SUMMARY
Community antibiotic utilization and its relationship with trachoma has been poorly characterized in areas with endemic trachoma. A survey of all drug-dispensing facilities in an area of rural Ethiopia was conducted. Antibiotic use was calculated using both retrospective and prospective methodology, and expressed as defined daily doses (DDDs). Overall antibiotic consumption estimates ranged from 2.91 to 3.07 DDDs per 1000 person days. Macrolide antibiotics accounted for 0.01 to 0.02 DDDs per 1000 person days. Each additional DDD of antibiotic use per 1000 person days was associated with a 15.0% (95% CI −19.7 to −10.3) decrease in the prevalence of clinically active trachoma among children under 10 years of age after adjusting for age, gender, altitude and the distance to nearest town. Increased background community antibiotic use may therefore be an aspect of socioeconomic development that can partially explain why trachoma prevalence has decreased in some areas in the absence of a trachoma program. The low volume of macrolide consumption in this area suggests that selection for nasopharyngeal pneumococcal macrolide resistance after mass azithromycin treatments likely has little clinical significance.
Keywords: Pharmacies, Defined Daily Dose, Chlamydia, Azithromycin, Mass Drug Ddministration, Africa
1. Introduction
Trachoma, caused by infection with ocular strains of chlamydia, is the leading cause of infectious blindness worldwide.1 Components of the WHO’s SAFE strategy, including mass antibiotic distributions, facial hygiene promotion, and environmental improvements, are effective in reducing the burden of ocular chlamydial infection and trachoma.2 Even in the absence of a designated trachoma control program, reductions in trachoma prevalence have been reported in some areas of Africa and Asia in recent years.3–5 This secular trend has been attributed to inherent benefits of economic development, including improved hygiene and sanitation. Additionally, increased antibiotic use may also accompany development, and may account for some of the observed secular trend.6
Antibiotic consumption in trachoma-endemic areas of Africa has been poorly characterized. In this study, our goal was to quantify the amount of antibiotic use in a rural area of Ethiopia in order to analyze whether community antibiotic use was associated with clinically active trachoma or ocular chlamydial infection, and to compare the amount of background antibiotic use with the volume of antibiotics used for mass azithromycin distributions.
2. Materials and methods
We conducted a series of cluster-randomized clinical trials for trachoma from May 2006 until November 2009 in the Goncha Siso Enese woreda, Amhara Region, Ethiopia. A woreda is a government-defined district, which is divided into multiple smaller geographic units known as kebeles. Kebeles are in turn subdivided into 3 smaller units called subkebeles. Our study area encompassed 72 contiguous subkebeles from 25 of the 38 kebeles of Goncha Siso Enese woreda. Subkebeles in the study area were homogeneously rural and poor, and trachoma was hyperendemic. No mass azithromycin treatments had been distributed previously. The study design has been described in detail elsewhere.7 Briefly, each subkebele was randomized to one of six different trachoma treatment strategies, which included varying frequencies and target populations for mass azithromycin distributions. We excluded subkebeles near the woreda center, Gindewoin, since urban communities are thought to have a lower prevalence of trachoma.8,9 We also excluded inaccessible communities, defined as being located greater than a three-hour walk from the closest point an automobile could reach. As part of the baseline assessment for the clinical trial, local workers from the Ethiopian Ministry of Health performed a population census of the study area. From this census, we estimated the population of each of the 25 kebeles in the study area. Three subkebeles were part of the study area but not included in the trial; the population for these subkebeles was imputed as the mean of the remaining subkebeles in the kebele. Approximately 2–4 weeks later, health workers performed conjunctival examination and swabbing in 60 of the subkebeles on a random sample of 50 children aged 0–9 years from each subkebele. The remaining 12 subkebeles were randomized to an untreated control group, and had similar monitoring performed 12 months later. From these 72 subkebeles, we determined the prevalence of clinically active trachoma and chlamydial infection among 0–9 year-old children before azithromycin treatment. Clinically active trachoma was defined as follicular trachomatous inflammation (TF) and or intense trachomatous inflammation (TI) according to the simplified WHO grading system.10 Conjunctival swabs were transported to San Francisco for pooled PCR testing for Chlamydia trachomatis using the AMPLICOR platform (Roche Diagnostics, Branchburg, NJ, USA). The transport, storage, pooling, and processing of samples has been described previously.7 The subkebele prevalence of chlamydial infection was obtained with maximum likelihood estimation; the number of positive individual samples most likely to have resulted in the observed pooled PCR results was chosen as the estimate for that village.11,12
We surveyed all drug-dispensing facilities that community members living in the 72 subkebeles of our study area would have access to, including governmental health centers, private pharmacies and private medical clinics. Facilities were located both within and outside of the study area (Figure 1). The survey was completed at the conclusion of the trial in November 2009, 42 months after the baseline monitoring and initial azithromycin treatment. The survey consisted of three parts: the facility worker was asked to rank each of eight classes of antibiotics from the most dispensed (rank no. 1) to least dispensed (no. 8); the worker reviewed the facility invoices to determine the quantity of each antibiotic ordered during the preceding six months; and for seven consecutive days, the facility worker prospectively recorded the dose and volume of each antibiotic dispensed, as well as the patient age and kebele in which the patient lived.
