recovery are scarce. Gotlib et al.5 prospectively tracked 730 women from pregnancy through the early postpartum period on a number of psychosocial variables, including age, duration of marriage, education, marital status, at-home parenthood, number of prior children, marital satisfaction, perceived stress, baseline depressive symptoms, parental bonding experienced in the family of origin, dysfunctional attitudes, and coping styles. Although those who recovered differed from those whose depressive symptoms persisted on initial analyses of marital satisfaction and perceived stress, regression analysis failed to identify any significant predictors of recovery. Similarly, Andersson et al.4 more recently found age, marital and economic status, alcohol and tobacco use, parity, baseline body mass index (BMI), and chronic disease during pregnancy did not differentiate between those with continued PPD or anxiety symptoms and those who recovered. In their research, history of psychiatric illness before pregnancy was the only factor that predicted continued symptoms.
Further, these longitudinal studies have overlooked several biopsychosocial factors that may be salient to understanding the trajectory of perinatal depressive symptoms. For instance, general population studies and, to a far lesser extent, research involving perinatal women have demonstrated associations between perinatal exercise behaviors and mental health. Although not restricted to women with elevated prenatal depressive symptoms, one longitudinal investigation indicated that prenatal physical activity and body image were significantly associated with lower severity of PPD.6 A recent analysis of cross-sectional data from the Pregnancy Risk Assessment Monitoring System (PRAMS) did not find a significant relationship between exercise and feeling down during the postpartum but did detect an association with symptoms of restricted interest and pleasure following childbirth.7 Further research is warranted to evaluate the relationship between physical activity and PPD in women with elevated symptoms during pregnancy. Postpartum division of labor, romantic relationship behaviors, and previous history of perinatal depression have been similarly absent in recovery research.
Given the paucity of information on the trajectory of depressive symptoms from pregnancy through postpartum and the limited success of previous research to identify significant predictors of recovery based on demographic characteristics, this study aimed to (1) examine rates of depression symptom recovery from 32 weeks of gestation through 12 weeks postpartum and (2) identify demographic, psychologic, environmental, or social predictors of symptom improvement. Because of the limited research on recovery from PD, we hypothesized there would be differences between those whose symptoms resolved postpartum and those who experienced PPD but did not make any specific predictions as to the direction of these differences.
Materials and Methods
Recruitment and study sample
The screening process and recruitment criteria have been elsewhere described.8 Briefly, this report includes analysis of data obtained from two studies of perinatal mental health. In Study 1, after giving informed consent, pregnant women (n=27) completed a 15-minute screen at their obstetric visits to determine preliminary eligibility. From this pool, eligible women with and without elevated PD were recruited for a longitudinal study of prenatal and postpartum maternal and child biopsychosocial outcomes. In Study 2, a similar population (n=14) was recruited for participation in a randomized controlled psychotherapy trial. The recruitment and data collection procedures, eligibility criteria, and measures collected were otherwise identical for both studies.
At 32 weeks pregnant, women completed additional psychologic, interpersonal, and resource-related self-report instruments. Twelve weeks postpartum, women repeated the assessment battery and a division of labor measure. Women were paid $135–$180 for their contributions to this research. Trained clinicians with at least a master's level degree completed assessments. The University of Michigan Medical School Institutional Review Board approved all screening and research procedures.
One hundred fifty-four women enrolled in Studies 1 and 2. Of these, 57 participants (37%) had Beck Depression Inventory-II (BDI-II)9 scores ≥10 during the prenatal baseline interview and were categorized as experiencing PD. Of these women, 28% either left (n=14) the studies or did not complete the 12-week postpartum assessment but continued participation at later points in the study (n=2), resulting in a sample size of 41 women with complete data. As the purpose of this report is to better understand the outcomes for women experiencing PD, we did not analyze data from the 97 women enrolled in Studies 1 and 2 who did not endorse elevated PD (prenatal BDI-II <10).
