Abstract
Natural killer (NK) cell responses are determined by signals received through activating and inhibitory cell surface receptors. Ly49H is an NK cell-specific activating receptor that accounts for the genetic resistance to murine cytomegalovirus (MCMV). The Ly49H receptor has been shown to interact with two adapter proteins (DAP12 and DAP10). In the context of MCMV infection, interaction of m157 (the MCMV-encoded ligand for Ly49H) with Ly49H results in activation of Ly49H-expressing NK cells. Chronic exposure of Ly49H with m157, however, induces tolerance in these same cells. The mechanism of this tolerance remains poorly understood. Using a transgenic mouse model, we demonstrate that induction of tolerance in Ly49H+ NK cells by chronic exposure to m157, in vivo, requires signaling through the Ly49H adaptor protein DAP12, but not the DAP10 adaptor protein. Furthermore, mature Ly49H-expressing NK cells from wild-type mice can acquire a tolerant phenotype by twenty-four hours post transfer into a transgenic C57BL/6 mouse that expresses m157 (m157-Tg). The tolerant phenotype can be reversed, in vivo, if tolerant NK cells are transferred to mice that do not express the m157 protein. Thus, continuous activating receptor engagement can induce a transient tolerance in mature NK cells in vivo. These observations provide new insight into how activating receptor engagement shapes NK cell function and has important implications in how NK cells respond to tumors and during chronic viral infection.
Introduction
Natural killer (NK) cells represent an important cellular component of the innate immune system. These cells play a central role in the immune response to viral infection and tumor surveillance. Using activating and inhibitory receptors expressed on their cell surface, NK cells distinguish normal “self” cells from abnormal cells by scanning the molecules expressed on the cell surface of potential target cells. The integration of both positive and negative stimuli determines if the NK cell will become activated during an effector response. NK cell effector responses include the release of cytokines and chemokines that shape the subsequent immune response as well as release of cytotoxic granules, which result in the destruction of the target cell.
During times of stress and/or infection, host cells can over express certain endogenous self-ligands, which can be recognized by specific activating receptors expressed on the NK cell. For instance, the NKG2D receptor interacts with RAE1α–ε, H60, and Mult1 (1); CD226 recognizes Necl-5 and Nectin-2 (2); and CRTAM recognizes Necl-2 (2). In addition to activating receptors that recognize “induced-self” molecules, NK cells express activating receptors that recognize non-self molecules. For example, NKP46 is believed to recognize the influenza virus hemaglutinin and Ly49D recognizes a xenogeneic major histocompatability complex (MHC) class I molecule (3, 4). In addition, Ly49H recognizes the murine cytomegalovirus (MCMV) encoded protein m157 (5–8). Thus, NK cell activating receptors can recognize both self and non-self ligands.
In general, NK cell activating receptors contain short cytoplasmic tails and require association with adaptor proteins in order to transduce signal once the receptor has been engaged. One set of adaptor proteins known to associate with activating receptors (including FcεRI-γ, CD3-ζ and DAP12) contains immunoreceptor tyrosine-based activation motifs (ITAMS) (9). Engagement of the receptor results in phosphorylation of the ITAM tyrosine by Src family kinases (mutations of ITAM tyrosine abolishes their signaling function). The phosphorylated tyrosine serves as a binding site for spleen tyrosine kinase (syk) and ζ-chain-associated kinase of 70 kDa (ZAP-70) (10, 11). Eventually, signaling cascades result in cytoskeletal reorganization, transcription of a number of cytokines and chemokines, as well as, release of cytotoxic granules.
The Ly49H activation receptor, which is expressed on a subset of NK cells in C57BL/6 mice, is coupled to the DAP12 adaptor protein. Recent work suggests that the Ly49H activating receptor can also associate with the DAP10 adaptor protein, but the significance of this interaction remains unclear (12–14). The only known ligand for the Ly49H receptor is the MCMV encoded protein, m157 (5–7). The Ly49H receptor, due to its interaction with m157, accounts for genetic resistance to MCMV (15, 16). During MCMV infection, there is an initial proliferation of the generalized NK cell population followed by specific proliferation of the Ly49H+ subset of NK cells. In addition to proliferation, the Ly49H+ NK cells are activated as a result of the m157/Ly49H interaction (they produce cytokines and are able to kill specific targets) (17). More recent work has demonstrated that continuous engagement of m157 with the Ly49H activating receptor results in tolerance of the Ly49H-expressing NK cells (18, 19). In vitro and in vivo studies by other groups have demonstrated a similar induction of tolerance in NK cells through continuous engagement of another NK cell activating receptor, NKG2D (12, 20–22). Thus, stimulation through NK activation receptors can function to activate as well as decrease NK cell function.
Multiple transgenic mouse models have demonstrated that continuous expression of ligands for NK cell activating receptors results in NK cell defects in vivo (18, 21, 22). Initial studies addressing the mechanism in which chronic activating receptor engagement results in NK cell dysfunction have all been in vitro and involved using NK cells that had been stimulated with cytokines (a necessity when culturing NK cells in vitro) (12, 20). To better understand the mechanism and extent of activating receptor-induced tolerance in vivo, we took advantage of our previously described m157-Tg model system (18). Using this system, we demonstrate that Ly49H activating receptor-induced NK cell tolerance in vivo requires signaling through the receptor via the DAP12 adaptor protein. In addition, tolerance can be induced in mature NK cells in vivo demonstrating that activating receptor-induced tolerance is not developmentally regulated. Finally, we demonstrate that activating receptor-induced tolerance can be reversed in vivo.
