Abstract
Balb/c mice, which are T-helper lymphocyte 2 (Th2) responders, are highly susceptible to infectious and non-infectious heart diseases, whereas C57BL/6 mice (Th1 responders) are not. Angiotensin II (Ang II) is not only a vasopressor but also a pro-inflammatory factor that leads to cardiac hypertrophy, fibrosis and dysfunction. We hypothesized that Ang II exacerbates cardiac damage in Balb/c but not in C57BL/6 mice even though both strains have a similar level of hypertension. Twelve-week-old male C57BL/6J and Balb/c mice received either vehicle or Ang II (1.4mg kg−1 day−1, s.c. via osmotic minipump) for 8 weeks. At baseline, Balb/c mice exhibited the following: (1) a lower heart rate; (2) an enlarged left ventricular chamber; (3) a lower ejection fraction and shortening fraction; and (4) twice the left ventricular collagen deposition of age-matched C57BL/6J mice. Angiotensin II raised systolic blood pressure (to ~150 mmHg) and induced cardiomyocyte hypertrophy in a similar manner in both strains. While C57BL/6J mice developed compensatory concentric hypertrophy and fibrosis in response to Ang II, Balb/c mice demonstrated severe left ventricular chamber dilatation, wall thinning and fibrosis, leading to congestive heart failure as evidenced by dramatically decreased ejection fraction and lung congestion (significant increase in lung weight), which are both characteristic of dilated cardiomyopathy. Our study suggests that the Th phenotype plays an active role in cardiac remodelling and function both in basal conditions and in hypertension. Angiotensin II-induced dilated cardiomyopathy in Balb/c mice is an ideal animalmodel for studying the impact of the adaptive immune system on cardiac remodelling and function and for testing strategies to prevent or treat hypertension-associated heart failure.
T-helper lymphocytes (Th) are divided into two main subtypes,Th1 andTh2, according to their distinct cytokine profiles (Mosmann et al. 1986; Rogge, 2002). The Th1 cytokines include interleukin-2 and interferon-γ, which suppress Th2 response; while typical Th2 cytokines are interleukin-4 (IL-4) and interleukin-10, which inhibit Th1 (Paul & Seder, 1994). Imbalance of Th1–Th2 plays an important role in the pathophysiology of heart disease (Okura et al. 1998; Shimizu et al. 2004; Yu et al. 2006). Susceptibility to heart disease varies between strains, because each has a distinct immunological phenotype. Autoimmune- and coxsackievirus (Cox) B-induced myocarditis can be induced in Th2-dominant mice, including A/J (Afanasyeva et al. 2001), Balb/c (Huber & Lodge, 1986; Liao et al. 1993) and DBA/2 mice (Huber & Lodge, 1986); however, Th1-dominant C57BL/6 mice are reportedly resistant to heart disease (Huber & Lodge, 1986; Liao et al. 1993). Balb/c mice are also susceptible to dilated cardiomyopathy (DCM) induced by immunization with ADP/ATP carrier peptides (Liao et al. 2005) or disruption of the gene encoding the negative immunoregulatory receptor PD-1 (Nishimura et al. 2001). Although the genetic background of the host may influence the pathogenesis of heart disease, the immune system has also been implicated in its initiation and development, because anti-mouse thymocyte serum and the monoclonal antibody against Iad (a major histocompatibility complex class II molecule) effectively prevented Cox B-induced myocarditis in both DBA/2 and Balb/c mice (Huber & Lodge, 1986) and because intact Balb/c mice were found to be more susceptible to infection with Leishmania major and developed severe footpad lesions, whereas Balb/c mice rendered deficient in Vβ4+CD4+ T cells developed a Th1 response and were resistant to infection (Himmelrich et al. 2000). However, there have been very few studies of the effects of the predominant Th1 or Th2 subset on cardiac damage and dysfunction during hypertension.
Angiotensin II (Ang II), a potent vasopressor, has been implicated in the inflammatory process (Suzuki et al. 2003; Androulakis et al. 2009). As Th1/Th2 imbalance plays a role in the pathophysiology of cardiovascular remodelling and dysfunction (Okura et al. 1998; Shimizu et al. 2004; Yu et al. 2005; Yu et al. 2006) and Balb/c mice are susceptible to heart disease (Huber & Lodge, 1986; Liao et al. 1993), we hypothesized that in Ang II-induced hypertension the Th2-dominant Balb/c strain would develop exacerbated cardiac remodelling and impaired cardiac function, whereas the Th1-dominant C57BL/6 strain would be protected. In the present study, we evaluated the effect of chronic infusion of Ang II on cardiac remodelling and function in Balb/c and C57BL/6 mice.
Methods
Animals and experimental design
Twelve-week-old male C57BL/6J and Balb/c mice (27–29 g; Jackson Laboratory, Bar Harbor, ME, USA) were divided into the following four groups: (1) C57BL/6J vehicle; (2) C57BL/6J Ang II; (3) Balb/c vehicle; and (4) Balb/c Ang II. Mice were anaesthetized with sodium pentobarbital (50 mg kg−1 i.p.), and an osmotic minipump (Alzet, Cupertino, CA, USA) was implanted under aseptic conditions to deliver Ang II (1.4mg kg−1 day−1; Bachem, Torrance, CA, USA) or vehicle (saline plus 0.01 n acetic acid) for 8 weeks. This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee.