Figure 1.
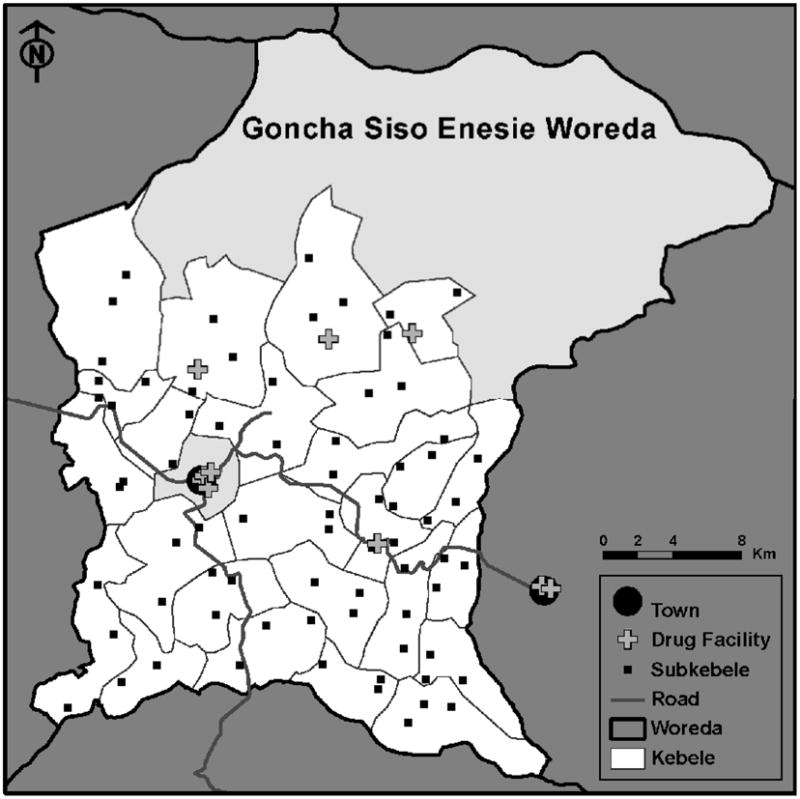
Map of the woreda (district) of Goncha Siso Enesie in the Amhara Region of Ethiopia. The study area is highlighted in white. Each of the 25 kebeles in the study area was divided into three subkebeles; subkebele markers on the map correspond to the locations where trachoma monitoring occurred. The central kebele (grey) contained the town of Gindewoin, and was not included in the study area. There were 12 drug-dispensing facilities accessible to the study area, including six facilities located in Gindewoin.
We calculated the average rank given to each antibiotic class among the 12 facilities. Any antibiotics not ranked by a facility were treated identically, and assigned the average value of the remaining ranks. We quantified antibiotic consumption by calculating defined daily doses (DDDs); DDDs were developed by the World Health Organization as a way of standardizing drug utilization.13 Each drug has a specific DDD, which corresponds to the average daily maintenance dose for that drug’s major indication. Using the appropriate anatomical therapeutic chemical (ATC) code for each antibiotic (http://www.whocc.no/atc_ddd_index/), we converted grams of antibiotics into DDDs. We calculated DDDs of antibiotics consumed in the 72-subkebele study area from the invoice data (referred to as the Invoice method), and from the week-long record of dispensed antibiotics (referred to as the Dispensed method). We did not know the proportion of total antibiotics that were dispensed to the study area for the Invoice method, so we assumed this proportion was similar to that of the Dispensed method, and weighted the estimates accordingly. We displayed the total DDDs dispensed by each facility per day, as estimated by both methods. For the Dispensed method, we also showed the number of prescriptions dispensed per day, and displayed age-stratified DDDs and prescriptions. We calculated the DDDs per 1000 person days for each antibiotic listed in the survey according to each method, using population estimates from the clinical trial. We had information on the age and kebele address for the Dispensed method only; therefore, we used the Dispensed method to calculate DDDs per 1000 person days stratified by age group (0–9 years versus 10 years and older) and by kebele. We calculated DDDs per 1000 person days for the kebele containing the town of Gindewoin using population estimates from the Goncha Siso Enese woreda health office. Percentile confidence intervals were estimated for the invoice method by resampling the total number of invoices from a Poisson distribution, and resampling the DDD for each invoice from the available data (9999 repetitions). For the Dispensed method, confidence intervals were estimated by resampling the daily dispensed DDDs (9999 repetitions).