Material and Methods
Mice
The m157-Tg mouse has been previously described (18). Mice expressing a nonfunctional mutated form of DAP12 (DAP12ki), as well as, the DAP10 knockout mice (DAP10ko) have both been previously described (23, 24). All mice used in these experiments were C57BL/6 background. The m157-Tg mice were crossed with DAP12ki and DAP10ko mice to generate mice that were heterozygous at the DAP12 and DAP10 locus respectively. These mice were mated with DAP12ki or DAP10ko mice to generate the m157Tg mice in the homozygous DAP12ki and DAP10ko backgrounds. The transgenic mice were maintained on the DAP12ki and DAP10ko background by mating the m157Tg mice in the DAP12ki and DAP10ko backgrounds with non-Tg DAP12ki and DAP12ko mice respectively. Mice were maintained under specific pathogen-free conditions and used after they reached 8 weeks of age. The Animal Studies Committee at Washington University (St. Louis, MO) approved all animal studies.
Antibodies
The following antibodies were obtained from EBioscience or BD Pharmingen: APC-PK136 (anti-NK1.1), PerCP-Cy5.5-145-2C11 (anti-CD3), Alexa488-XMG1.2 (anti-IFN-γ), PacBlue-XMG1.2 (anti-IFN-γ) and FITC-1D4B (anti-CD107a). Biotinylated-3D10 (anti-Ly49H) was purified from hybridomas by the RDCC antibody production core and conjugated to biotin using EZ-Link Sulfo-NHS-LC-LC-Biotin (Pierce) according to manufacturer’s protocol. Purified 4E4 (anti-Ly49D) and PK136 (anti-NK1.1) were obtained from hybridomas by the RDCC antibody production core.
Adoptive transfer experiments
Spleen cell suspensions were generated by harvesting spleens into R10 media (RPMI, 10%FCS) through a 70 μM Nylon cell strainer (BD Falcon). Following passage through the strainer, splenocytes were centrifuged and red blood cells were lysed by resuspending pelleted cells in one ml of red blood cell lysing solution (SIGMA) and incubating for 5 minutes at room temperature. The cells were then washed twice with R10, counted and resuspended in R10 at 107/ml. Donor splenocytes were labeled using the Vybrant CFDA SE Cell tracer kit (Molecular Probe) per the manufacturer’s protocol. Briefly, splenocytes were washed with PBS and then resuspended in 1 μm CFSE (diluted in PBS) at a concentration of 100x106 cells/ml for 15 minutes in the dark at room temperature. The reaction was stopped with R10 media. Cells were washed with PBS and then resuspended at 250x106 cells/ml. Prior to injection, cells were assessed for CFSE labeling as well as NK cell percentage by flow cytometry. In all injections NK cells made up 2-4% of the splenic lymphocyte population. Mice were injected intravenously in the tail vein with 200 μl of labeled splenocytes (approximately 50x106 total splenocytes). Mice were then harvested at the indicated time points.
Cytokine assays
Spleen cells suspensions were generated as described above. To coat plates, appropriate antibody was diluted to 2 μg/ml in PBS. One ml of antibody (2 μg) was placed in 6 well tissue culture plates (Techno Plastic Product) and incubated at 37°C for 90 minutes. After incubation, the plates were washed with PBS three times prior to use for stimulation assays. For stimulation of NK cells, 1 ml of splenocytes (107 cells/ml in R10) were incubated in 6 well plates coated with anti-NK1.1 mAb or anti-Ly49D mAb for 1 hr and then further incubated in the presence of a 833-fold dilution of stock GolgiPlug (BD Pharmingen) for an additional 6–8 hours, as previously described (25). Stimulation with RMA and RMAm157 cells was performed as previously described (18). Splenocytes were stained for NK1.1, CD3 and Ly49H using the antibodies described above. To block nonspecific binding of antibodies to Fc receptors, all antibodies were diluted in the presence of mAb 2.4G2 (anti-Fcγ receptor II/III, ATCC). Cells were fixed and permeabilized with Cytofix/Cytoperm kit (BD Pharmingen) and then stained with either Alexa488-XMG1.2 (anti-IFN-γ, BD Pharmingen) or PacBlue-XMG1.2 (anti-IFN-γ, BD Pharmingen) diluted in perm/wash buffer (BD Pharmingen). Cells were analyzed using a FACSCalibur or FACSCanto cytometer (BD Biosciences) gating on NK1.1+, CD3−populations.
Degranulation assays
Spleen cell suspensions (at a concentration of 107 cells/ml) were generated as described above. YAC target cells were resuspended at a concentration of 106 cells/ml in R10 containing 2X monensin (Ebioscience). Equal volumes (100 μl each) of spleen cells and YAC cells were incubated in 96 v-bottom wells (NUNC) for 2 hours at 37°C. Following incubation, the cells were stained for NK1.1, CD3, Ly49H and CD107a using the antibodies described above.
Statistical analysis
The data were analyzed with Microsoft Excel X for Mac (Microsoft). Unpaired, two-tailed t test was used to determine statisticallysignificant differences between experimental groups. Error bars in the figures represent the standard error of the mean (SEM).