Measures
Sociodemographics, mental health history, and treatment use
Women completed the General Health Questionnaire (GHQ), a study-specific demographic screening survey assessing age, ethnicity, cohabitation status, income, parity, use of medication or psychotherapy for depression, and frequency of exercise behaviors (scores range from 1, never exercise, to 5, every day). Self-reported past history of major depressive disorder (MDD) was assessed with two items specific to either lifetime or postpartum experiences. The items were worded as whether (within the specified time frame) “you had 2 weeks or more when nearly every day you felt sad, blue, or depressed or in which you lost all interest in things like work?” The sensitivity of this item as a screener for MDD has been found to range between 0.83 and 0.94.10
Depression
In order to assess the severity of depressive symptoms, the BDI-II9 was administered at 32 weeks of gestation and again 12 weeks postpartum. The BDI-II is a commonly used 21-item measure of depression. Items are scored 0–3 and summed, with higher scores reflecting greater depression severity. The BDI-II has demonstrated good reliability and validity in community, psychiatric, and medical populations.11 Research describes myriad adverse physiologic and psychologic outcomes for mothers and their offspring associated with elevated symptoms of depression, even in the absence of a clinical diagnosis of a current major depressive episode.12 Research assessing the psychometric properties of the BDI-II with postpartum women experiencing depressive episodes of mild or greater severity determined ≥10 as the optimum threshold value, with a sensitivity of 100% and specificity of 98.15%.13 In this study, BDI-II scores ≥10 were used to (1) select participants with PD and (2) dichotomize postpartum data to create a recovered depression (RD) group and a comparison group of women exhibiting sustained depression (SD). In the present study, the internal consistency reliability alphas were good during pregnancy (0.85) and postpartum (0.91).
Life events
The Life Events (LE) stress scale of the Parenting Stress Index, 3rd ed.,14 provides a structured method of inquiry to evaluate psychosocial events experienced by the immediate family during the past 12 months. We modified this measure to include 22 total potential life events that also incorporate infant safety and well-being. In the current study, the internal consistency reliability was adequate during pregnancy (0.78).
Family relationships, resources, supports, and division of labor
The Intimate Relations Questionnaire (IRQ)15 was used to evaluate emotions and behaviors in the context of romantic relationships. The IRQ is a 25-item self-report survey. Responses to questions consist of 9-point Likert items from 1 (not at all or never) to 9 (very much or extremely). Scoring of the questionnaire yields four subscale scores: love (i.e., belongingness and closeness), conflict-negativity (e.g., disagreements or expression of negative emotions), ambivalence (i.e., confusion, weighing advantages and benefits of the relationship), and maintenance (e.g., communication and problem-solving behaviors). Internal reliability consistencies varied by subscale and were 0.9, 0.74, 0.62, and 0.72, for the love, conflict, ambivalence, and maintenance subscales, respectively.
The Family Resource Scale (FRS)16 is a 30-item assessment of the sufficiency of basic needs (e.g., food and shelter) and additional household resources (i.e., entertainment, travel). Items are scored 1 to 5, with higher scores reflecting greater adequacy of resources. A not applicable (NA) option is included for items to denote items that do not apply. Four items (8, 10, 20, and 21) commonly receive NA ratings. In such instances, these responses are given a score of 5 based on the inference of the original scale developers that these scores reflect adequate access to this resource.
The Family Support Scale (FSS)17 is an 18-item assessment of the perceived utility of interpersonal resources. Potential allies, such as family members, co-workers, and professional service providers, are rated on a scale of 1 (not at all helpful) to 5 (extremely helpful). The measure has demonstrated good reliability and validity. In this study, the internal reliability alpha was adequate (0.72).
Division of labor was evaluated using an adapted survey of partners' relative participation in domestic activities.18,19 This scale was modified for use in this study to incorporate 10 items detailing general household tasks (e.g., washing dishes) and 9 items specific to division of infant care activities (e.g., changing diapers, bathing the baby). Items are scored using 5-point Likert items, with 1 representing tasks that are almost always completed by male partners and 5 denoting tasks that are almost always completed by female participants. In this study, postpartum internal consistency reliability alphas were adequate (0.78) and good (0.85) for the household and child care subscales, respectively.