We used linear regression to investigate the association between antibiotic consumption and trachoma in the 72 subkebeles included in the clinical trial. We analyzed the data at the subkebele level to maximize the amount of information for our regression model, assuming that each subkebele had the same amount of antibiotic consumption as the kebele to which it belonged. We used the log-transformed pre-treatment prevalence of chlamydial infection from each subkebele as the response variable, and the subkebele-specific DDDs of antibiotic use among 0–9 year old children (per 1000 person days, as estimated from the Dispensed method) as the primary explanatory variable. We adjusted for the number of children aged 0–9 years, number of females, altitude, and distance to the nearest town in an attempt to control for socioeconomic and demographic differences between the subkebeles. A similarly adjusted analysis was performed using the log-transformed pre-treatment prevalence of clinically active trachoma as the response variable. Inherent in this analysis is the assumption that antibiotic consumption had not been altered over the 3.5 years of the study by the clinical trial interventions. In order to test this assumption, we performed an ANOVA to test whether the mean DDDs dispensed per subkebele differed among study arms.
Finally, DDDs of azithromycin were estimated for an annual mass azithromycin distribution, using data from the baseline treatment. We analyzed the treatment log from 24 subkebeles of two treatment arms that received a single mass azithromycin treatment. The total volume of azithromycin distributed to these subkebeles was tabulated and converted to DDDs per 1000 person days. Note that the antibiotics consumed as part of mass treatments were not included in the regression analyses described above.
3. Results
Characteristics of the 72 subkebeles included in the study area are described in Table 1. We conducted surveys at all 12 operating facilities that dispensed drugs to individuals living within the clinical trial study area (Figure 1). An additional facility existed, but was not being staffed, and had not been staffed for several months. Of the 12 facilities, four were located within the study area, and eight were outside of, but adjacent to, the study area. Six of the facilities were located in the town of Gindewoin, which was not in a study kebele, but was located in the geographic center of the study area. Five of the facilities were at a government health center, two were private pharmacies, and five were private clinics. Facility workers were asked to rank antibiotic classes from most to least dispensed, based only on their impression from working at the facility. On average, facility workers thought penicillin antibiotics were dispensed most frequently, followed by sulfonamide, tetracycline, chloramphenicol, and fluoroquinolone antibiotics (Table 2).
Table 1.
Characteristics of 72 subkebeles in a rural area in Ethiopia
| Subkebele characteristic | Mean ± SD |
|---|---|
| Population, persons | 1478.3±551.5 |
| No. 0–9 years, % | 32.4±2.3 |
| No. female, % | 50.1±1.5 |
| Distance to nearest town, km | 8.8±3.2 |
| Altitude, m | 2562.1±222.4 |
| Prevalence of clinically active trachoma, %a | 70.6±19.5 |
| Prevalence of ocular chlamydia, %a | 43.6±15.0 |
Estimated from a random sample of 0–9 year-old children from each subkebele before mass antibiotic distributions
Table 2.
Facility workers’ opinions of the most to least frequently dispensed antibiotics in a rural area of Ethiopia.
| Rank | Antibiotic | Mean Rank Score |
|---|---|---|
| 1 (Most often) | Penicillins | 1.5 |
| 2 | Sulfonamides | 2.7 |
| 3 | Tetracyclines | 4.2 |
| 4 | Chloramphenicol | 4.2 |
| 5 | Fluoroquinolones | 4.5 |
| 6 | Macrolides | 5.7 |
| 7 | Cephalosporins | 6.2 |
| 8 (Least often) | Aminoglycocides | 7.0 |
We calculated DDDs of antibiotic consumption for our study area from retrospective invoice records and from a prospective log of antibiotics dispensed during one week. Estimates of DDDs of total antibiotic use are shown for each facility in Table 3. The methods correlated well (r=0.77). As shown in Table 3, most antibiotics were procured through a government health post, although a sizable number were purchased through private medical clinics. Children aged 0–9 years consumed 29.5% of dispensed prescriptions, although these prescriptions constituted only 14.7% of total DDDs due to the reduced dosing (Table 4).