Results
Signaling through the Ly49H receptor is necessary to induce Ly49H-mediated NK cell tolerance
Cell surface expression of the Ly49H activating receptor as well as signaling through the receptor requires association of Ly49H receptor with the adapter molecule DAP12. Upon engagement of the Ly49H activating receptor, the DAP12 adapter molecule is phosphorylated, which begins an intracellular signaling cascade resulting in the activation of the NK cell.
Recent work has demonstrated that continuous engagement of NK cell activating receptors results in NK cell tolerance both in vivo and in vitro. Though in vitro studies suggest that signaling through activating receptors is important for tolerance to be induced, it remained unclear if this was the case in vivo (12, 20). To determine if signaling through the Ly49H receptor (not just receptor engagement) was important to induce tolerance in the NK cells in vivo, we bred the m157-Tg mouse onto a DAP12ki background. The DAP12ki mouse is a DAP12 loss-of-function mutant where the wild-type C-terminal Y75-R86 amino acid stretch (YSDLNTQRQYR) of the DAP12 protein is replaced with G75-I90 (GLQEFIEDEKKKRNSI). This mutation results in the loss of tyrosine residues, whose phosphorylation are important for signaling mediated by DAP12 (23).
The breeding of the m157-Tg mouse onto the DAP12ki background resulted in a mouse that ubiquitously expressed the m157 protein but also contained NK cells in which the Ly49H activating receptor was associated with a mutant from of the DAP12 protein that could not be phosphorylated and thus would not allow for signal transduction upon engagement of the Ly49H receptor. Although the DAP12ki mutant does not allow for signaling through the Ly49H receptor, it does allow for expression of the receptor on the NK cell surface. Similar to the downregulation of the Ly49H receptor in m157-Tg mice in the C57Bl/6 background, we observed decreased levels of the Ly49H receptor in m157-Tg mice in the DAP12ki backgrounds, suggesting that engagement with the m157 ligand was taking place (Fig. 1A).
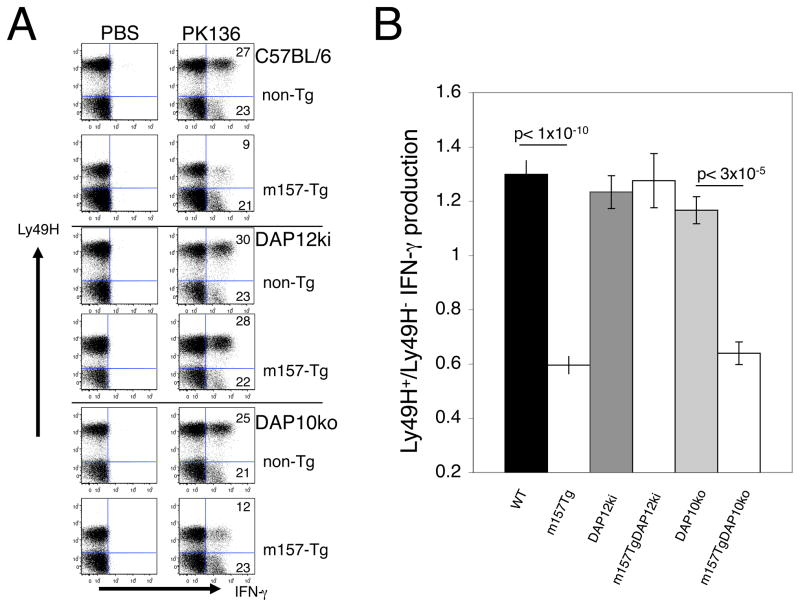
FIGURE 1. DAP12 but not DAP10 signaling is required for induction of NK cell tolerance.
(A) Representative dot plots demonstrating IFN-γ production by freshly isolated splenocytes from non-Tg or m157-Tg mice stimulated with plate bound anti- NK1.1 mAb (PK136). The numbers represent the percentage of Ly49H+ or Ly49H− NK cells producing IFN-γ. The dot plots were gated on NK cells (NK1.1+, CD3−lymphocytes). NK cells were obtained from non-Tg and m157-Tg mice in the C57BL/6, DAP12ki, and DAP10ko backgrounds. (B) The ratio of the percentage of IFN-γ–producing Ly49H+ NK cells to the percentage of IFN-γ –producing Ly49H− NK cells in non-Tg and m157-Tg mice in the C57BL/6, DAP12ki and DAP10ko backgrounds. The graph represents the mean ± the SEM. Ratios were calculated after stimulation with anti-NK1.1 mAb (PK136). The number of mice used in each group: WT=20, m157Tg=28, DAP12ki=12, m157TgDAP12ki=11, DAP10ko=6 and m157TgDAP10ko=5)
As previously demonstrated, upon stimulation of NK cells through a Ly49H-independent manner with plate bound anti-NK1.1 mAb, Ly49H+ NK cells produce less IFN-γ as compared to Ly49H− NK cells in m157-Tg mice in the C57BL/6 background (18). Ly49H+ NK cells and Ly49H− NK cells from non-Tg mice in the C57BL/6 background produced similar levels of IFN-γ. Interestingly, Ly49H+ NK cells from both m157-Tg and non-Tg mice in the DAP12ki background produced similar amounts of IFN-γ to Ly49H− NK cells (Fig 1B). This demonstrates that in the absence of signaling through the Ly49H receptor, tolerance is no longer established in the NK cells despite the fact that activating receptor engagement is still taking place. Thus, DAP12 signaling is required for Ly49H-mediated NK cell tolerance.