Splenic cell interferon-γ and interleukin-4 detection
Splenic cells were obtained from 12-week-old male C57BL/6J and Balb/c mice overdosed with pentobarbital sodium (200 mg kg−1, i.p.). For this, the spleen was minced into small pieces on ice with a sterile pair of scissors and passed through a 70 µm nylon mesh. Red blood cells were removed by osmotic lysis and washed twice with ice-cold PBS. Cells were seeded at a density of 2 × 105 cells per well in a 96-well plate and cultured in Roswell Park Memorial Institute (RPMI) 1640 containing 25mm Hepes, 10% fetal bovine serum, 2mm l-glutamine, 5 × 10−5 m 2-mercaptoethanol and 0.1% penicillin/streptomycin. Cells were stimulated with Dynabeads mouse T-activator CD3/CD28 (Invitrogen,Carlsbad, CA,USA). Interferon-γ and IL-4 in the conditioned medium were measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA) after incubation for 48 and 96 h, respectively.
Measurement of blood pressure and cardiac function
Systolic blood pressure (SBP) and heart rate (HR) were measured weekly in conscious mice using a non-invasive computerized tail-cuff system (BP-2000; Visitech, Apex, NC,USA) as described previously (Krege et al. 1995).They were trained for 7 days by measuring SBP and HR daily, after which both parameters were recorded weekly. Left ventricular (LV) systolic (LVDs) and diastolic dimensions (LVDd), posterior wall thickness (PWT), LV ejection fraction (EF) and shortening fraction (SF) were measured in conscious mice with a Doppler echocardiographic system equipped with a 15 MHz linear transducer (Acuson c256, Mountain View, CA, USA) as described previously (Yang et al. 1999; Xu et al. 2002).
Organ harvest
At the end of the experiment, animals were anaesthetized with pentobarbital sodium (50 mg kg−1, i.p.). The heart, lung and liver were rapidly excised, and the LV (including the septum), lung and liver were weighed. Half of the LV was fixed in 10% formaldehyde solution for morphological studies. The right leg was excised, the muscles of the tibia were removed, and tibia length (TL) was measured.
Morphological study
For myocyte cross-sectional area (MCSA) and interstitial collagen fraction (ICF), 6-µm-thick sections were deparaffinized, rehydrated and stained with Picrosirius Red using a modification of Sweat and Puchtler’s method (Sweat et al. 1964). Sections were postfixed inBouin’s fluid, followed by staining with iron Haematoxylin to show the nuclei. They were then stained with 0.1% Picrosirius Red for 1 h and washed twice with 0.5% acetic acid. Images were obtained with a computerized digital camera (Spot Diagnostic, Dexter, MI, USA).Myocytes whose nuclei were visible and whose shapes were either round or triangular were selected; the MCSA of ~150 cells was measured by computer-based planimetry (Jandel Scientific, Corte Madera, CA, USA).Collagen morphometry was examined under a microscope (Nikon E600) using normal light and analysed with SigmaScan Pro (Jandel Scientific).
Statistical analysis
Systolic blood pressure and HR were analysed using ANOVA for repeated measures. As interactions between SBP and HR with time frequently occurred, individual group comparisons at each time point were made by ANOVA using contrast statements, in which a pooled estimate of variance among all four groups is used for the prespecified contrasts. Comparisons across time were examined using Student’s paired t tests. All other variables were either not normally distributed or the standard deviation varied enough to degrade standard parametric testing; here we used a Kruskal–Wallis test followed by examination of pairwise comparisons using the Mann–Whitney procedure.
For all analyses we used Hochberg’s multiple comparisons procedure to establish significance, thereby adjusting our rejection level to ensure that an overall 0.05 α level would be maintained. We employed SAS version 9.2 (SAS Institute, Cary, NC, USA), taking P <0.05 as significant. Data are reported as means ± SEM.
Results
Splenic cell cytokines
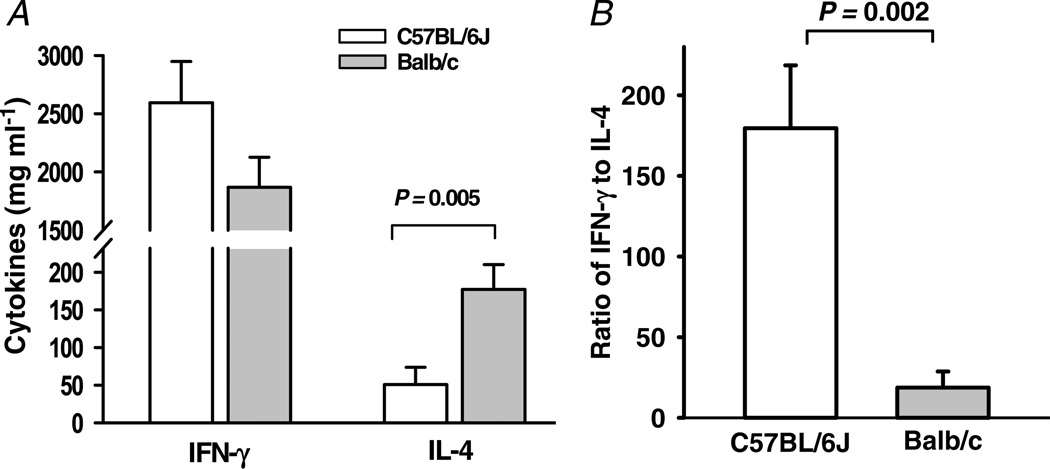
Interferon-γ and IL-4 profiles of C57BL/6J and Balb/c mice are shown in Fig. 1A. Interleukin-4 was significantly higher in Balb/c mice (P = 0.005), whereas interferon-γ tended to be higher in C57BL/6J mice, although the difference was not statistically significant (P = 0.13). The ratio of IFN-γ to IL-4 was about 180 in C57BL/6 mice, but only 19 in Balb/c mice (P = 0.002; Fig. 1B). These data confirm that C57BL/6J mice are Th1 dominant and Balb/c mice are Th2 dominant.
Figure 1. Interferon-γ (IFN-γ) and interleukin-4 (IL-4) content in the conditioned medium of splenic cells from C57BL/6J and Balb/c mice.