Statistical analysis
An initial comparison was conducted to determine if women enrolled in Studies 1 and 2 differed on baseline depressive symptoms. No significant difference on BDI-II scores was noted (F=0.861, df=1, 38, p=0.359). Subsequently, we collapsed the data from these studies for analyses. Women with BDI-II scores ≥10 at the 12-week postpartum assessment were categorized in an SD group (n=25, 61%). Those with postpartum BDI-II scores <10 were classified in an RD group (n=16, 39%). Within-group and between-group t tests were used to evaluate differences in depression symptom severity between RD and SD groups and to analyze changes from pregnancy to postpartum.
Regarding predictors of recovery, we aimed to identify variables that would distinguish between SD and RD groups. As a preliminary step, continuous data were analyzed using between-group t tests. Ordinal variables were assessed with the Mann-Whitney U test. Given the small sample size of the present study, Fisher's exact test was used to examine nominal data and, in a subsample of multiparous women, the effect of previous history of PPD on recovery. Based on the results of the initial analyses, a logistic regression analysis was conducted using variables with significant between-group differences. Statistics were conducted using SPSS 18.0 software.
Results
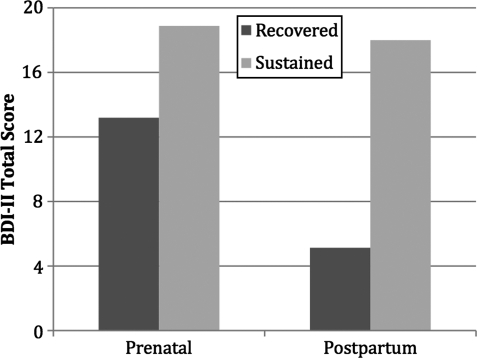
The mean prenatal BDI-II score was 16.67 (standard deviation [SD] 6.9). The SD group had average pregnancy and postpartum BDI-II scores of 18.88 (SD 7.6) and 18 (SD 6.9), respectively (t=0.61, df=24, p=0.549, d=0.12), a 0.88-point decline. In contrast, the RD group BDI-II mean was 13.19 (SD 3.6) during pregnancy. These BDI-II scores among the RD group decreased 8.04 points to a mean of 5.13 (SD 3) postpartum. Significant and large differences were observed from pregnancy to postpartum for the RD group using a paired-sample t test (t=7.248, df=15, p<0.001, d=1.81). Between-group t tests detected statistically significant and large differences between SD and RD groups during pregnancy (t=2.8, df=39, p=0.008, d=1.02) and postpartum (t=7.06, df=39, p<0.001, d=2.61). Figure 1 illustrates between-group symptom severity during pregnancy and postpartum.
FIG. 1.
Prenatal and postpartum Beck Depression Inventory-II (BD-II) symptom severity for recovered and sustained symptom groups.
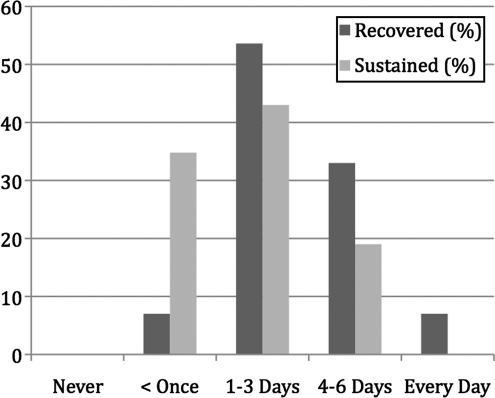
Given the observed differences between the RD and SD groups, we aimed to identify characteristics that distinguished between those who recovered and those with sustained symptoms using t tests, Mann-Whitney U tests, and Fisher's exact test as appropriate. Demographic and psychosocial characteristics are summarized in Table 1. Among the variables explored, only severity of depression during pregnancy (t=2.8, df=39, p=0.008), frequency of physical exercise (U=95, p=0.046) (Fig. 2), and cohabitation (married or live-in partner) status were statistically different between the RD and SD groups (p=0.031, Fisher's exact test). Fifty-four percent of participants used psychotherapy and 32% used psychotropic medications during the course of the study. Women completed an average of eight sessions (range 0–25). Neither engagement in psychotherapy (p=0.331, Fisher's exact test), the number of sessions attended (t=1.02, df=39, p=0.313), nor the use of psychotropic medications (p=503, Fisher's exact test) were associated with recovery status.
Table 1.