In addition to IFN-γ production, we assessed degranulation of NK cells upon stimulation with YAC target cells (another Ly49H-independent stimulation). Degranulation of NK cells was determined by the appearance of cell surface LAMP-1 (CD107a) following stimulation with YAC target cells. Degranulation of Ly49H+ NK cells from m157-Tg mice was significantly decreased compared to non-Tg mice in the C57BL/6 background. Similar to IFN-γ production, degranulation was not altered by the presence of the m157 transgene in the DAP12ki background (Supplemental figure 1). This further demonstrates the importance of DAP12 signaling in the induction of Ly49H- mediated tolerance.
Signaling through DAP10 is not important to induce Ly49H-mediated NK cell tolerance
Recent work suggests that the adaptor molecule DAP10 can also associate with the Ly49H activating receptor (12–14). In order to determine if interaction and signaling through DAP10 plays a role in the induction of Ly49H-mediated NK cell tolerance, the m157-Tg mice were bred to the DAP10ko background. Molecular confirmation of the m157-Tg mice in the DAP10ko background was carried out by PCR (data not shown) as previously described (24). Similar to the C57Bl/6 and DAP12ki background, down regulation of the Ly49H receptor was seen in m157-Tg in the DAP10ko background when compared to non-Tg mice from a similar background (Fig. 1A). Upon stimulation, the Ly49H+ NK cells from m157-Tg mice in the DAP10ko background produced decreased amounts of IFN-γ as compared to non-Tg mice in the DAP10ko background. This demonstrates that in the absence of the DAP10 adapter protein, the continuous engagement of Ly49H with its ligand results in a Ly49H-mediated tolerance of the NK cells. Thus, DAP10 is not required for Ly49H-mediated NK cell tolerance.
Ly49H-mediated NK cell tolerance can be induced in mature NK cells
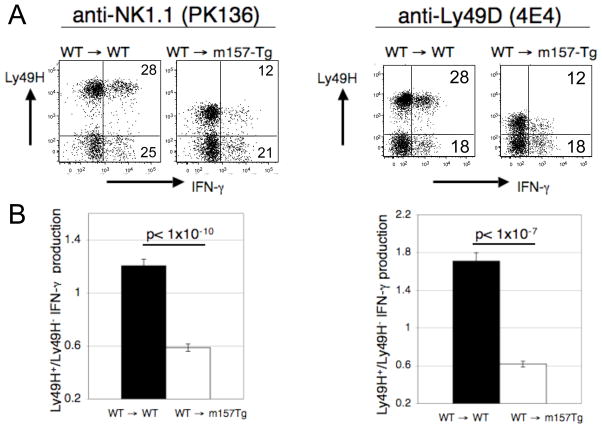
In order to determine if receptor engagement/signaling during NK cell development was required for the induction of Ly49H-mediated NK cell tolerance, we performed adoptive transfer of labeled mature splenic NK cells into both m157-Tg and WT recipients. Transfer of WT NK cells into m157-Tg but not WT recipient mice resulted in downregulation of the Ly49H receptor (Fig 2A). By seven days post transfer, Ly49H+ NK cells from donor NK cells transferred into m157-Tg mice produced less IFN-γ than Ly49H− NK cells. Ly49H+ and Ly49H− NK cells from donor NK cells transferred into non-Tg mice produced similar levels of IFN-γ [Fig 2B and Tripathy et al. (18)]. Decreased IFN-γ production by Ly49H+ NK cells was seen upon transfer into m157-Tg mice when NK cells were stimulated with either plate-bound anti-NK1.1 or anti-Ly49D (Figs. 2A and 2B). This demonstrates that NK cell tolerance can be mediated through continuous engagement of activating receptor in mature NK cells.
FIGURE 2. Mature donor WT Ly49H+ NK cells become tolerant upon transfer into m157-Tg mice.
(A) Representative dot plots demonstrating IFN-γ production by freshly isolated splenocytes stimulated with plate bound anti-NK1.1 mAb (PK136) or anti- Ly49D mAb (4E4). The numbers represent the percentage of Ly49H+ or Ly49H− NK cells producing IFN-γ. The dot plots were gated on donor NK cells (NK1.1+, CD3−, CFSE+ cells). Donor cells were assessed 7 days post transfer. (B) The ratio of the percentage of IFN-γ producing Ly49H+ NK cells to the percentage of IFN-γ producing Ly49H− NK cells from WT→ WT (n=8) or WT→ m157-Tg (n=18) mice following stimulation with plate bound anti-NK1.1 mAb. (C) The ratio of the percentage of IFN-γ producing Ly49H+ NK cells to the percentage of IFN-γ producing Ly49H− NK cells from WT→ WT (n=5) or WT→ m157-Tg (n=8) mice following stimulation with plate bound anti-Ly49D. Donor cells in (A), (B) and (C) were assessed 7 days post transfer. The results are presented as the mean ± SEM.