Table 3.
Defined Daily Doses (DDD) recorded by drug-dispensing facilities in a rural area of Ethiopia
| Facility Type | Facility | DDD recorded, per day
|
|
|---|---|---|---|
| Invoice methoda | Dispensed methodb | ||
| Government | 1 | 93.7 | 102.6 |
| 2 | 64.9 | 24.5 | |
| 3 | 25.4 | 21.2 | |
| 4 | 11.3 | 18.1 | |
| 5 | 4.5 | 11.9 | |
| 6 | 0 | 3.5 | |
| 7 | 1.7 | 0 | |
| Private Clinic | 8 | 37.0 | 28.6 |
| 9 | 17.1 | 35.5 | |
| 10 | 12.2 | 34.2 | |
| 11 | 12.0 | 18.7 | |
| Private Vendor | 12 | 58.0 | 21.1 |
|
| |||
| Total | 337.9 | 319.7 | |
Average DDD inventory per day, as assessed from invoice records from the previous six months.
Average DDD dispensed per day, as assessed during a one-week period.
Table 4.
Proportion of Defined Daily Doses (DDD) and prescriptions dispensed per day in a rural area of Ethiopia, classified according to age of consumer and dispensing facility type
| Characteristic | Proportion of total
|
|
|---|---|---|
| DDDs | Prescriptions | |
| n=319.7a | n=43.6a | |
| Age | ||
| 0–9 years | 14.7% | 29.5% |
| 10–19 years | 15.3% | 13.8% |
| 20–29 years | 30.1% | 24.3% |
| 30–39 years | 12.9% | 11.5% |
| ≥40 years | 26.7% | 20.7% |
Average number dispensed per day, as assessed during a one-week period.
Table 5 shows the number of DDDs per 1000 person days of each class of antibiotic consumed in the study area separately for the entire population (109 895 persons), the population of children aged 0–9 years (34 421 persons), and the population of individuals 10 years and up (75 474 persons). We estimated that overall antibiotic utilization was 2.91 DDDs per 1000 person days (95% CI 2.52 to 3.32) based on dispensed antibiotics, and 3.07 DDDs per 1000 person days (95% CI 1.91 to 4.23) based on invoices. Antibiotic consumption was lower in children (1.37 DDDs per 1000 person days based on dispensed antibiotics, Table 5). Penicillins were most widely used, followed by fluoroquinolones, sulfonamides, and tetracyclines. Macrolide consumption was quite low (0.01 to 0.02 DDDs per 1000 person days). Antibiotic utilization was not different when comparing the six treatment arms of the clinical trial, either for the entire population (P=0.93) or for children aged 0–9 years (P=0.59). Antibiotic utilization was higher in an adjacent kebele containing the town of Gindewoin (9.28 DDDs per 1000 person years of antibiotics [95% CI 7.04 to 11.75]); this town was the largest population center in the woreda and was excluded from the study area.
Table 5.
Antibiotic utilization in a rural area of Ethiopia, classified according to antibiotic class and age
| Antibiotic | All ages
|
Children 0–9
|
Individuals ≥10
|
|||
|---|---|---|---|---|---|---|
| DDDs
|
DDDsb | Rx c | DDDsb | Rxc | ||
| Invoice methoda | Dispensed methodb | |||||
| Penicillins | ||||||
| Amoxicillin | 0.59 | 0.47 | 0.17 | 13 | 0.61 | 33 |
| Ampicillin | 0.61 | 0.18 | 0.16 | 15 | 0.18 | 17 |
| Cloxacillin | 0.07 | 0.24 | 0 | 0 | 0.36 | 25 |
| Penicillin | 0.59 | 0.72 | 0.55 | 16 | 0.80 | 37 |
| Fluoroquinolones | ||||||
| Ciprofloxacin | 0.47 | 0.37 | 0 | 0 | 0.54 | 37 |
| Norfloxacin | 0.07 | 0.08 | 0 | 0 | 0.11 | 8 |
| Sulfonamides | ||||||
| Cotrimoxazole | 0.35 | 0.27 | 0.36 | 37 | 0.21 | 18 |
| Tetracyclines | ||||||
| Doxycycline | 0.18 | 0.42 | 0.06 | 1 | 0.59 | 19 |
| Tetracycline | 0.08 | 0.05 | 0 | 0 | 0.08 | 3 |
| Amphenicols | ||||||
| Chloramphenicol | 0.03 | 0.03 | 0.02 | 2 | 0.03 | 5 |
| Aminoglycocides | ||||||
| Gentamicin | 0.02 | 0.04 | 0.05 | 5 | 0.03 | 7 |
| Macrolides | ||||||
| Erythromycin | 0.01 | 0.02 | 0 | 0 | 0.03 | 1 |
| Nitroimidazoles | ||||||
| Metronidazole | 0.007 | 0.02 | 0 | 0 | 0.03 | 3 |
| Cephalosporins | ||||||
| Ceftriaxone | 0.001 | 0.004 | 0.004 | 1 | 0.004 | 1 |
|
| ||||||
| All antibiotics | 3.07 | 2.91 | 1.37 | 90 | 3.60 | 214 |
DDDs per 1000 person days purchased by the drug-dispensing facilities over 6 months
DDDs per 1000 person days dispensed over one week to either the total population (109 895), the population of children aged 0–9 years (34 421), or the population of individuals aged 10 years and older (75 474)
Antibiotic prescriptions recorded over one week to the populations noted above DDD=Defined daily dose; Rx=prescriptions
In regression analyses adjusted for age, gender, altitude, and distance to the nearest town, each additional DDD of antibiotic use among 0–9 year-old children (per 1000 person days) was associated with a 15.0% (95% CI −19.7 to −10.3) decrease in clinically active trachoma, and a non-significant 0.5% (95% CI −9.1 to 8.2) decrease in the prevalence of ocular chlamydia.