Psychosocial Characteristics of Recovered Depression and Sustained Depression Participants
| Continuous predictor variables | RD Mean (SD) | SD Mean (SD) | t | p |
|---|---|---|---|---|
| BDI-II | 13.19 (3.6) | 18.88 (7.6) | 2.8 | 0.008** |
| Age | 32 (4) | 29 (5) | 1.93 | 0.061 |
| Number of children | .81 (.8) | .95 (.7) | −0.54 | 0.593 |
| Number of pregnancies | 3.6 (2) | 2.5 (1) | 2.01 | 0.053 |
| FRS | 124 (16) | 117 (17) | 1.10 | 0.279 |
| FSS | 30.7 (12) | 24.1 (11) | 1.68 | 0.102 |
| LE | 2.7 (2) | 4.6 (3) | −1.97 | 0.057 |
| DOL (postpartum) | ||||
| Household tasks | 3.9 (1) | 3.1 (2) | 1.41 | 0.166 |
| Child care tasks | 5.3 (2) | 3.9 (3) | 1.94 | 0.060 |
| IRQ | ||||
| Love | 70.9 (15) | 72.5 (13) | −0.37 | 0.716 |
| Maintenance | 27.3 (6) | 27.0 (7) | 0.10 | 0.923 |
| Conflict | 21.9 (6) | 22.3 (8) | −0.14 | 0.888 |
| Ambivalence | 13.0 (8) | 13.1 (6) | −0.04 | 0.968 |
| Nominal predictor variables | RD % | SD % | p (FET) | |
|---|---|---|---|---|
| Psychotropic medications | 25 | 38 | 0.503 | |
| Psychotherapy utilization | 44 | 62 | 0.331 | |
| Percent Caucasian | 69 | 83 | 0.444 | |
| Tobacco use | 7 | 33 | 0.104 | |
| Percent married/cohabitating | 100 | 72 | 0.031* | |
| Lifetime depression | 53 | 81 | 0.141 | |
| Past postpartum depressiona | 25 | 75 | 0.020* |
| Ordinal predictor variables | RD % | SD % | U | p |
|---|---|---|---|---|
| Income | 121.5 | 0.689 | ||
| Very low (<$25,000) | 7 | 0 | ||
| Low ($25,000–49,999) | 7 | 26 | ||
| Moderate ($45,000–64,999) | 14 | 11 | ||
| High (>$65,000) | 71 | 63 | ||
| Exercise frequency | 95.0 | 0.046* | ||
| Never | 0 | 0 | ||
| Less than once a week | 7 | 38 | ||
| 1–3 days a week | 53 | 43 | ||
| 4–6 days a week | 33 | 19 | ||
| Every day | 7 | 0 |
The Division of Labor (DOL) scale was collected postpartum. All other reported statistics are based on data collected during pregnancy.
Parous pregnant women only.
p<0.05; **p<0.01.
BDI-II, Beck Depression Inventory-II; FET, Fisher's exact test, two-sided; FRS, Family Resource Scale; FSS, Family Support Scale; IRQ, Intimate Relations Questionnaire; LE, Life Events stress subscale; RD, recovered depressive symptoms; SD, sustained depressive symptoms; (SD), standard deviation.
FIG. 2.
Weekly exercise frequency for participants with recovered vs. sustained symptoms.
A logistic regression analysis was performed using the variables identified as significant distinguishers between RD and SD groups to evaluate their predictive utility. However, no women who recovered during the postpartum period identified as noncohabitating. Odds ratios (ORs) may be inappropriately inflated in such cases of sparse data (e.g., cells with zero observations).20 Consequently, BDI-II scores during pregnancy and exercise activity were incorporated into the logistic regression analysis, and the marital/cohabitation status variable was omitted. The resulting model was significant (chi-square=13.25, df=2, p=0.001). Lower BDI-II scores during pregnancy (OR 1.23, 95% confidence interval [CI] 1.03-1.47) and higher frequency of prenatal exercise behaviors (OR 0.27, 95% CI 0.0.08-0.92) were significant predictors of postpartum recovery. A Hosmer-Lemshow test was not significant (chi-square=3.194, df=6, p=0.794), suggesting adequate goodness-of-fit of the overall model. Summary data from the logistic regression are presented in Table 2.