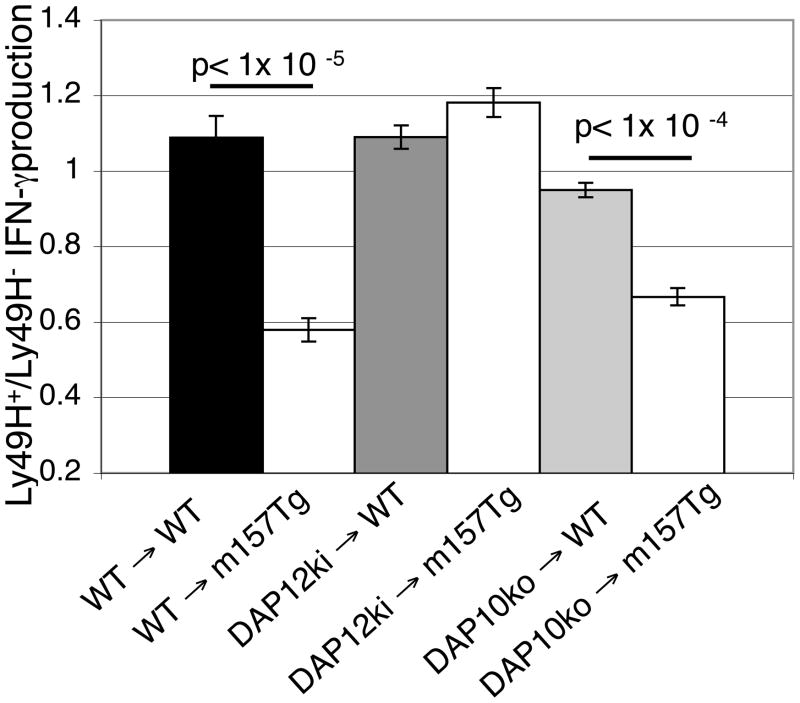
To determine if signaling through either DAP12 or DAP10 adaptor proteins is important for the induction of Ly49H-mediated NK cell tolerance seen in adoptively transferred mature NK cells, we transferred donor mature splenic NK cells from DAP12ki and DAP10ko mice into non-Tg or m157-Tg recipient mice. Donor Ly49H+ NK cells from both DAP12ki and DAP10ko backgrounds demonstrated downregulation of the Ly49H receptor upon transfer into m157-Tg recipient mice as compared to transfer into WT recipient mice (Fig. 3A). Unlike donor cells from the C57Bl/6 and DAP10ko background, DAP12ki Ly49H+ donor NK cells produced similar levels of IFN-γ when transferred into m157-Tg or non-Tg mice. This result, along with the previous results, clearly demonstrate that DAP12-mediated signaling through the Ly49H receptor, and not engagement alone, is necessary to induce Ly49H-mediated NK cell tolerance.
FIGURE 3. Dap12 signaling required for mature NK cells to become tolerant upon transfer into m157-Tg mice.
(A) Representative dot plots demonstrating IFN-γ production by freshly isolated splenocytes stimulated with plate bound anti-NK1.1 mAb. The numbers represent the percentage of Ly49H+ or Ly49H− NK cells producing IFN-γ. The dot plots were gated on donor NK cells (NK1.1+, CD3−, CFSE+ cells). (B) The ratio of the percentage of IFN-γ producing Ly49H+ NK cells to the percentage of IFN-γ producing Ly49H− NK cells from DAP12ki→ WT (n=6), DAP12ki → m157-Tg (n=10), DAP10ko→ WT (n=4), DAP10ko → m157-Tg (n=5) mice following stimulation with plate bound anti-NK1.1 (PK136). Donor cells in (A) and (B) were assessed 7 days post transfer. The results are presented as the mean ± SEM.
Ly49H-mediated NK cell tolerance occurs quickly following adoptive transfer of mature NK cells
To better understand the time course in which Ly49H-mediated NK cell tolerance can be induced, we examined donor WT NK cells at six hours and twenty-four hours post transfer into WT or m157-Tg recipient mice. At six hours post transfer, there was no difference in IFN-γ production by donor WT NK cells transferred into either WT or m157-Tg recipients upon stimulation with plate bound PK136. Furthermore, there was no baseline IFN-γ production or difference in NK cell activation marker expression (CD69) or NK cell maturation marker (Mac1) in the two groups of mice, even though downregulation of the Ly49H receptor was noted (Supplemental figure 2). Twenty-four hours after transfer of donor NK cells from the C57BL/6 and DAP10ko background, differences in IFN-γ production could be detected in Ly49H+ NK cells upon transfer into m157-Tg mice (Fig 4). No difference in IFN-γ production was seen at twenty-four hours when NK cells from DAP12ki mice were used as the donors. In addition, at 24 hours, there was no baseline production of IFN-γ by donor WT NK cells upon transfer into m157-Tg recipients. Finally, there was no difference in NK cell activation marker (CD69) or NK cell maturation marker (Mac1) expression in the WT donor NK cells upon transfer into m157-Tg or WT recipient mice (Supplemental figure 3).
FIGURE 4. Mature donor WT Ly49H+ NK cells display tolerant phenotype within 24 hours of transfer into m157-Tg mice.
The ratio of the percentage of IFN-γ producing Ly49H+ NK cells to the percentage of IFN-γ producing Ly49H− NK cells from WT→ WT (n=6), WT → m157-Tg (n=8), DAP12ki→ WT (n=4), DAP12ki → m157-Tg (n=5), DAP10ko→ WT (n=4), DAP10ko → m157-Tg (n=6) mice following stimulation with plate bound anti-NK1.1 mAb (PK136). Donor cells were assessed 24 hours post transfer. The results are presented as the mean ± SEM.