In the trachoma program, 24 subkebeles received a single azithromycin treatment at baseline.14 According to the treatment log, 31 171 of 36 148 individuals received treatment (antibiotic coverage 86.2%), consuming a total of 12 778 tablets of 250mg azithromycin, and 41,552 ml of 200mg/5ml azithromycin syrup. Assuming one mass azithromycin treatment per year, this distribution was equivalent to 5.9 DDDs of azithromycin per 1000 person days.
4. Discussion
Surveys of pharmacies in a rural area of Ethiopia revealed very low community antibiotic consumption of only 2.91 to 3.07 DDDs per 1000 person days. This figure is much lower than in Europe and the United States, where recent estimates of outpatient antibiotic usage have ranged from 10 to 30 DDDs per 1000 person days.15 It is also much lower than a similar study in Nepal, which found consumption of all antibiotics to be the equivalent of 8.2 DDDs per 1000 persons per day.6
The low level of antibiotic consumption in this study area may be one factor contributing to the persistence of endemic trachoma in the study area. In a separate report, we showed that the pre-treatment prevalence of trachoma was high in the study communities, with almost half of children aged 1–10 years infected with ocular chlamydia.7 Furthermore, there was no evidence of a secular trend in the area—the mean prevalence of infection in 1–10 year-old children was 48.4% (95% CI 42.9 to 53.9%) in 12 untreated communities at baseline, and 45.6% (95% CI 36.7% to 54.5%) in 12 different untreated communities 12 months later.7 Secular trends have been suspected to contribute to trachoma reduction in other parts of the world. For example, studies in Malawi and the Gambia conducted over decades demonstrated a dramatic decrease in the prevalence of trachoma in the absence of any trachoma control programs.3, 4 A study in Nepal found that one-fifth of the decrease in trachoma could be attributed to neither antibiotic treatments nor to seasonal effects, providing evidence of a secular trend.5 It is tempting to speculate that part of the secular trend observed for trachoma in other parts of the world may be due to increased background antibiotic use in the community, unrelated to trachoma programs; if so, this might also explain the lack of a secular trend in this region of Ethiopia, where antibiotic utilization is quite low.
Previous reports have noted an increase in nasopharyngeal pneumococcal macrolide resistance following mass azithromycin distributions.16,17 This is perhaps not surprising, given the volume of antibiotics distributed by trachoma programs. In the current study, we estimated that a single annual mass azithromycin distribution represents twice the level of overall background antibiotic consumption, and 30 times the level of macrolide consumption. However, the current study also suggests that the selection of pneumococcal strains resistant to azithromycin may be of little clinical significance, since macrolides are so rarely dispensed in this area. Furthermore, penicillin resistance has not yet been shown to increase following mass azithromycin treatments.17 This is fortunate, since penicillins are by far the most commonly prescribed antibiotic in the area, and would likely be the treatment of choice for infections presumably caused by pneumococcus.