A, IL-4 was more than threefold higher in Balb/c mice (n = 9–10). B, the ratio of IFN-γ to IL-4 was significantly higher in C57BL/6J mice.
Systolic blood pressure and heart rate
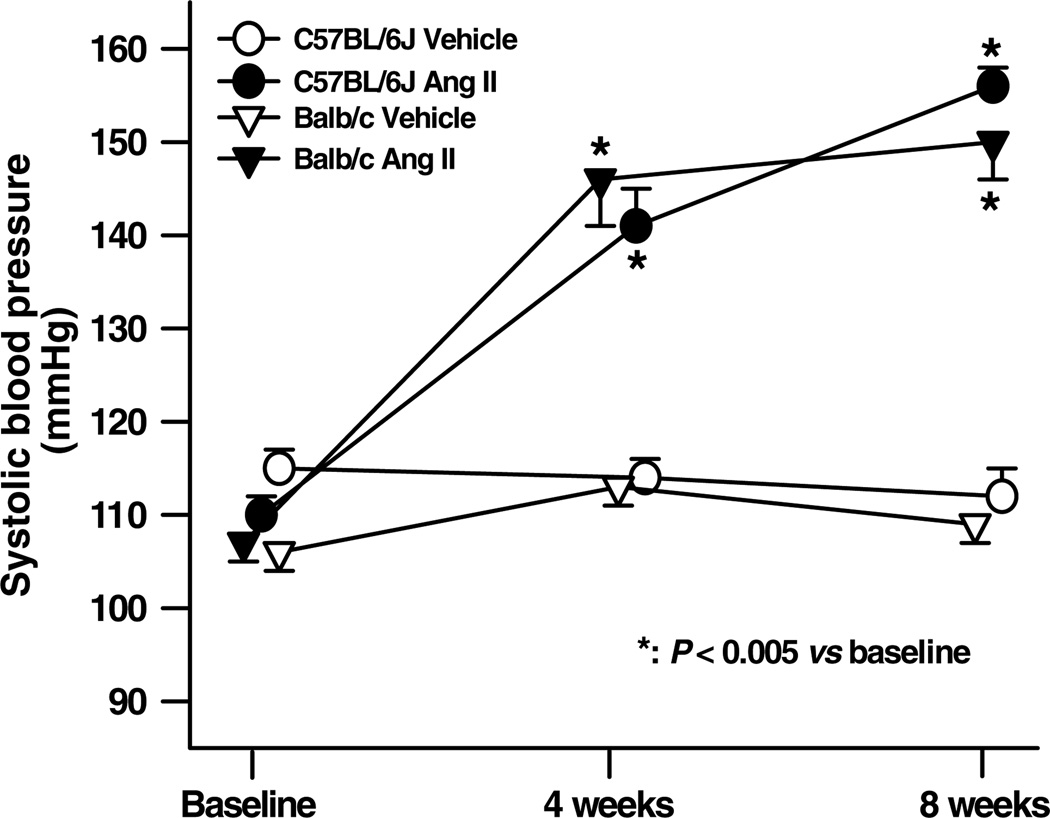
Both basal SBP and the increase seen with Ang II were similar for both strains (Fig. 2). Basal HR was lower in Balb/c mice (556 ± 9 versus 489 ± 11 beats min−1, P <0.05), while Ang II increased HR in Balb/c mice (542 ± 16 beats min−1, P <0.01), but had no effect on C57BL/6J mice.
Figure 2. Effect of angiotensin II (Ang II) on systolic blood pressure in C57BL/6J and Balb/c mice.
Angiotensin II induced hypertension to a similar extent in both strains (n = 16–19).
Body, left ventricular, lung and liver weight
Angiotensin II significantly reduced body weight in both C57BL/6J and Balb/c mice, but had no effect on TL (Table 1); therefore, organ weight was normalized to TL instead of body weight. Angiotensin II significantly increased LV weight, LV weight/TL and lung weight/TL in both strains. While the increases in LV weight and LV weight/TL were more obvious in C57BL/6J mice (P <0.005), Balb/c mice had a higher lung weight/TL (P <0.005; Table 1). Liver weight/TL did not differ among groups.
Table 1.
Body, heart, lung and liver weight in C57BL/6J and Balb/c mice treated with angiotensin II (Ang II)
| Parameter | C57BL/6J vehicle |
Balb/c vehicle |
C57BL/6J Ang II | Balb/c Ang II |
|---|---|---|---|---|
| Body weight (g) | 29.4 ± 0.5 | 29.6 ± 0.5 | 26.9 ± 0.4* | 25.9 ± 0.4† |
| Tibia length (mm) | 18.3 ± 0.1 | 18.4 ± 0.1 | 18.2 ± 0.1 | 18.4 ± 0.1 |
| Left ventricular weight (mg) | 95.5 ± 1.6 | 91.6 ± 1.5 | 132.9 ± 4.5* | 116.5 ± 3.1†‡ |
| Left ventricular weight/tibia length (mg mm−1) | 5.44 ± 0.09 | 5.10 ± 0.1 | 7.87 ± 0.32* | 6.32 ± 0.23†‡ |
| Lung weight/tibia length (mg mm−1) | 9.01 ± 0.17 | 9.10 ± 0.24 | 10.68 ± 0.33* | 13.72 ± 0.91†‡ |
| Liver weight/tibia length (mg mm−1) | 75.6 ± 2.5 | 74.7 ± 1.9 | 78.1 ± 1.9 | 69.5 ± 3.1 |
P < 0.005, C57BL/6J Ang II versus C57BL/6J vehicle.
P < 0.005, Balb/c Ang II versus Balb/c vehicle.
P < 0.05, C57BL/6J Ang II versus Balb/c Ang II.