Ly49H-mediated NK cell tolerance is reversible in vivo
To determine if the Ly49H-mediated induced tolerance was fixed, we adoptively transferred splenocytes from m157-Tg donor mice into m157-Tg or WT recipient mice. Seven days after transfer, we assessed donor NK cells for the expression of Ly49H and for their ability to produce IFN-γ following stimulation through the NK1.1 activating receptor. By seven days, donor m157-Tg cells transferred into WT recipient mice expressed normal levels of Ly49H on their cell surface (Fig 5A). In addition, m157-Tg donor NK cells transferred into WT recipient mice produced significantly more IFN-γ as compared to identical donor cells transferred into m157-Tg recipient mice (Fig 5B). To address kinetics of this gain of function, we assessed donor m157-Tg NK cells at twenty-four and seventy-two hours after transfer. By twenty-four hours, donor m157-Tg NK cells transferred into WT recipients were expressing almost normal levels of Ly49H as compared to donor m157-Tg NK cells transferred into m157-Tg recipients. In addition, donor m157-Tg NK cells transferred into WT recipients were starting to produce more IFN-γ upon stimulation through the NK1.1 activating receptor than those transferred into m157-Tg recipients. Similar results were seen at seventy-two hours (Supplemental figure 4). Thus, upon removal of activating receptor stimulation NK cells regain normal function, suggesting that Ly49H-mediated NK tolerance is not a fixed defect in the NK cells.
FIGURE 5. Reversal of tolerance upon transfer of m157-Tg NK cells into WT mice.
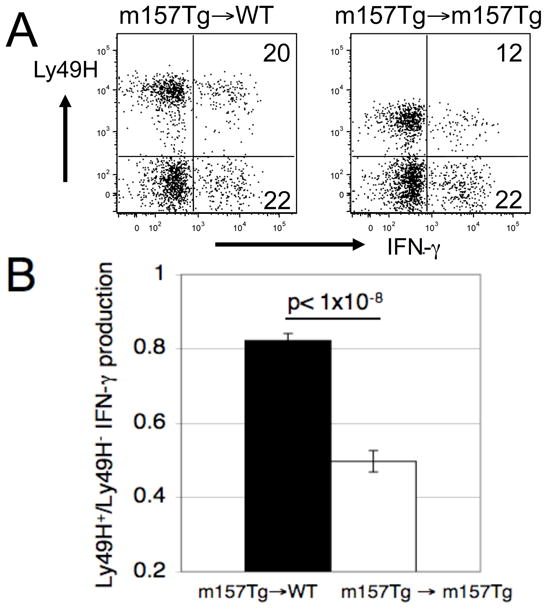
(A) Representative dot plots demonstrating IFN-γ production by freshly isolated splenocytes stimulated with plate bound anti-NK1.1 mAb. The numbers represent the percentage of Ly49H+ or Ly49H− NK cells producing IFN-γ. The dot plots were gated on donor NK cells (NK1.1+, CD3−, CFSE+ cells). (B) The ratio of the percentage of IFN-γ producing Ly49H+ NK cells to the percentage of IFN-γ producing Ly49H− NK cells from m157-Tg→ WT (n=15) or m157-Tg→ m157-Tg (n=7) mice following stimulation with plate bound anti-NK1.1 mAb. Donor cells in (A) and (B) were assessed 7 days post transfer. The results are presented as the mean ± SEM.
Discussion
The chronic engagement of the Ly49H activating receptor with m157 results in the impairment of NK cell function in vitro and in vivo (18, 19). In the studies described in this report, we demonstrate that NK cell impairment resulting from sustained engagement of Ly49H activating receptor in vivo requires signaling through the DAP12 adaptor molecule and is independent of DAP10. In addition, we demonstrate that Ly49H-mediated NK cell tolerance can be established in mature NK cells and that the Ly49H-mediated NK cell tolerance is reversible in vivo.
The use of an in vivo transgenic mouse model offers advantages over in vitro models to study activation receptor-induced NK cell hyporesponsiveness. Primarily, NK cell activation assays in these experiments were performed on freshly isolated NK cells without the need for additional cytokines necessary for growing NK cells in vitro. Although it is necessary to use cytokines in order to grow in vitro, it is unclear if these lymphokine-activated killer cells truly represent NK cell function in a mouse or human. In addition, freshly isolated murine NK cells are poor effector cells when used in killing assays. To generate more efficient killing, NK cells are grown in high levels of IL-2 for extended periods of time. In these experiments we focused on IFN-γ production and degranulation because such assays could be performed reproducibly with freshly isolated NK cells. The use of freshly isolated splenocytes likely represents true NK cell function and physiology better than cytokine stimulated cells in vitro.
Studies of transgenic mice expressing ligands (Rae-1ε, MICA) for the NKG2D activation receptor provide additional evidence for NK cell tolerance due to engagement of an activation receptor (21, 22). In addition to decreased levels of NKG2D receptor levels on the NK cell surface, sustained expression of Rae-1– resulted in a defect in natural cytotoxicity to Rae1-expressing targets, as well as more general impairment in NK cell function, such as reactivity against MHC class I-deficient targets (22). Similarly, sustained MICA expression resulted in defects in NK cell cytotoxicity against MICA-expressing target cells (21). These results are similar to the Ly49H downregulation and the defects in the Ly49H+ NK cells that we observed in the m157-Tg mice (18).