In this study, increased antibiotic use was associated with lower levels of clinically active trachoma. Clinically active trachoma is characterized by inflammatory sequelae of the conjunctivae that result from infection with ocular strains of chlamydia. It is possible that over the long-term, those communities with higher antibiotic utilization more effectively clear the strains of chlamydia that cause ocular inflammation, and thus have lower rates of clinically active trachoma. It is also quite possible that antibiotic consumption is simply a proxy for socioeconomic development. We attempted to adjust for several potential sociodemographic confounders to address this possibility, though unmeasured confounders could still account for some or all of the observed association. Furthermore, the communities in this study were randomly selected and the demographic and socioeconomic makeup was very similar, which should minimize confounding to some extent. Although we might have expected the prevalence of ocular chlamydia to also be associated with antibiotic utilization, it is likely that clinically active trachoma is a long-term, slowly-changing index of infection, which might be more sensitive to inherent community characteristics such as antibiotic utilization. Ocular chlamydia infection, on the other hand, is of shorter duration and might be more likely to fluctuate in the short term, potentially explaining the lack of association found in this study.
We found that antibiotic utilization was much higher in the kebele that contained the largest town in the area. This is not surprising, since six of the 12 facilities were located in this town. Although we did not examine children from the town, many studies have shown that rates of trachoma are lower in urban areas compared to rural areas.8,9,18–20 There are many aspects of urban life that might play a role in preventing transmission of ocular chlamydia, such as improved access to water and sanitation, and improved education and hygiene practices.21 Nonetheless, the much higher utilization of antibiotics, many of which are effective against chlamydia, may also explain some of the reduction of trachoma among town-dwellers.
Antibiotic utilization can be calculated by various methods, including drug expenditures, days of therapy, prescribed daily doses, and defined daily doses.22–25 Here, we calculated DDDs per 1000 person days based on the sum of the number of antibiotics dispensed during one week, and separately by the sum of antibiotics purchased during the previous six months. We found very good agreement between the two methods, lending validity to each of the methods. In addition, the number of DDDs that was calculated generally agreed with the ranking that the pharmacists performed, which gives further support to the content validity of these methods. It is important to note that the DDD methodology was designed to make long-term comparisons between different regions, countries, or settings. The DDD method has several limitations.25, 26 For example, DDDs are based on average doses for healthy adults, and therefore underestimate antibiotic utilization in individuals taking smaller doses, such as children or those with renal failure. This was evident in our study, where children accounted for 30% of all dispensed antibiotic prescriptions, but only half as many DDDs. In addition, DDDs are not established for certain types of medications, such as topical preparations. Despite its limitations, however, the DDD method provides a well characterized and frequently used technique for quantifying antibiotic use.
One strength of this study is the inclusion of every facility that would have dispensed antibiotics to the study area. We included commercial pharmacies, government health centers and private health clinics. We included facilities present in the study area and those located outside the study area that would nonetheless draw customers from the study area. There is little trade for antibiotics outside the surveyed drug facilities.
The chief weakness of this study is the timing of the pharmacy survey. We calculated antibiotic utilization at month 42 of the clinical trial, but analyzed for associations with baseline prevalence of clinically active trachoma and ocular chlamydia. Although it would have been ideal to conduct the pharmacy survey at the baseline visit, it is unlikely that the general antibiotic utilization patterns changed significantly over time, since we did not observe a secular trend in the trial,7 antibiotic consumption was so low, and the list of essential drugs published by the Drug Administration and Control Authority of Ethiopia was similar throughout the study. Moreover, the average number of DDDs dispensed in each study arm was not significantly different, suggesting that the clinical trial interventions themselves did not differentially alter antibiotic consumption.
In conclusion, we found that antibiotic utilization in a region of rural Ethiopia was quite low—approximately 3 DDDs per 1000 person days. Antibiotic use was associated with reduced prevalence of clinically active trachoma, raising the possibility that background antibiotic use could play a role in reducing the prevalence of trachoma outside of program activities (i.e., a secular trend). Indeed, the low levels of antibiotic use observed in this study area may help explain the overall lack of a secular trend in this region. In addition, the low level of macrolide use suggests that any nasopharyngeal pneumococcal azithromycin resistance seen after mass azithromycin treatments may have limited clinical significance. Further study of antibiotic utilization in trachoma endemic areas will be useful in characterizing the role of background antibiotic use in trachoma control.