Table 2.
Summary of Logistic Regression Analysis to Predict Postpartum Depression Status
| Predictor | β | β SE | Wald | p | Odds ratio | 95% CI |
|---|---|---|---|---|---|---|
| Constant | 0.997 | 0.62 | 0.23 | 0.632 | NA | NA |
| Prenatal BDI-II | 0.21 | 0.09 | 5.46 | 0.019 | 1.23 | 1.03-1.47 |
| Exercise | −1.29 | 0.62 | 4.38 | 0.036 | 0.27 | 0.08-0.92 |
| Test | chi-square | p | ||||
|---|---|---|---|---|---|---|
| Overall model evaluation | 13.25 | 0.001 | ||||
| HL goodness-of-fit test | 3.19 | 0.784 |
Cox and Snell R2=0.308. Nagelkerke R2=0.414.
BSE, β standard error; CI, confidence interval; HL, Hosmer-Lemeshow; NA, not applicable.
In addition to these analyses, we evaluated the impact of prior self-reported PPD in multiparous women (n=28); 9 of 13 women (69%) without a prior history of PPD recovered. In contrast, of the 15 women who acknowledged a prior history of PPD, only 3 (20%) recovered by the follow-up assessment (p=0.02, Fisher's exact test).
Discussion
The first aim of this study was to examine postpartum rates of recovery from elevated depressive symptoms experienced during late pregnancy. Fewer than 40% of women showed a decline in BDI-II scores to the degree that they were classified as recovered, and the majority experienced ongoing depressive symptoms during the perinatal period. Further, there was no observed difference in prenatal and postpartum symptom severity in the SD group. This suggests the naturalistic course in this group is relatively static. In contrast, those endorsing fewer symptoms during the third trimester of pregnancy were typified by a more transient presentation and reported a 61% reduction in symptom severity during the course of this study. Despite the potential attributions of certain depressive symptoms (e.g., changes in energy, mood fluctuations) as transitory and pregnancy specific,21 this study indicates most women will experience symptoms for an extended period (e.g., >5 months).
The rate of recovery in this study is lower than that reported by earlier research.3,4 This may be explained, in part, by the methods used to assess depressive symptoms and, by extension, differing thresholds of symptom severity in identifying cases and evaluating recovery. Further, in the study by Andersson et al.,4 participants with either depression or anxiety diagnoses were combined, limiting the ability to draw comparative conclusions.
The second aim of the study was to investigate factors that may differentiate the course of prenatal depressive symptoms into the postpartum period. Based on bivariate analyses, three psychosocial characteristics were associated with recovery: prenatal severity of depressive symptoms, exercise frequency, and cohabitation status. Absence of a previous history of PPD in multiparous women, although not a history of lifetime depression, discriminated between those more likely to recover and women with sustained elevations. Multivariate analyses supported the relationships among prenatal symptom severity, exercise frequency, and recovery.
Prior research has described significant negative associations between engagement prenatal exercise and postpartum symptoms of depression6,7 that are supported by this study. However, the relationship of prenatal exercise behaviors to symptom course has not previously been evaluated in prospective studies examining multiple biopsychosocial variables that may highlight the comparative magnitude of associations between myriad factors and recovery status. Unfortunately, as many as 60% of women are inactive during pregnancy, and many previously active women significantly curtail or cease exercising, particularly in later trimesters.22,23 Continued research is warranted to further evaluate the influence of exercise and the use behavioral activation strategies more broadly defined in PD recovery.
Regarding marital and live-in partner status, those with a cohabitating partner had a 50% chance of recovery. In contrast, all single women experienced a sustained elevation in depressive symptoms. Noncohabitation has been associated, although not always consistently, with depression during pregnancy24 and postpartum.2 Interestingly, despite the divergent rates of recovery based on marital status, we did not observe significant differences on other scales related to either the romantic relationships (Division of Labor [DOL], IRQ) or resources and support available to the family (FRS, FSS). Thus, the presence of a partner in the home appears more salient to resolution of depressive symptoms than the dynamics of that relationship, distribution of household tasks, or the fiscal or social support resources available to the family investigated in this study.