Following engagement of the Ly49H receptor with m157, there is a decrease in the level of Ly49H receptor on the cell surface of the NK cell (7, 18). It remained unclear if the engagement of the receptor alone or if signaling through the receptor was necessary for activation-receptor induced tolerance to occur. Our studies clearly demonstrate that signaling through activating receptor, using the DAP12 adaptor protein, is important for tolerance to be induced in the NK cell. Neither the presence of the ligand nor engagement of the receptor with the ligand was enough to induce NK cell defects. This was demonstrated by the fact that NK cells from m157-Tg mice in the DAP12ki background underwent down regulation of Ly49H after receptor engagement as compared to NK cells from DAP12ki mice, yet both sets of NK cells produced similar amounts of IFN-γ following stimulation. Similar results were seen when we assessed degranulation of Ly49H+ NK cells in response to stimulation with YAC target cells.
Down regulation of the Ly49H receptor was not as robust in the m157-Tg mice in the DAP12ki background as compared to the C57BL/6 background. The loss-of- function of the DAP12 protein in the DAP12ki background results in a signaling defect, which likely decreases internalization of the receptor upon engagement. However, there is still some downregulation of the Ly49H receptor seen in the m157-Tg mice in the DAP12ki background because m157 engagement of the Ly49H receptor likely decreases recognition of the receptor by the anti-Ly49H antibody. Of note, the Dap12ki NK cells produced higher levels of IFN-γ upon stimulation by NK1.1 as compared to C57BL/6 mice. This is similar to previous studies in which NK cells from DAP12 KO mice demonstrated enhanced IFN-γ production upon stimulation by NK1.1 compared to WT mice (26).
Recent studies suggest that functional activity of mature NK cells can be reset when the cells are exposed to a different MHC class I environment. Transfer of mature splenic NK cells from MHC class I- sufficient background to an MHC class I-deficient host resulted in loss of function of the donor NK cells, while transfer of splenic NK cells from MHC class I- deficient background to an MHC class I-sufficient host resulted in gain of function in the donor NK cells (27, 28). Thus, mature NK cell function appears to have some plasticity in regards to host MHC class I environment.
In agreement with these findings, our studies also demonstrate that mature NK cell can reset their functional activity when exposed to a different environment, specifically mature NK cells can acquire a tolerant phenotype upon transfer into mice that express activating receptor ligands. In addition, even though donor WT NK cells appeared to engage ligand as early as six hours post transfer into m157-Tg recipient mice (as demonstrated by the down modulation of the Ly49H receptor), they did not produce IFN-γ at baseline and there was no difference in the expression of activation markers on donor WT NK cells that were transferred into either WT or m157-Tg mice. This suggests that the donor Ly49H+ NK cells are not becoming activated upon transfer to m157-Tg recipient mice. Instead, the Ly49H+ NK cells that had matured in an m157-free environment became less responsive to stimulation after transfer into m157-Tg mice. This demonstrates that activation receptor induced hyporesponsiveness is not developmentally regulated and can be induced by activating receptors that do not recognize “self” proteins.
Not only can the hyporesponsiveness be induced in mature splenic NK cells, but it is also reversible in vivo. When splenocytes from m157-Tg mice were transferred into wild-type recipient mice, Ly49H expression levels increased on the cell surface of the NK cells and they regained function. This recovery appeared to occur within twenty-four hours post transfer. This is in agreement with previously published in vitro data (12, 18, 20). This also suggests that trans interactions of the Ly49H receptor with m157 on another cell are significantly more important than cis interactions in the induction of NK cell tolerance.
The fact that mature NK cells can become hyporesponsive upon engagement of an activating receptor is quite intriguing. Recent studies suggest that NK cells have an ability to adapt to previous stimuli and change subsequent responses (29, 30). Activating receptor-induced tolerance may represent another adaptive response of the NK cell. The induced hyporesponsiveness may be an adaptive response that provides a control mechanism to limit tissue damage by NK cells during responses to infection. However, it is possible that such adaptive responses may result in NK cells adapting to a tumor or a chronic infection rather than attacking them.
There is evidence in both mouse and human models suggesting induced NK cell hyporesponsiveness plays a role in the pathogenesis of disease. For example, the majority of NK cells in patients with acute myeloid leukemia, unlike healthy individuals, display low levels of NCRs (natural cytotoxicity receptors) and have an associated functional defect (31). Similarly, patients with HIV infection have NK cells that display low NCR levels and also have decreased function (32, 33). The basis for the decrease in NCR on the NK cells in these situations remains unclear, but it is possible that the continuous engagement of the receptors with their ligand is responsible for the low levels of the receptor and associated functional defects. In fact, recent work demonstrating that human NK cells expressing an activating receptor (KIR2DS1) together with its human leukocyte antigen (HLA) class I molecule ligand (HLA-C2), are hyporesponsive to stimulation with cellular targets (34), suggests that continuous activating receptor engagement in human NK cell can indeed result in their functional defect.