Acknowledgments
We thank the data safety and monitoring committee including William Barlow (University of Washington, Washington, DC, USA; Chair), Donald Everett (National Eye Institute, Bethesda, MD, USA), Larry Schwab (International Eye Foundation, Kensington, MD, USA), Arthur Reingold (University of California, Berkeley, CA, USA), and Serge Resnikoff (WHO, Geneva, Switzerland), who were generous with their time and advice and met before, during, and after this study; the head of the Goncha woreda health office Tadege Alemayehu; the head of the Amhara Regional Health Bureau Asrat Genet Amnie; the Ethiopian Ministry of Health; the nurses and health workers who helped collect samples for the study, including Azmeraw Adgo, Melese Temesgen, Gebeyehu Sibhatu, Manahlosh Berihun, Temesgen Demile, Melkam Andualem, Mitselal Abrhale, Banchu Gedamu, Tessema Eneyew, and Muluken Gobezie; and the following individuals for providing data for and assistance with the study: Amsalu Abate, Etsubedink Anteneh, Almaz Ashebir, Sewmehon Dagnachew, Temesgen Demile, Yebeltal Ewonetu, Getasew Gelaye, Bekalu Getu, Hiwot Girma Guaide, Taddesse Mitiku (MD), Adugna Mulugeta, Tigist Azene Tamir.
Funding: The National Institutes of Health (Bethesda, MD, USA) was the primary supporter of this work, through grants NEI U10 EY016214 and K23 EY019071. We thank the International Trachoma Initiative (Atlanta, GA, USA) for the generous donation of azithromycin for the underlying clinical trial, the Bernard Osher Foundation (San Francisco, CA, USA), That Man May See (San Francisco, CA USA), the Bodri Foundation (San Francisco, CA, USA), the Harper Inglis Trust (Sunnyvale, CA, USA), the South Asia Research Fund (San Francisco, CA, USA), and Research to Prevent Blindness (New York, NY, USA).
Footnotes
Conflicts of Interest: None declared
Ethical approval: Approval for the study was obtained by the Committee for Human Research at the University of California, San Francisco, and by the Ethiopian Science and Technology Commission, and included verbal consent for adult study participants, and verbal consent from guardians of minors.
Authors’ contributions: BA, NES, and JDK designed the study protocol; AA and TG collected population data; BA, TB, MZ, YA, and DH collected trachoma prevalence data; BA, TB, ARL, NES, and JDK collected pharmacy data; BA and JDK performed the statistical analyses and interpretation of the data. BA and JDK drafted the manuscript. All authors read and approved the final manuscript. JDK is guarantor of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Emerson PM, Burton M, Solomon AW, Bailey R, Mabey D. The SAFE strategy for trachoma control: Using operational research for policy, planning and implementation. Bull World Health Organ. 2006;84:613–9. doi: 10.2471/blt.05.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolin PJ, Faal H, Johnson GJ, Minassian D, Sowa S, Day S, et al. Reduction of trachoma in a sub-Saharan village in absence of a disease control programme. Lancet. 1997;349:1511–2. doi: 10.1016/s0140-6736(97)01355-x. [DOI] [PubMed] [Google Scholar]
- 4.Hoechsmann A, Metcalfe N, Kanjaloti S, Godia H, Mtambo O, Chipeta T, et al. Reduction of trachoma in the absence of antibiotic treatment: evidence from a population-based survey in Malawi. Ophthalmic Epidemiol. 2001;8:145–53. doi: 10.1076/opep.8.2.145.4169. [DOI] [PubMed] [Google Scholar]
- 5.Jha H, Chaudary JS, Bhatta R, Miao Y, Osaki-Holm S, Gaynor B, et al. Disappearance of trachoma from Western Nepal. Clin Infect Dis. 2002;35:765–8. doi: 10.1086/342298. [DOI] [PubMed] [Google Scholar]
- 6.Chidambaram JD, Bird M, Schiedler V, Fry AM, Porco T, Bhatta RC, et al. Trachoma decline and widespread use of antimicrobial drugs. Emerg Infect Dis. 2004;10:1895–9. doi: 10.3201/eid1011.040476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.House JI, Ayele B, Porco TC, Zhou Z, Hong KC, Gebre T, et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet. 2009;373:1111–8. doi: 10.1016/S0140-6736(09)60323-8. [DOI] [PubMed] [Google Scholar]
- 8.Zerihun N. Trachoma in Jimma zone, south western Ethiopia. Trop Med Int Health. 1997;2:1115–21. doi: 10.1046/j.1365-3156.1997.d01-211.x. [DOI] [PubMed] [Google Scholar]