Finally many women, despite reporting elevated levels of depressive symptoms during pregnancy, do not necessarily warrant immediate liaison to mental healthcare. From a risk-and-resilience perspective, individual, social, or environmental characteristics may confer buffering effects that yield more transient symptom trajectories. Thus, it may be helpful to further develop and research decision-making models that match intervention recommendations to individual risk, such as stepped-care models. In considering these results, development of such predictive profiles may benefit from incorporating additional data beyond prenatal depressive symptom severity.
Consistent with older research in the area of perinatal recovery, most demographic factors evaluated in this research did not evidence strong relationships with the course of depressive symptoms. Although life stressors, lack of social support, and relationship quality have been identified as salient determinants of elevated depression in pregnancy,24 none of these factors were associated with postpartum recovery. Given the limited sample size, our results are preliminary. Thus, negative findings may not be robust and necessitate replication with attention to power and sample size. However, it is important to consider that the same factors related to the onset of symptoms may not necessarily be those related to persistence.
Future studies are needed to assess a broader variety of potential predictors. Exercise frequency was identified as a pertinent predictor in this study. By extension, a focus on other types of behavioral activation strategies may provide direction for additional research beyond the demographic and interpersonal factors that have formed the basis of most recovery research to date. Further, our participants were principally higher-income Caucasian women. Additional research is needed to determine if these results extend to women with other demographic characteristics. This research emphasized factors associated with divergent postpartum trajectories (recovered vs. sustained symptoms) in women with elevated perinatal symptoms of depression. Further research examining predictors of postpartum outcomes in women with nonelevated prenatal symptoms would be illustrative.
The overall rationale of this study was to identify potential predictors of recovery in the larger sample, all of which had the identical assessment protocol. The ancillary open treatment arm was added because it was observed that most women receiving treatment were not improving. One relevant observation is that not all women get better despite treatment. Thus, our principal aim was to examine factors (other than, but including, treatments received) that relate to improved treatment. Although no statistical differences were noted between RD and SD groups based on engagement in psychotherapy or the number of sessions attended, treatment use is a potential confounding factor. Future research is necessary to determine if factors associated with recovery differ between treated and untreated populations.
In considering our results, several weaknesses of the study are noteworthy. The small sample size in this study resulted in reduced power to detect potential key variables with less substantial effects and, as noted, limited our ability to evaluate the impact of cohabitation status more comprehensively. Second, the psychometric properties of study-specific or modified scales (e.g., GHQ, DOL, and LE) have not been fully evaluated. Third, recovery and sustained group membership was determined using self-report of mild or greater depressive symptoms. Thus, it is not clear how these results may differ for women meeting diagnostic thresholds for major depressive episodes. Fourth, a third of our participants were enrolled in a psychotherapy trial (Study 2). Finally, 12-week postpartum data were not available for 28% of those with elevated prenatal symptoms, either because of attrition or a missed data point, which may limit the generalizability of results if women with incomplete data differed systematically.
Despite these concerns, this research offers several contributions. First, this study used a longitudinal design to identify determinants of perinatal recovery from depressive symptoms. Second, identification of significant relationships among symptom severity, past history of PPD, and cohabitation status with postpartum symptom outcomes offers feasibly assessed characteristics for identifying women during pregnancy who may require additional surveillance or liaison to mental healthcare. Recognition of exercise frequency as predictive of recovery highlights the potential utility of behavioral healthcare recommendations.