A large number of tumor cells express NKG2D ligands constitutively (35–38). The fact that NKG2D ligand expressing tumors progress suggests that NKG2D function may be impaired during tumor progression. In vitro studies have demonstrated that chronic engagement of cell bound NKG2D ligand can result in NK cell hyporesponsiveness (12, 20). Furthermore, analysis of lung adenocarcinoma infiltrating NK cells revealed decreased cytotoxicity associated with low NKG2D (39). These findings, along with our adoptive transfer studies, suggest that normal NK cells could be made hypofunctional by the engagement of an activating receptor in a setting where activation of the NK cell would be the desired outcome. These issues become extremely relevant when one considers using NK cell based therapies in the treatment of cancer and other diseases. Interestingly, recent work has demonstrated that in a transgenic mouse model where NK cells were continuously stimulated through an NKG2D receptor ligand (Rae-1), NKG2D-independent function remained intact in vivo. In particular, response to MCMV infection was unaltered in the Rae-1 Tg mice (40). It is possible that in the context of viral infection, the inflammatory milieu and released cytokines can break activating receptor induced NK cell tolerance. Clearly, further studies are required to better understand the factors in vivo that determine the outcome of activating receptor engagement (activation versus induced hyporesponsiveness).
Supplementary Material
Acknowledgments
The authors would like to thank Wayne M. Yokoyama (Washington University, St. Louis, MO) for his helpful discussions and for providing the 4E4 (anti-Ly49D), 3D10 (anti-Ly49H) and PK136 (anti NK1.1) mAbs. The DAP12ki mice were a generous gift from Eric Vivier (CNRS-INSERM-Universitede la Mediterranee, France). We are grateful to Marco Colonna and Susan Gilfillan (Washington University, St. Louis, MO) for providing the DAP10ko mice. We thank Anthony French, Rodney Newberry, Julie Elliot, Megan Cooper and Deborah Lenschow for their comments on this manuscript.
This research was supported by the Washington University Pilot and Feasibility program of the RDCC (P30 AR48335) and an award from the Edward Mallinckrodt, Jr. Foundation.
Abbreviations used in this paper
- MCMV
murine cytomegalovirus
- WT
wild type
- Tg
transgenic
References
- 1.Bahram S, Inoko H, Shiina T, Radosavljevic M. MIC and other NKG2D ligands: from none to too many. Curr Opin Immunol. 2005;17:505–509. doi: 10.1016/j.coi.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359–366. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 4.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, Bar-Ilan A, Bloushtain N, Lev M, Joseph A, Kedar E, Porgador A, Mandelboim O. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 5.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy SK, Smith HR, Holroyd EA, Pingel JT, Yokoyama WM. Expression of m157, a murine cytomegalovirus-encoded putative major histocompatibility class I (MHC-I)-like protein, is independent of viral regulation of host MHC-I. J Virol. 2006;80:545–550. doi: 10.1128/JVI.80.1.545-550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis AH, Guseva NV, Ball BL, Heusel JW. Characterization of murine cytomegalovirus m157 from infected cells and identification of critical residues mediating recognition by the NK cell receptor Ly49H. J Immunol. 2008;181:265–275. doi: 10.4049/jimmunol.181.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason LH, Willette-Brown J, Taylor LS, McVicar DW. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol. 2006;176:6615–6623. doi: 10.4049/jimmunol.176.11.6615. [DOI] [PubMed] [Google Scholar]
- 11.McVicar DW, Burshtyn DN. Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Sci STKE. 2001;2001:re1. doi: 10.1126/stke.2001.75.re1. [DOI] [PubMed] [Google Scholar]
- 12.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 13.Orr MT, Sun JC, Hesslein DG, Arase H, Phillips JH, Takai T, Lanier LL. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tassi I, Le Friec G, Gilfillan S, Takai T, Yokoyama WM, Colonna M. DAP10 associates with Ly49 receptors but contributes minimally to their expression and function in vivo. Eur J Immunol. 2009;39:1129–1135. doi: 10.1002/eji.200838972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 16.Cheng TP, French AR, Plougastel BF, Pingel JT, Orihuela MM, Buller ML, Yokoyama WM. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics. 2008;60:565–573. doi: 10.1007/s00251-008-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 18.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–1717. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 21.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 23.Tomasello E, Desmoulins PO, Chemin K, Guia S, Cremer H, Ortaldo J, Love P, Kaiserlian D, Vivier E. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 24.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 26.Takaki R, Watson SR, Lanier LL. DAP12: an adapter protein with dual functionality. Immunol Rev. 2006;214:118–129. doi: 10.1111/j.1600-065X.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 27.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 32.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 33.De Maria A, Moretta L. NK cell function in HIV-1 infection. Curr HIV Res. 2008;6:433–440. doi: 10.2174/157016208785861221. [DOI] [PubMed] [Google Scholar]
- 34.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 115:1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 35.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 37.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 38.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Le Maux Chansac B, Moretta A, Vergnon I, Opolon P, Lecluse Y, Grunenwald D, Kubin M, Soria JC, Chouaib S, Mami-Chouaib F. NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol. 2005;175:5790–5798. doi: 10.4049/jimmunol.175.9.5790. [DOI] [PubMed] [Google Scholar]
- 40.Champsaur M, Beilke JN, Ogasawara K, Koszinowski UH, Jonjic S, Lanier LL. Intact NKG2D-independent function of NK cells chronically stimulated with the NKG2D ligand Rae-1. J Immunol. 2010;185:157–165. doi: 10.4049/jimmunol.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.