Cardiac remodelling and function
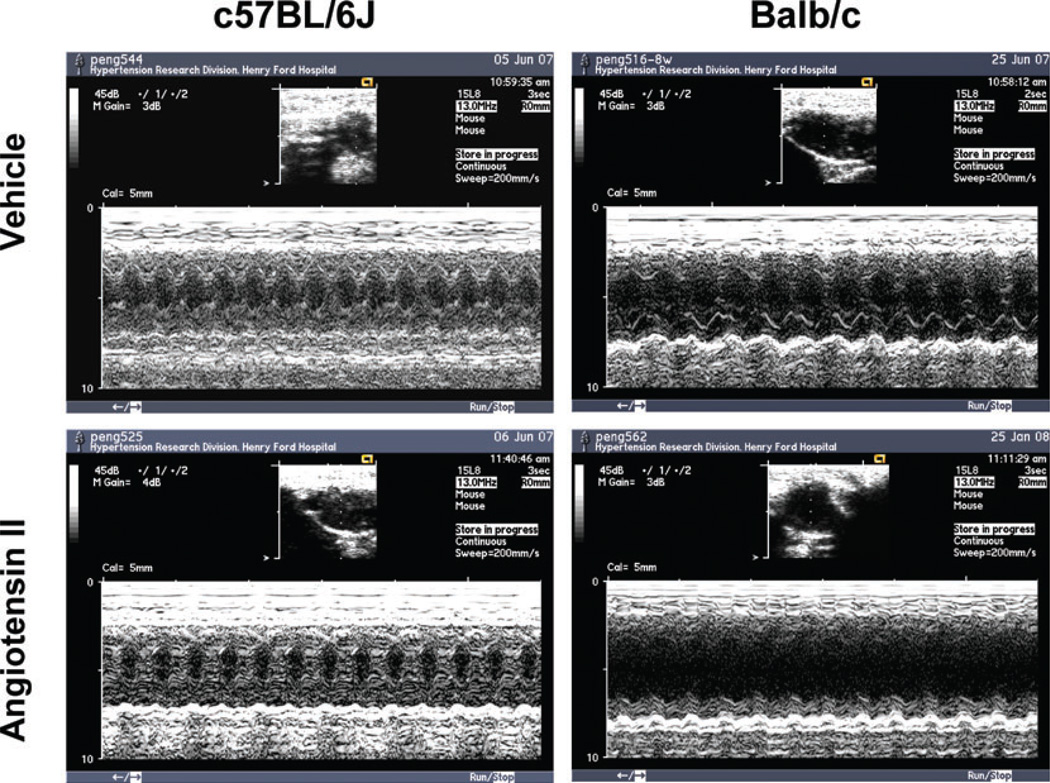
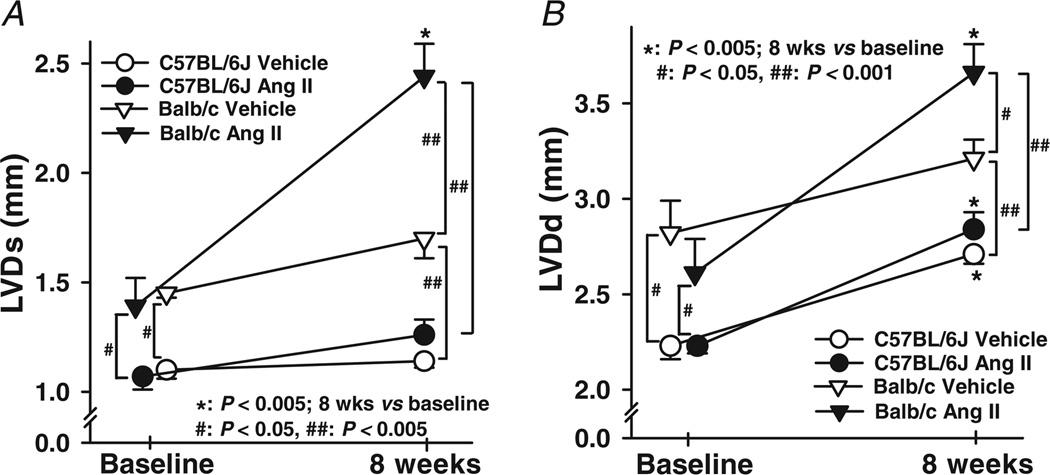
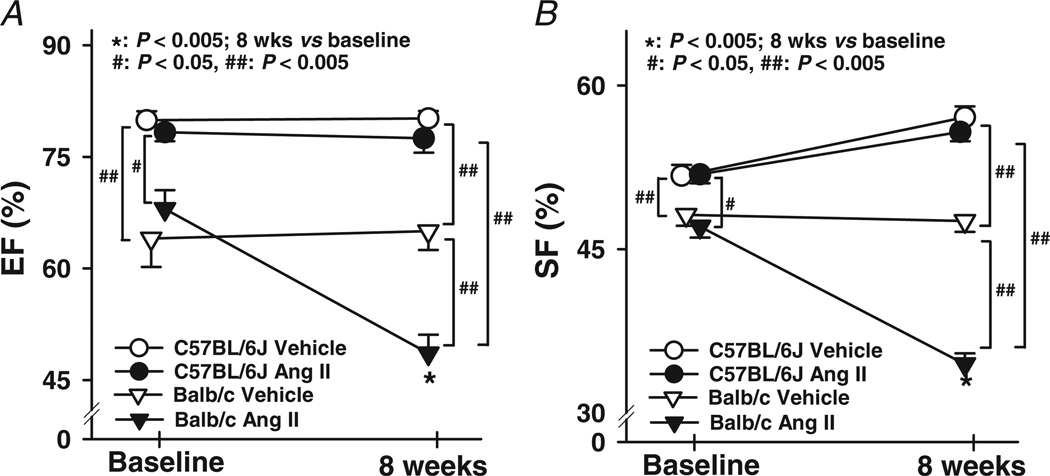
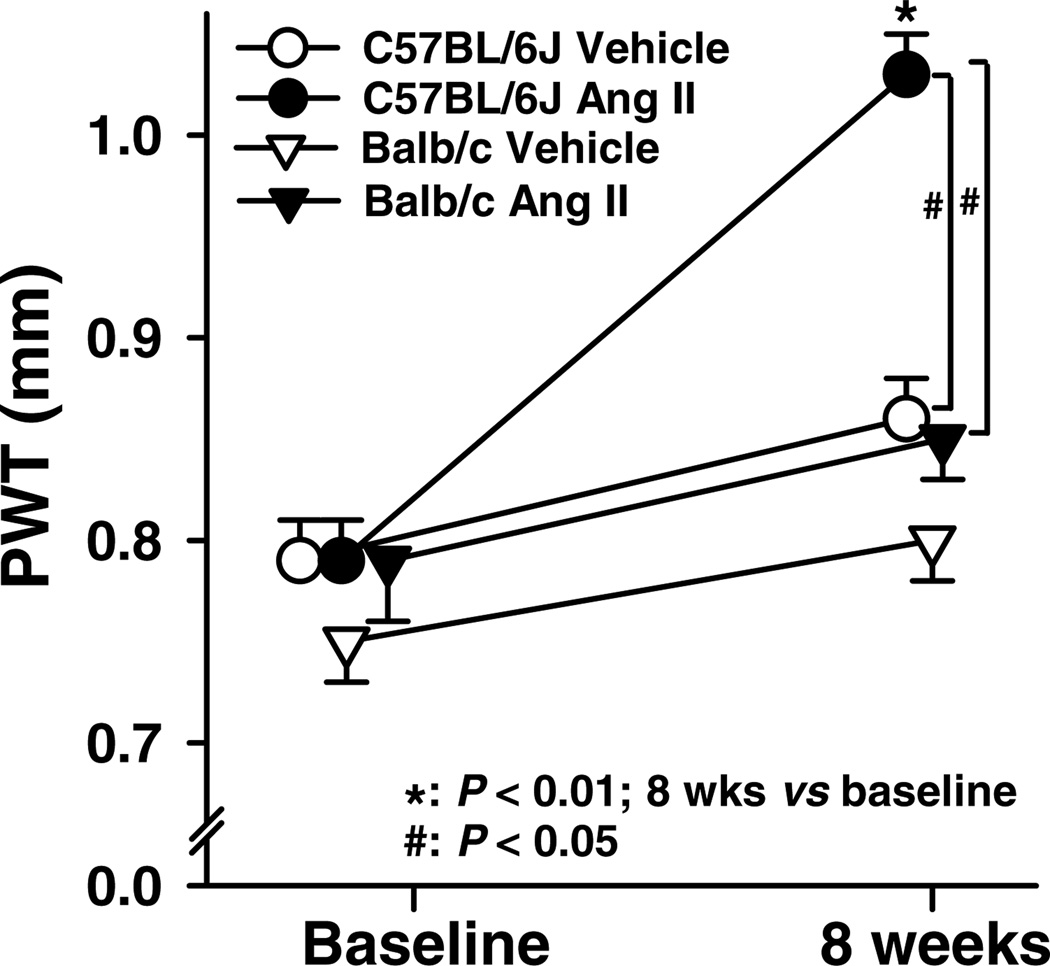
Basal LVDs was significantly larger in Balb/c mice and increased further with Ang II, which was not seen in C57BL/6J mice (Figs 3 and 4A). In Balb/c mice, basal LVDd was also greater and increased markedly with Ang II (Figs 3 and 4B). Basal EF and SF were significantly lower in Balb/c mice, and both deteriorated further with Ang II, which again was not seen in C57BL/6J mice (Fig. 5). These data suggest that Balb/c mice have inheritable LV dilatation and impaired LV contractile function and are more susceptible to Ang II-induced cardiac remodelling and functional damage than C57BL/6J mice. Basal LV PWT did not differ between Balb/c and C57BL/6J mice, while Ang II significantly increased PWT in C57BL/6J but not Balb/c mice (Fig. 6), indicating compensatory concentric hypertrophy (which was not seen in Balb/c mice).
Figure 3. Representative M-mode echocardiography on conscious C57BL/6J and Balb/c mice given vehicle (upper panels) or Ang II (lower panels) for 8 weeks.
Note the clear left ventricular chamber enlargement in Balb/c versus C57BL/6J mice, which was exaggerated in Balb/c but not C57BL/6J mice with Ang II-induced hypertension.
Figure 4. Changes in left ventricular systolic (LVDs) and diastolic dimension (LVDd) in C57BL/6J and Balb/c mice treated with Ang II.
Vehicle-treated Balb/c mice had higher LVDs and LVDd both at baseline and after 8 weeks, while LVDd in vehicle- or Ang II-treated C57BL/6J mice was increased at 8 weeks as well. Angiotensin II markedly increased LVDs and LVDd further in Balb/c but not C57BL/6J mice (n = 25–29).
Figure 5. Effect of Ang II on left ventricular ejection fraction (EF) and shortening fraction (SF) in C57BL/6J and Balb/c mice.
Balb/c mice had lower EF and SF than C57BL/6J mice both at baseline and after 8 weeks. Angiotensin II dramatically decreased EF and SF in Balb/c mice, but had no effect on C57BL/6J mice (n = 25–29).
Figure 6. Posterior wall thickness (PWT) of the left ventricle in C57BL/6J and Balb/c mice treated with Ang II.