Acknowledgments
Financial support was provided by the National Institute of Mental Health grants MH065062 and MH072673 and the National Institutes of Health grant UL1RRR024986. Additional bridge funding was provided by the University of Michigan Depression Center and the Department of Psychiatry. We thank Joan Zhao and Susie Hamilton for assistance with database management.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gaynes BN. Gavin N. Metlzer-Brody S, et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;(Feb):1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck CT. Predictors of postpartum depression: An update. Nurs Res. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Gotlib IH. Whiffen VE. Mount JH. Milne K. Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57:269–274. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- 4.Andersson L. Sundstrom-Poromaa I. Wulff M. Astrom M. Bixo M. Depression and anxiety during pregnancy and six months postpartum: A follow-up study. Acta Obstet Gynecol Scand. 2006;85:937–944. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- 5.Gotlib IH. Whiffen VE. Wallace PM. Mount JH. Prospective investigation of postpartum depression: Factors involved in onset and recovery. J Abnorm Psychol. 1991;100:122–132. doi: 10.1037//0021-843x.100.2.122. [DOI] [PubMed] [Google Scholar]
- 6.Downs DS. DiNallo JM. Kirner TL. Determinants of pregnancy and postpartum depression: Prospective influences of depressive symptoms, body image satisfaction, and exercise behavior. Ann Behav Med. 2008;36:54–63. doi: 10.1007/s12160-008-9044-9. [DOI] [PubMed] [Google Scholar]
- 7.Ersek JL. Brunner Huber LR. Physical activity prior to and during pregnancy and risk of postpartum depressive symptoms. J Obste Gyneco Neonatal Nurs. 2009;38:556–566. doi: 10.1111/j.1552-6909.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 8.Marcus SM. Lopez JF. McDonough S, et al. Depressive symptoms during pregnancy: Impact on neuroendocrine and neonatal outcomes. Infant Behav Dev. 2011;34:26–34. doi: 10.1016/j.infbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck AT. Steer RA. Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 10.Rost K. Burnam MA. Smith GR., Jr Development of screeners for depressive disorders and substance disorder history. Med Care. 1993;31:189–200. doi: 10.1097/00005650-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.Muzik M. Marcus SM. Heringhausen JE. Flynn H. When depression complicates childbearing: Guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36:771–788. doi: 10.1016/j.ogc.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmud WMRW. Awang A. Herman I. Mohamed MN. Analysis of the psychometric properties of the Malay version of Beck Depression Inventory II (BDI-II) among postpartum women in Kedah, northwest of peninsular Malaysia. Malaysian J Med Sci. 2004;11:19–25. [PMC free article] [PubMed] [Google Scholar]
- 14.Abidin RR. Parenting stress index. 3rd. Odessa, FL: Psychological Assessment Resouces; 1995. [Google Scholar]
- 15.Braiker H. Kelley H. Conflict in the development of close relationships. In: Burgess R, editor; Huston T, editor. Social exchange and developing relationships. New York: Academic Press; 1979. pp. 135–168. [Google Scholar]
- 16.Dunst CJ. Leet HE. Measuring the adequacy of resources in households with young children. Child Care Health Dev. 1987;13:111–125. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 17.Dunst CJ. Jenikins V. Trivette C. Family Support Scale: Reliability and validity. J Individual Fam Community Wellness. 1984;1:45–52. [Google Scholar]
- 18.Belsky J. Lang ME. Huston T. Sex typing and division of labor as determinants of marital change across the transition to parenthood. J Pers Soc Psychol. 1986;50:517–522. doi: 10.1037//0022-3514.50.3.517. [DOI] [PubMed] [Google Scholar]
- 19.Belsky J. Spanier G. Rovine M. Stability and change in marriage across the transition to parenthood. J Marriage Fam. 1983;45:855–865. [Google Scholar]
- 20.Greenland S. Schwartzbaum JA. Finkle WD. Problems due to small samples and sparse data in conditional logistic regression. Am J Epidemiol. 2000;151:531–539. doi: 10.1093/oxfordjournals.aje.a010240. [DOI] [PubMed] [Google Scholar]
- 21.Marcus SM. Depression during pregnancy: Rates, risks, and consequences—Motherisk Update 2008. Can J Clin Pharmacol. 2009;16:e15–22. [PubMed] [Google Scholar]
- 22.Poudevigne MS. O'Connor PJ. A review of physical activity patterns in pregnant women and their relationship to psychological health. Sports Med. 2006;36:19–38. doi: 10.2165/00007256-200636010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Sternfeld B. Quesenberry CP., Jr Eskenazi B. Newman LA. Exercise during pregnancy and pregnancy outcome. Med Sci Sports Exerc. 1995;37:634–640. [PubMed] [Google Scholar]
- 24.Lancaster CA. Gold KJ. Flynn HA. Yoo H. Marcus SM. Davis MM. Risk factors for depressive symptoms during pregnancy: A systematic review. Am J Obstet Gynecol. 2010;202:5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]