- 9.Mesfin MM, de la Camera J, Tareke IG, Amanual G, Araya T, Kedir AM. A community-based trachoma survey: prevalence and risk factors in the Tigray region of northern Ethiopia. Ophthalmic Epidemiol. 2006;13:173–81. doi: 10.1080/09286580600611427. [DOI] [PubMed] [Google Scholar]
- 10.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–83. [PMC free article] [PubMed] [Google Scholar]
- 11.Diamant J, Benis R, Schachter J, Moncada J, Pang F, Jha HC, et al. Pooling of Chlamydia laboratory tests to determine the prevalence of ocular Chlamydia trachomatis infection. Ophthalmic Epidemiol. 2001;8:109–17. doi: 10.1076/opep.8.2.109.4156. [DOI] [PubMed] [Google Scholar]
- 12.Melese M, Chidambaram JD, Alemayehu W, Lee DC, Yi EH, Cevallos V, et al. Feasibility of eliminating ocular Chlamydia trachomatis with repeat mass antibiotic treatments. JAMA. 2004;292:721–5. doi: 10.1001/jama.292.6.721. [DOI] [PubMed] [Google Scholar]
- 13.Madaras-Kelly K. Optimizing antibiotic use in hospitals: the role of population-based antibiotic surveillance in limiting antibiotic resistance. Insights from the society of infectious diseases pharmacists. Pharmacotherapy. 2003;23:1627–33. doi: 10.1592/phco.23.15.1627.31967. [DOI] [PubMed] [Google Scholar]
- 14.Stoller NE, Gebre T, Ayele B, Zerihun M, Assefa Y, Habte D, et al. Efficacy of latrine promotion on reinfection with ocular chlamydia after mass antibiotic treatment: a cluster randomized trial. Int Health. 2011 doi: 10.1016/j.inhe.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goossens H, Ferech M, Coenen S, Stephens P. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis. 2007;44:1091–5. doi: 10.1086/512810. [DOI] [PubMed] [Google Scholar]
- 16.Leach AJ, Shelby-James TM, Mayo M, Gratten M, Laming AC, Currie BJ, et al. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24:356–62. doi: 10.1093/clinids/24.3.356. [DOI] [PubMed] [Google Scholar]
- 17.Haug S, Lakew T, Habtemariam G, Alemayehu W, Cevallos V, Zhou Z, et al. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis. 2010;51:571–4. doi: 10.1086/655697. [DOI] [PubMed] [Google Scholar]
- 18.Taylor HR, Fox SS, Xie J, Dunn RA, Arnold AL, Keeffe JE. The prevalence of trachoma in Australia: the National Indigenous Eye Health Survey. Med J Aust. 2010;192:248–53. doi: 10.5694/j.1326-5377.2010.tb03501.x. [DOI] [PubMed] [Google Scholar]
- 19.Jie Y, Xu L, Ma K, Zhang S, Zhu J, Jonas JB. Prevalence of trachoma in the adult Chinese population. The Beijing Eye Study. Eye (Lond) 2008;22:790–1. doi: 10.1038/sj.eye.6702857. [DOI] [PubMed] [Google Scholar]
- 20.Uzma N, Kumar BS, Khaja Mohinuddin Salar BM, Zafar MA, Reddy VD. A comparative clinical survey of the prevalence of refractive errors and eye diseases in urban and rural school children. Can J Ophthalmol. 2009;44:328–33. doi: 10.3129/i09-030. [DOI] [PubMed] [Google Scholar]
- 21.House J, Gaynor B, Taylor H, Lietman TM. The real challenge: can we discover why trachoma is disappearing before it’s gone? Int Ophthalmol Clin. 2007;47:63–76. doi: 10.1097/IIO.0b013e318074e01b. [DOI] [PubMed] [Google Scholar]
- 22.Miller GE, Hudson J. Children and antibiotics: analysis of reduced use, 1996–2001. Med Care. 2006;44:I36–44. doi: 10.1097/01.mlr.0000208142.53164.76. [DOI] [PubMed] [Google Scholar]
- 23.Kuster SP, Ruef C, Ledergerber B, Hintermann A, Deplazes C, Neuber L, et al. Quantitative antibiotic use in hospitals: comparison of measurements, literature review, and recommendations for a standard of reporting. Infection. 2008;36:549–59. doi: 10.1007/s15010-008-7462-z. [DOI] [PubMed] [Google Scholar]
- 24.de With K, Bestehorn H, Steib-Bauert M, Kern WV. Comparison of defined versus recommended versus prescribed daily doses for measuring hospital antibiotic consumption. Infection. 2009;37:349–52. doi: 10.1007/s15010-008-8138-4. [DOI] [PubMed] [Google Scholar]
- 25.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664–70. doi: 10.1086/511640. [DOI] [PubMed] [Google Scholar]
- 26.Natsch S, Hekster YA, de Jong R, Heerdink ER, Herings RM, van der Meer JW. Application of the ATC/DDD methodology to monitor antibiotic drug use. Eur J Clin Microbiol Infect Dis. 1998;17:20–4. doi: 10.1007/BF01584358. [DOI] [PubMed] [Google Scholar]