Basal PWT was similar in C57BL/6J and Balb/c mice. Angiotensin II increased PWT significantly in C57BL/6J but not Balb/c mice (n = 25–29).
Cardiomyocyte size and interstitial collagen
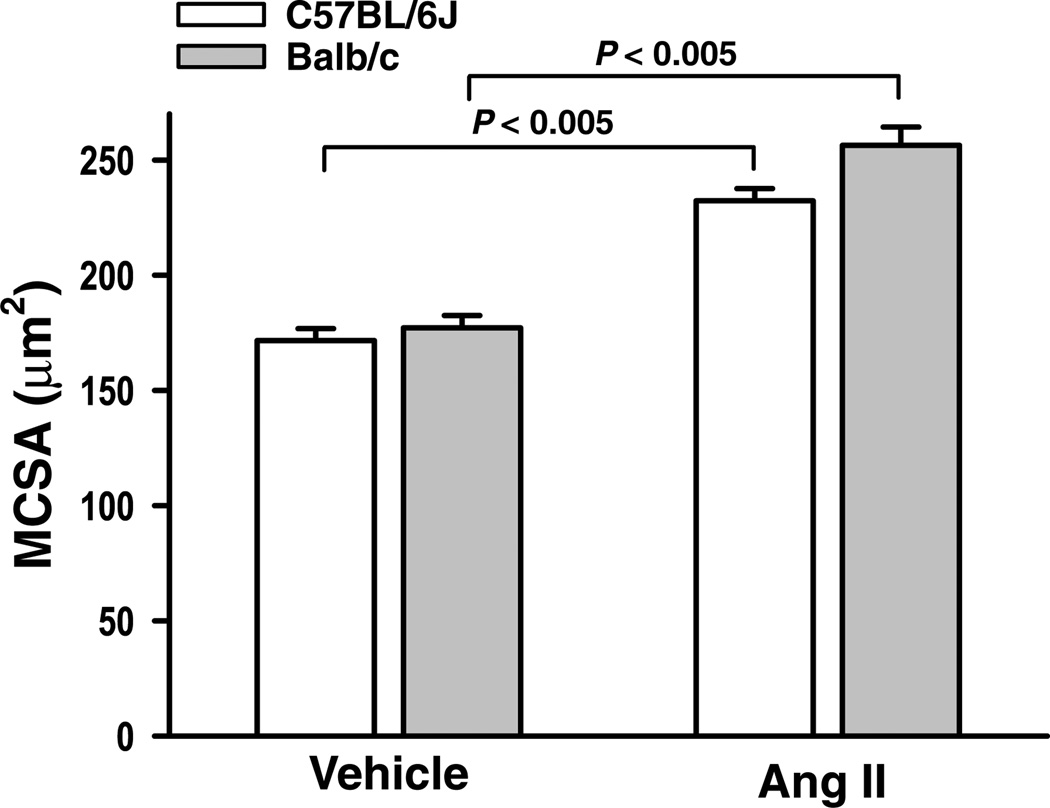
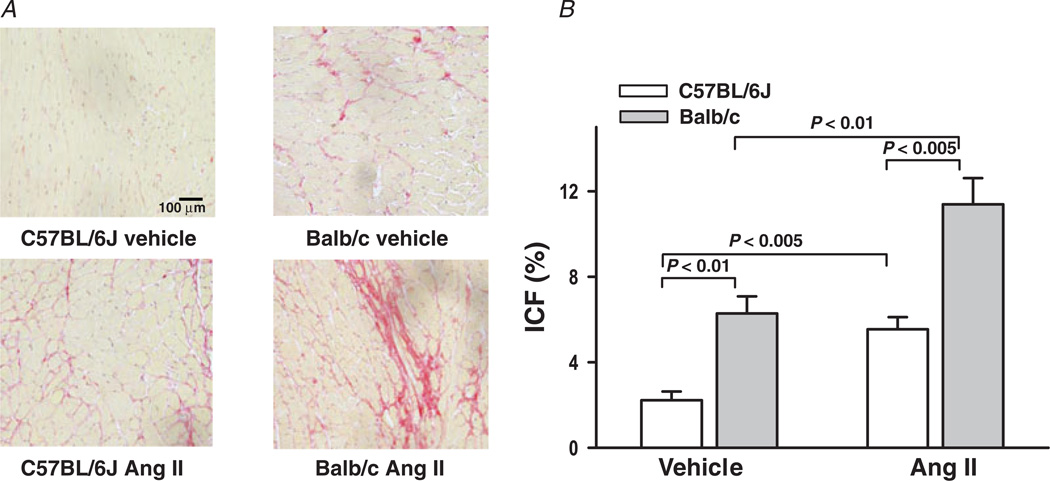
There was no difference in MCSA between C57BL/6J and Balb/c mice treated with vehicle (Fig. 7). Angiotensin II increased MCSA to a similar extent in both strains. Left ventricular interstitial collagen fraction (ICF) was significantly higher in Balb/c mice given vehicle (6.28 ± 0.86 versus 2.22 ± 0.41%; P <0.01). Angiotensin II doubled ICF in both strains, but collagen scarring was only apparent in Balb/c mice (Fig. 8A), suggesting loss of myocytes and subsequent collagen replacement (P <0.005; Fig. 8B).
Figure 7. Myocyte cross-sectional area (MCSA) of the left ventricle in C57BL/6J and Balb/c mice treated with Ang II.
The MCSA was similar in both C57BL/6J and Balb/c mice. Angiotensin II significantly increased MCSA to a similar extent in both strains (n = 7–12).
Figure 8. Interstitial collagen fraction (ICF) of the left ventricle in C57BL/6J and Balb/c mice treated with Ang II.
A, interstitial collagen stains red when treated with Picrosirius Red. B, quantitative data on ICF. Vehicle-treated Balb/c mice had greater collagen deposition at baseline, while Ang II increased ICF significantly in both strains (n = 7–10).
Discussion
In order to identify the Th phenotype of the mice used in this study, IFN-γ (a typical Th1 cytokine) and IL-4 (a typical Th2 cytokine) were measured in conditioned medium of cultured splenic cells. Interferon-γ was 1.4-fold higher in C57BL/6J mice, whereas IL-4 was more than threefold higher in Balb/c mice, making the ratio of IFN-γ to IL-4 10-fold higher in C57BL/6J mice. These results are consistent with other reports (Mills et al. 2000; Yu et al. 2006) and confirm that C57BL/6 mice are Th1 dominant, while Balb/c mice are Th2 dominant. In basal conditions, 12-week-old Balb/c mice exhibited the following characteristics: (1) a lower heart rate; (2) an enlarged LV chamber; (3) a lower LV EF and SF; and (4) twice the LV collagen deposition of C57BL/6J mice. Similar findings were reported by Yu et al. (2006), who observed that 4-week-old Balb/c mice had a lower EF and increased LV end-diastolic and LV end-systolic volume. More importantly, when haemodynamic loading was applied by chronic infusion of Ang II, Balb/c mice exhibited greater LV remodelling and dysfunction as characterized by dilatation, reduced EF, greater interstitial fibrosis and fibrotic scar formation. Taken together, these findings demonstrate that Balb/c mice (Th2 responders) exhibit cardiac remodelling and deterioration of cardiac function in physiological conditions and are susceptible to Ang II-induced pressure overload, leading to exacerbated LV remodelling and damage and ultimately DCM, while C57BL/6J mice (Th1 responders) develop concentric hypertrophy, which may compensate for Ang II-induced hypertension.
It is not clear why Balb/c mice are prone to cardiac fibrosis, LV chamber dilatation and dysfunction, whereas C57BL/6J are not. Numerous studies have shown that C57BL/6J mice infused with high doses of Ang II for periods of 4–8 weeks maintain either normal cardiac function (ejection and/or shortening fractions; Xu et al. 2008; Haudek et al. 2010; Harding et al. 2011) or only a minimal decrease in ejection fraction (Zhang et al. 2010). Overall, C57BL/6J mice exhibited robust resistance towards developing heart failure when they were subjected to sustained hypertension for reasons that remain unclear. In contrast, studies have shown that transcription of several genes known to be involved in wound healing and fibrosis is upregulated by Th2 cytokines. In Th2-polarized mice infected with Schistosoma mansoni, large granulomas with massive collagen deposition formed in the liver (Hoffmann et al. 2001) and lung (Sandler et al. 2003), together with upregulation of genes important for the fibrotic response. Those reports provide further proof that fibrogenesis is intimately linked with a Th2 response. T-helper lymphocyte 2 dominance has been implicated in DCM, as the severity of DCM in patients with cardiac insufficiency has been attributed to increased Th2-type CD4+ T lymphocyte infiltration of cardiac tissue (Kuethe et al. 2006). Consistent with our findings, Yu et al. (2005) reported that induction of the Th2 phenotype by infecting C57BL/6J mice with LP-MB5 retrovirus decreased both diastolic and systolic function. They further showed that the Th2-responder Balb/c mice exhibited increased ventricular volume and decreased EF compared with the Th1-responder C57BL/6J mice, along with reduced cross-linked collagen (Yu et al. 2006). Decreased collagen cross-linkage could alter the integrity of the myocardial fibrillar network, causing myocyte slippage and eventually LV chamber dilatation, which may explain the changes in cardiac remodelling and function that we observed in Balb/c mice.
Disruption of the gene encoding the negative immunoregulatory receptor PD-1 has been shown to induce DCM in Balb/c mice, while knocking out recombinase-activating gene 2 (RAG-2−/−) in Balb/c mice with PD-1−/− prevented the development of DCM (Nishimura et al. 2001). As RAG-2−/− mice lack mature lymphocytes (Shinkai et al. 1992), one could attribute DCMin Balb/c PD-1−/− mice to T and/or B lymphocytes, as evidenced by the fact that it was successfully transferred from mice with DCM into RAG-2−/− by infusion of spleen or bone marrow cells (Nishimura et al. 2001). As Balb/c mice are Th2 responders, polarized cytokine production by Th1/Th2, as well as their distinct genetic background and predisposition, may play an important role in the onset and development of heart disease.
Angiotensin II acts not only as a vasopressor but also as a paracrine and autocrine hormone to promote cell growth, apoptosis, inflammation and tissue damage that ultimately lead to hypertrophy, fibrosis and heart failure (Paul et al. 2006; Androulakis et al. 2009). Studies have shown that mice with the Th2 phenotype are predisposed to heart disease (Huber&Lodge, 1986; Liao et al. 1993; Afanasyeva et al. 2001; Liao et al. 2005), although their susceptibility to Ang II-induced cardiac damage (or lack of it) is not known. In the present study, we examined cardiac structural and functional responses to Ang II-induced hypertension in both C57BL/6J and Balb/c mice and found that following a similar increase in blood pressure C57BL/6J mice developed compensatory concentric hypertrophy (indicated by significantly increased LV PWT and well-preserved LV function) and fibrosis, whereas Balb/c mice exhibited eccentric hypertrophy and DCM as evidenced by a greatly increased LV chamber dimension, thinner posterior wall, greater LV collagen deposition, fibrotic scarring and lower EF and SF. Interestingly, the increase in MCSA in response to Ang II was similar between strains, but the increase in LV weight was significantly less in Balb/c mice. One possible explanation for this inconsistency is that Balb/c mice may exhibit greater loss of cardiomyocytes, which would account for their lower heart weight and thinner posterior wall. The severe fibrosis and apparent scarring may indicate replacement of cardiomyocytes by fibrotic tissue. It is also unclear why Balb/c mice are predisposed to DCM with Ang II-induced hypertension; however, this could be due to Th2 dominance-related detrimental effects on the heart, as we have discussed above. One important determinant of LV contractile function is wall stress. According to Laplace’s law, wall stress correlates positively with LV pressure and chamber area and negatively with wall thickness. In our study, when systolic pressure in Balb/c mice is increased to the same level as in C57BL/6J mice, they already exhibit a small but significant increase in LV chamber dimension compared with C57BL/6J mice, which suggests that wall stress is elevated significantly and that these Balb/c mice are therefore predisposed to a decline in cardiac function after stress.
In conclusion, our mouse model of Ang II-induced DCM is an ideal means of assessing DCM related to Th1–Th2 imbalance. However, the mechanism(s) by which Balb/c mice develop DCM in response to Ang II-induced hypertension remain incompletely understood. Future studies exploring the mechanism(s) involved could lead to a greater understanding of the role of Th cells in cardiac remodelling and function and the development of more effective strategies to prevent or treat hypertension-associated heart failure.
Acknowledgements
This study was supported by Henry Ford Hospital Institutional Fund A10163.
References
- Afanasyeva M, Wang Y, Kaya Z, Park S, Zilliox MJ, Schofield BH, Hill SL, Rose NR. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulakis ES, Tousoulis D, Papageorgiou N, Tsioufis C, Kallikazaros I, Stefanadis C. Essential hypertension: is there a role for inflammatory mechanisms? Cardiol Rev. 2009;17:216–221. doi: 10.1097/CRD.0b013e3181b18e03. [DOI] [PubMed] [Google Scholar]
- Harding P, Yang XP, He Q, LaPointe MC. Lack of microsomal prostaglandin E synthase-1 reduces cardiac function following angiotensin II infusion. Am J Physiol Heart Circ Physiol. 2011;300:H1053–H1061. doi: 10.1152/ajpheart.00772.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelrich H, Launois P, Maillard I, Biedermann T, Tacchini-Cottier F, Locksley RM, Rocken M, Louis JA. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol. 2000;164:4819–4825. doi: 10.4049/jimmunol.164.9.4819. [DOI] [PubMed] [Google Scholar]
- Hoffmann KF, McCarty TC, Segal DH, Chiaramonte M, Hesse M, Davis EM, Cheever AW, Meltzer PS, Morse HC, 3rd, Wynn TA. Disease fingerprinting with cDNA microarrays reveals distinct gene expression profiles in lethal type 1 and type 2 cytokine-mediated inflammatory reactions. FASEB J. 2001;15:2545–2547. doi: 10.1096/fj.01-0306fje. [DOI] [PubMed] [Google Scholar]
- Huber SA, Lodge PA. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986;122:284–291. [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- Kuethe F, Braun RK, Foerster M, Schlenker Y, Sigusch HH, Kroegel C, Figulla HR. Immunopathogenesis of dilated cardiomyopathy. Evidence for the role of TH2-type CD4+T lymphocytes and association with myocardial HLA-DR expression. J Clin Immunol. 2006;26:33–39. doi: 10.1007/s10875-006-7585-x. [DOI] [PubMed] [Google Scholar]
- Liao L, Sindhwani R, Leinwand L, Diamond B, Factor S. Cardiac alpha-myosin heavy chains differ in their induction of myocarditis. Identification of pathogenic epitopes. J Clin Invest. 1993;92:2877–2882. doi: 10.1172/JCI116909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YH, Yuan J, Wang ZH, Cheng X, Zhang JH, Tian Y, Dong JH, Guo HP, Wang M. Infectious tolerance to ADP/ATP carrier peptides induced by anti-L3T4 monoclonal antibody in dilated cardiomyopathy mice. J Clin Immunol. 2005;25:376–384. doi: 10.1007/s10875-005-4187-y. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Okura Y, Takeda K, Honda S, Hanawa H, Watanabe H, Kodama M, Izumi T, Aizawa Y, Seki S, Abo T. Recombinant murine interleukin-12 facilitates induction of cardiac myosin-specific type 1 helper T cells in rats. Circ Res. 1998;82:1035–1042. doi: 10.1161/01.res.82.10.1035. [DOI] [PubMed] [Google Scholar]
- Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Rogge L. A genomic view of helper T cell subsets. Ann N Y Acad Sci. 2002;975:57–67. doi: 10.1111/j.1749-6632.2002.tb05941.x. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-γ signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–308. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Sweat F, Puchtler H, Rosenthal SI. Sirius Red F3BA as a stain for connective tissue. Arch Pathol. 1964;78:69–72. [PubMed] [Google Scholar]
- Xu J, Carretero OA, Liu YH, Shesely EG, Yang F, Kapke A, Yang X-P. Role of AT2 receptors in the cardioprotective effect of AT1 antagonists in mice. Hypertension. 2002;40:244–250. doi: 10.1161/01.hyp.0000029095.23198.ad. [DOI] [PubMed] [Google Scholar]
- Xu Z, Okamoto H, Akino M, Onozuka H, Matsui Y, Tsutsui H. Pravastatin attenuates left ventricular remodeling and diastolic dysfunction in angiotensin II-induced hypertensive mice. J Cardiovasc Pharmacol. 2008;51:62–70. doi: 10.1097/FJC.0b013e31815bb629. [DOI] [PubMed] [Google Scholar]
- Yang X-P, Liu YH, Rhaleb N-E, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48:98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
- Yu Q, Watson RR, Marchalonis JJ, Larson DF. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol. 2005;289:H643–H651. doi: 10.1152/ajpheart.00073.2005. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, Wu EX, Szalai AJ, Wong BC, Lau CP, Lan HY. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]